Student use of energy concepts from physics in chemistry courses
Megan L.
Nagel
*a and
Beth A.
Lindsey
*b
aDepartment of Chemistry, Penn State Greater Allegheny, McKeesport, Pennsylvania, USA. E-mail: mnagel@psu.edu
bDepartment of Physics, Penn State Greater Allegheny, McKeesport, Pennsylvania, USA. E-mail: bal23@psu.edu
First published on 9th October 2014
Abstract
This paper describes an interdisciplinary investigation of students' usage of ideas about energy from physics in the context of introductory chemistry. We focus on student understanding of the idea that potential energy is a function of distance between interacting objects, a concept relevant to understanding potential energy in both physical and chemical contexts. Data from student responses to written surveys and focus-group interviews reveal that students do not spontaneously make connections between ideas they have about energy from physics classes and the understanding of energy that they develop in chemistry. We describe the development of a sequence of questions that appears to aid students in drawing these connections appropriately. We also document students' as they are confronted with and struggle to resolve the mismatch between their energy ideas from physics and chemistry.
Introduction
Energy is an important concept in chemistry as well as in the other scientific disciplines. It has been promoted as a “unifying theme” (Lancor, 2012) and a “cross-cutting concept” for science education (NRC, 2012). This has led to increased attention for discipline-based education research activities both within the individual scientific disciplines (Brewe, 2011; Scherr et al., 2013; Lindsey, 2014) and spanning disciplinary boundaries (Dreyfus et al., 2012; Lancor, 2012; Cooper and Klymkowsky, 2013). Much of the research has focused on the limitations of student understanding of energy. For chemistry specifically, numerous reports have concluded that students lack a proper foundation for understanding a variety of energy topics, including ionization energy, bonding, enthalpy and Gibbs energy, particularly as they relate to what is happening at the atomic-molecular level (Boo, 1998; Tan and Treagust, 1999; Carson and Watson, 2002; Taber, 2003; Nilsson and Niedderer, 2014). This disconnect has been linked to the highly mathematical and algorithmic way students are initially exposed to energy topics in chemistry (Hadfield and Wieman, 2010; Sreenivasulu and Subramaniam, 2013) which does not allow students to develop a deeper conceptual understanding of energy that would aid in the application of these concepts to novel situations.In addition to the struggles reported within each individual discipline, there is also a lack of unity in the way energy is presented across disciplines. Current university-level science instruction includes the presentation of energy topics using highly discipline-specific frameworks. For students, this often results in a compartmentalized view of energy, particularly for the treatment of energy at the macro- and micro-scales (Dreyfus et al., 2012). This fragmented way of teaching about energy is in direct conflict with the most recent recommendations of the National Research Council's (NRC) Framework for K-12 Science Education, which advocates for an interdisciplinary approach to energy. The NRC comments (NRC, 2012) that all energy, regardless of scientific discipline, “is best modeled as motions of particles (kinetic) or as energy stored in fields (potential)” (p. 122) and that macroscale phenomena are best understood from the perspective of what is occurring at the microscale (p. 121).
Although the NRC recommendations are for K-12 instruction, the evidence clearly supports a need for research to develop curricula that help students establish a coherent framework for energy that spans multiple scientific disciplines at the university level as well. Some current work is focused on the development of whole-course reforms for this purpose (Cooper et al., 2012; Dreyfus et al., 2014a, 2014b). However, the goal set by this paper has a more narrow focus, as it seeks to explore how, with the proper scaffolding, a functional understanding of potential energy for chemical systems can be developed from students' existing knowledge of macroscopic systems, common in introductory physics courses. This exploration was guided by the following questions:
(1) Prior to any intervention, at the conclusion of General Chemistry I, can students properly recognize the changes in potential energy associated with the relative position of two electrostatically interacting objects?
(2) What correct reasoning patterns do students demonstrate with respect to gravitational potential energy, and how can these serve as a foundation for scaffolded instruction on potential energy in chemical contexts?
In this paper, we report our findings from these questions. The data we present include semi-structured group interviews in which students demonstrate their reasoning processes as they are completing a task that leads them to link energy topics in physics and chemistry. These reasoning processes, which we refer to as “in-the-moment reasoning,” provide insight into the progression of student ideas and illuminates where their understanding may falter. We will present evidence that our materials, based on a variation of the “Elicit–Confront–Resolve” model (McDermott, 1991), lead students to reconcile their incorrect ideas by using interdisciplinary examples. We also present our initial findings from the deployment of this scaffolded instruction in the classroom context.
Background and theory
Energy in basic chemistry instruction
Energy is relevant for numerous topics in chemistry, as it is discussed in multiple contexts throughout the chemistry curriculum. As others (Feynman et al., 1963; Papadouris and Constantinou, 2010; Quinn, 2014) have noted, however, providing a suitable operational definition for energy that spans the micro- to the macroscale is non-trivial, if not impossible. A relevant question thus becomes, what level of understanding of energy is necessary and sufficient for most applications within chemistry? A range of topics, including ionization energy, bonding, phase changes, and solution formation, involve systems of interacting charges. Thus, understanding energy changes associated with these phenomena hinges on an understanding of the potential energy that results from electrostatic (Coulombic) interactions. Specifically, students must recognize that the potential energy of a system of interacting charges depends on the distances between the charges and on whether the charges attract or repel one another. This understanding is necessary for explaining a wide range of chemical processes from the atomic-molecular perspective. However, the research literature documents numerous commonly-arising difficulties that suggest that students are not developing this understanding in typical General Chemistry courses (Boo, 1998; Chang, 1999; Ebenezer and Fraser, 2001; Teichert and Stacy, 2002; Galley, 2004). Because electrostatic potential energy is of such importance to the development of a functional understanding of the energetics of many chemical phenomena, we set out to investigate the level to which this idea is understood among General Chemistry students, and what can be done to improve student understanding of potential energy.Potential energy is commonly defined as the energy having to do with location relative to something else. More specifically, for two interacting charges (q1 and q2) separated by a distance R, the potential energy is given by U = kq1q2/R. If the two charges have the same sign, they will repel one another. In this case, the formula is positive and decreasing the distance between them will increase the potential energy of the system. If the charges have opposite signs, they will attract one another. In this case, the formula is negative, and increasing the distance between them increases the potential energy of the system. Many interactions in chemistry can be reduced to attracting charges coming together or separating, from the atomic scale (ionization) to the intramolecular scale (bonding) and the intermolecular scale (phase changes). While a student may recognize, at the macroscale, that a particular process releases energy to the surroundings (is exothermic), it is the atomic-level understanding of the electrostatic interactions of the system of particles that provides the necessary explanatory power for the observation that heat is released. Thus, in order to demonstrate a functional understanding of potential energy relevant for chemistry, we expect students to recognize that potential energy increases as the distance between attracted objects increases. We believe that an understanding of this fact is necessary for a functional understanding of potential energy, but we do not claim that understanding this fact is sufficient. We argue, however, that it provides a beneficial first step. We expect that the best way to help students gain an understanding suitable for electrostatic interactions at the atomic/molecular level is by allowing them to draw connections between these interactions and macroscopic gravitational interactions. This is indeed the approach taken in many introductory chemistry textbooks.
Most often, texts for General Chemistry (Brown et al., 2012; Tro, 2012; Zumdahl and Zumdahl, 2013) introduce and define energy at the beginning of a chapter on thermochemistry. A few paragraphs are devoted to the definition of potential energy and this definition is largely supported with macroscopic, gravitational examples, such as a ball of clay held above a hard surface or a roller coaster car at the top of a hill. Potential energy is an interaction energy – it is a property of a system of two or more interacting objects that arises from the forces those objects exert on one another and depends on the relative positions of the objects (Jewett, 2008b). For the macroscopic examples presented in chemistry texts, these features of potential energy are not necessarily obvious, nor is it clear how to connect the macroscopic examples provided to chemical systems. To the expert eye, gravitational potential energy is indeed very similar to electrostatic potential energy – the functional form of the gravitational potential energy of a system of two masses (m1 and m2) separated by a distance R is given by U = −Gm1m2/R (the negative sign indicates that gravity is always an attractive force). In the near-Earth case (i.e., in the limit where the distance of the object from the surface of the Earth is much less than the radius of the Earth), however, gravitational potential energy of an object of mass m interacting with the Earth can be represented as U = mgh, where h is the height of the object above some reference point. Near-Earth examples can thus obfuscate the similarity between gravitational potential energy and electrostatic potential energy. Only rarely do the texts explicitly state that the potential energy in the macroscopic examples is a function of gravitational forces between electrically neutral objects, while the potential energy of most chemical systems is governed by electrostatic forces, i.e., interactions between two charged particles. Instruction in chemistry largely assumes that students organically make the relevant connections between the macroscopic examples provided and systems at the atomic or molecular scale, and little additional attention is given to the matter.
An approach that expects students to make many relevant connections on their own may not be warranted by the students' experience. In many cases, when they begin a General Chemistry course, students have had no formal instruction on potential energy at the university level. Even those students who have already taken or are concurrently enrolled in an Introductory Physics course will most likely have been exposed primarily to mechanics; they will not have been instructed on the energy relationships of interacting charged particles. In most cases, these topics are reserved for electricity and magnetism, typically the second course in a traditional physics sequence. Recent work by Becker and Cooper (2014) has specifically targeted how students conceptualize the topic of potential energy at the atomic-molecular scale. Their finding suggest that rather than develop a cohesive and interdisciplinary understanding of electrostatic potential energy (and its relationship to force and stability), as traditional chemistry instruction would assume, students rely on often incorrect intuitions regarding potential energy and heuristics that lack any significant reasoning power (Becker and Cooper, 2014). This mismatch between expected knowledge and actual experience can be a significant roadblock for even the most motivated learners (Taber, 2001a, 2001b).
Furthermore, the physics education research (PER) literature suggests that even for those students who have completed the introductory physics sequence, potential energy remains a problematic topic (Loverude, 2005). Many students struggle to recognize that potential energy is a function of a system of objects, and instead describe it as a property of a single object (Jewett, 2008a; Lindsey et al., 2012), a view that is reinforced by the language used in many introductory courses (Jewett, 2008b). Even when there is a correct “systems” approach to potential energy, research has shown that students are repeatedly unable to identify how the potential energy of the system will change as the relative positions of objects in the system change. In attractive contexts involving both gravitational and electrostatic attractions, after all relevant instruction, nearly half of students incorrectly conclude that an increase in distance between the two interacting objects would result in a decrease in the potential energy of the system. Students use a variety of incorrect reasoning pathways to arrive at these responses (Lindsey, 2014).
Other research reveals additional difficulties with the concept of potential energy that have potential interdisciplinary consequences. For instance, many students find the idea of negative potential energy to be difficult (Stephanik and Shaffer, 2012; Dreyfus et al., 2013). While frequently avoided when dealing with near-Earth gravitational interactions (such as are typically used to introduce the idea of potential energy in chemistry textbooks), the description of potential energy as a negative function becomes important when distinguishing between the potential energy of a system of attracting objects versus the potential energy of a system of repelling objects – both of which appear in chemistry contexts. Students also frequently conflate energy with other concepts such as power, momentum, or force (Goldring and Osborne, 1994; Lindsey, 2014). Finally, many students do not distinguish appropriately between the changes in energy of a system and of its surroundings. This may relate to students' tendency to behave as though the idea that “energy is conserved” implies that the energy of any individual system must be constant, neglecting any energy transfers that may be happening between the system and its surroundings (Lindsey et al., 2009). Student difficulties of a similar nature have also been documented in the chemical education literature around thermodynamic topics including reaction enthalpy and calorimetry, thus demonstrating a widespread and interdisciplinary struggle with even the most basic of energy topics (Boo, 1998; Barker and Millar, 2000; Greenbowe and Meltzer, 2003).
Theoretical framework for research and curriculum development
Our approach to research and curriculum development is loosely based on the “constructivism” framework. As students learn, they are constantly building connections between new ideas and existing knowledge (Ausubel, 1968; Taber, 2001a, 2001b). (Note that we use the term “knowledge” loosely to represent ideas that students hold, which may or may not be canonically “correct.”) What is “learned,” and the connections that are made, may be context-dependent. Not only that, but the existing “knowledge” that is built upon may be different in different contexts and also dependent on how each individual frames the task at hand (Redish, 2014). For example, the private mental activities of an individual may differ from those expressed in a small group. Research suggests, however, that group interactions can provide a more natural setting for students to demonstrate their learning and expertise (Scherr, 2009). As students are asked to complete a task within a small group setting, we refer to the progression of thoughts that they display as “in-the-moment reasoning.” We acknowledge that the reasoning students display in this setting might be different from the reasoning that they would display on a similar written task completed individually, but we find value in examining the understanding that students develop as it is situated in their interactions with peers. Despite the context-dependent framing of tasks that students may exhibit, we and others (Heron, 2003b) have observed that certain ideas commonly arise in response to specific tasks; if these ideas are at odds with might be canonically deemed correct, we refer to them as “difficulties” that students commonly experience.Some curricula developed in the PER community that have had great success helping students address such difficulties follow the Elicit–Confront–Resolve model (McDermott, 1991, 2001). In this model, students are typically first presented with a question designed to evoke a response that reflects a common difficulty. Next, additional evidence is provided in the form of a simple experiment, demonstration or additional question that introduces obvious conflict with the initial response. Finally, students are led to resolve this contradiction using scientifically correct conceptions and arrive at a refined and scientifically accurate result. One set of instructional materials that frequently makes use of this model is Tutorials in Introductory Physics (McDermott and Shaffer, 2002) which is designed to help students develop a functional understanding of key physics concepts. This model has been applied successfully throughout the undergraduate physics curriculum, notably in addressing difficulties with electric circuits (Shaffer and McDermott, 1992), physical optics (Wosilait et al., 1999), static equilibrium (Ortiz et al., 2005) and special relativity (Scherr et al., 2002) among many other topics.
It is in light of this body of work that we seek to develop a series of scaffolded questions for use during basic chemistry instruction. This instructional sequence will guide students to make meaningful and coherent connections to their existing knowledge from other disciplines, while abandoning previous difficulties or refining previously held incorrect beliefs. We build these materials on a research base within both chemistry and physics that addresses the difficulties that commonly arise with energy and takes advantage of what students are capable of answering correctly on their own.
This study is guided by an iterative approach to research, instruction, and curriculum design (McDermott, 2014). In this approach, pretest materials that elicit the nature and prevalence of student difficulties are developed. Student responses on these materials help inform and guide both additional research (in the form of additional written questions and small-group interviews, which allow us to delve deeper into student understanding) and the initial development of instructional materials designed to target observed difficulties. These instructional materials are assessed and refined using a two-pronged approach that involves both student interviews and written responses to post-test questions. The post-test responses allow for a quantitative examination of changes in student understanding, while the interviews illuminate student reasoning patterns and can inform changes in the next iteration of the instructional materials. This method establishes a feedback loop which allows for continued refinement and analysis of the curricular materials.
Methods
Research setting and student population
Data were collected from students enrolled in three different courses: Introductory Chemistry, multiple sections of General Chemistry I, and General Chemistry II. All three of these courses were taught at Penn State Greater Allegheny, a small branch campus of The Pennsylvania State University located in southwestern Pennsylvania. Most students complete the first two years of their degree on the branch campus and then move to a larger campus to finish their major.The Introductory Chemistry course is offered each fall, and meets every week for two 50 minute lectures plus one two-hour lab. Typical class enrollments are between 35 and 45 students (approximately 45% female, 55% male). This course is designed for students with no chemistry background. In many cases, it serves as a preparatory course for the General Chemistry sequence, covering only the most basic of topics including atomic structure, naming, balancing chemical equations, and stoichiometry. Students with an interest in STEM majors but little or no high school chemistry experience are directed to take this course before enrolling in General Chemistry I. The remaining students are taking this as a terminal chemistry course to fulfill university General Education requirements. Not only is the chemistry background of students enrolled in this course considered sparse, the majority of students enrolled in this course have minimal physics background, with a very small minority (typically 3 or fewer students) concurrently enrolled in calculus-based Introductory Mechanics. Throughout this study, the course has been taught by one of the authors of this paper (MLN). The class is taught in a partially “flipped” manner. Students were required to view a 5–10 minute online lecture and complete a related short online assessment before arriving in class each day. Class itself made extensive use of interactive engagement techniques. Students worked in groups of three or four to complete an assigned task (designed to be a direct extension of content from the video). These activities were designed by the course instructor (MLN), and generally involved group discussion based on concepts from the pre-class video and group problem solving. Traditional lecture was used for any time remaining. Apart from the single class intervention described in the “An interdisciplinary instructional sequence in the classroom” section of this paper, the course did not have any special focus on interdisciplinary examples or additional attention paid to the topics of electrostatic interactions or potential energy. Traditional General Chemistry thermodynamic topics including enthalpy, calorimetry, and the energetics of solution formation were not covered in Introductory Chemistry and were not discussed during the semester, nor was there any time devoted to ionization energy or lattice energy. The topics of intermolecular forces and phase changes were briefly covered in the class sessions immediately following the energy intervention. The course included three unit exams and one cumulative final exam.
General Chemistry I is offered during both fall and spring semesters. This course meets three times each week for 50 minutes. Typical class enrollments are 40 to 60 students (again approximately 45% female, 55% male). Unlike Introductory Chemistry, this course requires some level of math and prior chemistry preparation. A minority of students (typically < 30%) enrolled in this course have completed or are concurrently enrolled in calculus-based Introductory Mechanics. Data were collected from two sections of General Chemistry I, taught by two different instructors (one of whom is an author of this paper, MLN). Each lecture section followed a similar schedule, covering the same set of topics, and both primarily used a traditional lecture format. For both sections of this course involved in the study, energy topics were introduced in a traditional manner and included the thermodynamics topics of calorimetry, reaction and bond enthalpies, as well as the energy of solution formation. Class time was also devoted to ionization energy and lattice energy as dictated by the course textbook. No supplemental instruction on potential energy topics or special attention to interdisciplinary examples was provided.
General Chemistry II is offered only during the spring semester. This course meets three times each week for 50 minutes, with a typical enrollment of 25 to 35 students. The majority of students enrolled in this course have completed or are concurrently enrolled in calculus-based Introductory Mechanics, while a smaller fraction are concurrently enrolled in or have completed calculus-based Introductory electricity and magnetism.
Data collection and analysis
Data from this study were collected from two main sources: student responses to written surveys, and semi-structured group interviews were conducted with groups of two to three students. An overview of the data streams is provided in Table 1. All research activities were deemed “exempt” by the Penn State Institutional Review Board, and every participant involved in the study explicitly agreed to participate by signing an Informed Consent form.| Data type | Population (course) | When? | When in semester? | Purpose |
|---|---|---|---|---|
| Written survey “Chemistry energy survey” | General Chemistry I | Spring and Fall of 2013 | End of semester | Evaluate students' understanding of energy following traditional instruction |
| Written survey “Energy pretest” | Introductory Chemistry | Fall 2013 | Following instruction on molecular shapes and polarity but prior to intermolecular forces and phase changes. | Identify correct ideas about energy that students can demonstrate |
| Semi-structured group interview | General Chemistry I and II | Spring 2014 | End of semester | Elicit student reasoning patterns about energy at the atomic-molecular and macroscopic scales |
All of the written survey data were open-coded to allow for the full range of common ideas and reasoning patterns to emerge. Constant comparative analysis of the data, allowed for the iterative refinement of codes as the two sets of data were examined in light of the interview data (Corbin and Strauss, 2008). This analysis facilitated the emergence of themes with regards to what the students were able to do and to not do in relation to potential energy. To ensure a high level of agreement with the coding scheme, two researchers independently coded ∼50% of the survey responses from one course section used in this study, and the initial agreement was 80%. Each individual's interpretation and application of the codes was then compared and 100% agreement was achieved after discussion. Based on the discussion, the codes were refined and applied to the remaining data sets by a single researcher.
In addition to the differences in chemistry experiences, students who volunteered from these two courses also represented a range of experience in physics, from students who had no post-secondary physics experience, to those with some university-level introductory mechanics, and also students who had completed introductory mechanics as well as introductory electricity and magnetism. Of those students who volunteered, the semi-structured group interview participants were selected and paired purposively to group students with similar physics and chemistry backgrounds together.
The semi-structured focus-group interviews had two major goals. The first goal was to explore student understanding of electrostatic potential energy in more depth than was allowed by the written questions. The second goal was to investigate how students reason with potential energy in gravitational contexts, and to monitor whether students could make explicit connections between potential energy at the atomic level and the macroscopic scale. Careful selection of interview groups allowed us to compare behaviors of students who had completed the instructional intervention during the previous semester with those who had not. Results from the interviews could thus inform our understanding of why a particular curricular intervention might be successful as well as to suggest future modifications to instructional materials, in keeping with an iterative approach to research and curriculum development. An interview protocol was developed that allowed us to carry out these two goals in tandem.
Interviews were conducted during the last two weeks of the spring 2014 semester. Each interview lasted approximately one hour. Rather than conducting one-on-one interviews with individual students, two to three students with similar chemistry and physics backgrounds worked together to complete the interview process. This focus-group setting was designed to have the structure and interviewer control of a one-on-one interview while also reaping some of the important benefits of recorded classroom activities (Chini et al., 2009). In this setting, conversations and questions between the students drive the interview, as opposed to interactions with the interviewer.
Five interviews were conducted, with 11 total students (5 female and 6 male). A description of each interview grouping is found in Table 2 (all student names are pseudonyms chosen to accurately reflect gender). Student interviews were video recorded, and the audio was transcribed verbatim using the transcription software, InqScribe. During the interview, each group was provided a single Livescribe pen to share (http://www.livescribe.com). The pen provided a back-up recording for audio and also created an electronic record of all written work generated from each interview. Additionally, a hard copy of the written work was collected at the completion of each interview.
| Interview group | Introductory Chemistry | General Chemistry I | General Chemistry II | Mechanics | Electricity and magnetism |
|---|---|---|---|---|---|
| a E – currently enrolled in the course, C – already completed the course. | |||||
| 1 Natalie and Carla | Ea | ||||
| 2 Jeremy and Roxanne | C | E | E | ||
| 3 Jerry, Lester, and Adam | C | E | C | E | |
| 4 Richard and Trisha | C | E | |||
| 5 Ronald and Amanda | C | E | |||
All interview recordings were reviewed in full by one of the researchers (MLN). In viewing the interviews, the researcher identified major themes that emerged as each group responded to the questions and attempted to reconcile their ideas of potential energy in chemical and gravitational contexts. These themes were found to be common across multiple groups, and representative interview quotes were chosen to represent all of the major themes. The interview quotes presented in the paper are done so unedited and collaboratively viewed by the investigators to ensure agreement of the interpretation. These quotes were also chosen in light of whether or not students were able to answer specific questions correctly on their first try.
Preliminary research
Demonstrating a gap in student understanding
To gauge student understanding of electrostatic potential energy in the context of chemistry, a short survey, which we will refer to as the “Chemistry Energy Survey,” was developed. The survey was administered in General Chemistry I near the end of the semester, after students had received all relevant instruction and had been tested on enthalpy changes and solution formation (see Table 1). It was therefore expected that students would be able to correctly answer questions requiring only the most basic understanding of energy changes of atomic-molecular level systems. The questions in this survey were based on the energy changes related to aqueous solution formation, as regularly described in a General Chemistry textbook (for instance, see Zumdahl and Zumdahl, 2013, p. 489, or Brown, 2012, p. 517). This particular scenario was chosen to avoid the known difficulties students have with the concept of energy as it relates to covalent bonding (Tan and Treagust, 1999; Teichert and Stacy, 2002; Galley, 2004). This scenario did, however, allow for the investigation of students' ability to recognize changes in energy associated with the relative positions of electrostatically attracted objects at the atomic-molecular scale.The first question on the Chemistry Energy Survey, which we will refer to as the “ions” question, is shown in Fig. 1. Students were asked to identify which of the configurations shown (a or b) has more energy. In the second question, the “water molecules” question, students were shown Fig. 2 they were again asked which configuration (a or b) has a greater energy. The students were instructed to provide an explanation for their selection in each case. Students were expected to recognize that the second configuration has a greater potential energy. In each case, the overall energy of the two configurations might be the same if this increase in potential energy was offset by a decrease in kinetic energy, but in practice we did not observe any students making this connection. Note that in the “ions” question the charges of the potassium and bromide ions are made explicit, whereas in the “water molecules” question, students must recognize the existence of the dipole moment of each individual water molecule and use this to make inferences about the electrostatic interaction between the molecules. Results from the Chemistry Energy Survey are shown in Table 3.
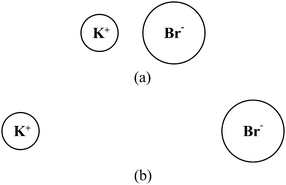 | ||
| Fig. 1 The “ions” question from the Chemistry Energy Survey. Students were asked whether the energy of the configuration is greater in (a) or (b), and to justify their response. | ||
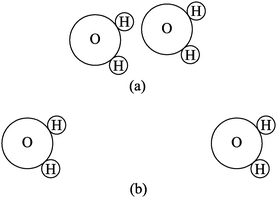 | ||
| Fig. 2 The “water molecules” question on the Chemistry Energy Survey. Students were asked whether the energy of the configuration is greater in (a) or (b), and to justify their response. | ||
In both questions, most students incorrectly identified the configuration with a smaller distance between the two objects as having greater energy. The most common justification for these incorrect responses was simply that the closer the objects are, the greater the energy of the system, without any further explanation. This response has been commonly observed in other contexts as well (Lindsey, 2014). Other common justifications provided by students to support their incorrect answers are summarized in Table 4. Students invoked a variety of concepts related to energy to justify their answer (including bonding, forces, and stability), but the vast majority did so incorrectly to support the wrong answer. The variety of justifications provided in support of the same response suggest that students might have an intuitive sense that “closer means more energy” and may search for a concept that might be used to justify that response, rather than starting from a concept and reasoning with that concept to develop a response. The phenomenon of “answer first” reasoning has been described in other contexts as well (Heron, 2003a; Kryjevskaia et al., 2014). In all of the responses for both questions, there was no evidence that students were drawing upon any knowledge of or experience with macroscopic systems to help inform their justifications.
| Student responses | |||
|---|---|---|---|
| Closer means greater energy (%) | Bonded, combined, or attached means greater energy (%) | Greater forces means greater energy (%) | |
| “Ions” question (N = 49) | 35 | 16 | 12 |
| “Water molecules” question (N = 41) | 49 | 7 | 20 |
A similar question was posed in the focus-group interview format. The “ions” question from the Chemistry Energy Survey was presented to students, but narrowed to instruct students to specify which scenario has greater potential energy. The following excerpt is from Interview Group 1, Natalie and Carla, who were in the penultimate week of General Chemistry I, but had not yet taken any university-level physics.
Carla: “I would think Fig. 1b [has greater potential energy] because if they're like separated then they have the opportunity to bond together whereas like it kind of looks like they're already bonded in Fig. 1. Wait, but then it would still be, see I don't know because then it would still have potential energy to like break apart.”
Note that Carla does initially respond correctly (indicating that Fig. 1b has greater potential energy), but then (as part of the same utterance) begins to talk herself out of this conclusion. The two students discuss this question at length, including the following representative snippet:
Natalie: “Um. So, Fig. 1a has more potential energy…”
Carla: “Stored in the bond?”
Natalie: “Possibly. Energy, um, what do you mean by energy stored in the bond, I guess.”
Carla: “Well because bonds store energy because it's at a more stable state than Fig. 1b. The breaking of the KBr compound will result in a more drastic…”
Natalie: “There's more free energy… when it's released? Well, I don't know.”
Carla: “What's the word for like… with a higher magnitude energy change versus the free K+ and Br− ions?”
Natalie: “Um also, um that would happen spontaneously [indicating the Fig. 1a configuration]. So I don't know if that has anything to do with it.”
The students ultimately use a variety of different concepts (including bonding, stability, free energy, and spontaneity) in an attempt to justify their incorrect answer. As has been reported in many cases (Boo, 1998; Teichert and Stacy, 2002; Galley, 2004) they discuss the idea that chemical bonds store energy and that energy is released if a bond is broken. Contrary to previous reports (Taber, 2009; Becker and Cooper, 2014) that students often use a relationship between low potential energy and greater stability as a heuristic for making predictions about energy changes, these students appear to associate greater stability with greater potential energy. While we do not claim that this observation is widely generalizable, this reasoning pattern was not altogether unique to this one interaction. It emerged in other interview groups and to a small extent on the written surveys (4 students provided this explanation for configuration 1a on the “ions” question), and this interview pair held tightly to this justification for their answer, as observed in their written response to the question. The pair finally agree that the first figure has more energy, beginning their written justification with: “Fig. 1 has more potential energy because it is at a more stable state than Fig. 2,” but Natalie also verbally concedes, “I don't actually know what potential energy means.”
In Interview Group 2, Jeremy and Roxanne similarly arrive at an incorrect answer to the “ions” question. These two students also use many reasons (including the space between the ions, stability, and the energy of bond formation) to justify their incorrect response to the question. Roxanne, who was enrolled in Introductory Mechanics when she completed the interview, makes explicit the disconnect that some students seem to experience between energy as it is treated in chemistry and in physics (see Dreyfus et al., 2012 for similar instances with Physics and Biology). At one point she states, “My head is going, PE = mgh and that's completely irrelevant to this situation.” Later in the same conversation, she says, “I'm not sure what potential energy is for chemistry.” Although the pair had previously had formal instruction on the topic of potential energy, it does not appear that they can easily translate any knowledge gained from that instruction to a system of electrostatically interacting objects in the context of chemistry.
Results from both the Chemistry Energy Survey and the interview questions reinforce the findings of others (Cooper and Klymkowsky, 2013; Dreyfus et al., 2014a, 2014b) that students do not spontaneously draw on energy ideas from physics classes and use these to reason about potential energy in chemistry. Instead, students must be supported in making these connections, as will be described in the next section.
Establishing a baseline for student understanding of energy
The results in the previous section suggest a need for curricula that help students reason about the energy changes associated with these simple atomic-molecular level systems. To aid in the design of the scaffolded sequence of questions, we administered the “Energy Pretest.” The pretest was created to explore what correct ideas students had regarding more familiar macroscopic systems, which might be exploited to help bridge the gap to related concepts in chemistry. The pretest was a survey administered in Introductory Chemistry prior to any formal instruction on the topic of energy. As students typically take Introductory Chemistry if they are considered underprepared for General Chemistry, we expected that this population might provide insights into the minimal knowledge that we could assume that students brought with them to a chemistry course. This knowledge could serve as a scaffold on which students could construct a more rigorous understanding of energy concepts across disciplines. We also included similar questions in the focus-group interviews designed to determine the appropriate levels of scaffolding.On the Energy Pretest, students were presented with a series of scenarios and asked to explain what is going on in each in terms of energy. Students were also instructed to consider using terms such as kinetic energy, potential energy, system, and surroundings as appropriate. The first scenario stated that, “An apple is hanging from the branch of a tree. The stem breaks. Describe what is going on from an instant just after the stem breaks until an instant just before the apple hits the ground.” Students were then asked a follow-up question in which they were to compare this situation to an identical apple hanging on a higher branch if its stem were to break.
The majority (80%) of students gave an indication that something (other than just its distance from the ground) was greater for the apple on the higher branch. Although students had been explicitly instructed to address energy in their response, many students did not do so. Nevertheless, when kinetic (26%) or potential (19%) energy was specifically invoked, it was identified as being greater for the apple on the higher branch. Both of these responses represent an appropriate understanding of how the distance between the apple and the ground influences the energy of the system. One representative student wrote, “The apple uses the saved up potential energy, but in this case, since it is higher, the apple will have more potential energy than the first apple hanging from a shorter distance.” Many students (16% of the total) who did not refer to energy explicitly did correctly cite a difference in speed or velocity of the two apples. This type of response is illustrated in the following example: “The apple will increase in velocity as it gets closer to the ground. The difference between the two apples is the [apple on the higher branch] will be travelling faster when it hits the ground compared to the [apple on the lower branch].” Although not every student could correctly articulate the difference between the two situations (possibly the result of minimal exposure to physics concepts), it should be noted that none of the students indicated that anything (kinetic energy, potential energy, velocity, etc.) was greater for the apple falling from the lower branch. This provides a strong contrast to the responses observed for the “ions” question and the “water molecules” question presented in the Chemistry Energy Survey (completed at the end of General Chemistry I), for which students consistently indicated that energy was greater when the attracting objects were closer together. These results support the idea that gravitational potential energy could indeed provide a foundation for the development of an understanding of electrostatic potential energy.
Investigating the utility of an interdisciplinary instructional sequence
The results of the Chemistry Energy Survey highlight the need for the development of curricular materials that address the lack of student understanding of potential energy. We propose that instructional materials that explicitly guide students to make connection between macroscopic gravitational contexts and the potential energy of chemical systems could be a viable route to address this need. In keeping with the iterative approach to research and curriculum development, we believe that the effects of these instructional materials are best studied using a two-pronged approach that includes both student interviews for understanding how students interact and reason about the materials and also classroom deployment of related curricular materials that provide quantitative feedback as to their success in a more traditional classroom setting. These two lines of data collection provide a feedback loop for the further development of curricular materials, and they are both described in detail below.Guiding students to make interdisciplinary connections: results from interviews
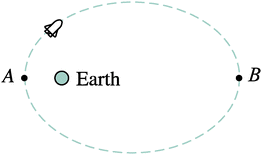
Based on the results of the energy pretest, it is clear that students typically do arrive in chemistry courses with some basic understanding of gravitational potential energy. The results of the chemistry energy survey administered at the end of General Chemistry I, however, as well as results from the focus-group interviews, make very clear that most students do not spontaneously draw on ideas about gravitational potential energy when reasoning about energy in chemistry contexts. The focus-group interviews provided a platform in which to delve deeper into student reasoning about energy by investigating how students' existing knowledge about gravitational potential energy could be leveraged to help them better reason about the energy associated with a system of electrostatically interacting objects. They also permitted us to monitor student reactions and the reasoning that was elicited as they moved between electrostatic and gravitational contexts.During the interview process, once it was established that a student group could not correctly identify which of Fig. 1a or b had a greater potential energy, the interview group was asked several follow-up questions based on gravitational potential energy. In the “space shuttle” question, presented first, the students were shown a space shuttle in a highly elliptical orbit around Earth (see Fig. 3). The students were asked whether the potential energy of the shuttle-Earth system increases, decreases, or stays the same as the shuttle moves from point A to point B. (The correct response is that the potential energy increases as the distance increases, just as in the “ions” and “water molecules” questions.) This question was chosen to follow the ion question to help serve as a bridge between the micro-scale electrostatic potential energy and macro-scale gravitational potential energy. We felt that it would fill this role because although it involves a gravitational interaction, in the “space shuttle” question, it is more clear that potential energy is due to the interactions of two objects, a fact not necessarily evident in most near-Earth gravitational examples.
Once students had answered the “space shuttle” question and given a reason justifying their response, they were presented with the “books on shelves” question (Fig. 4). This question showed two identical books on shelves of different heights. Students were asked to identify and justify which configuration has greater potential energy. The correct response is that the configuration with greater potential energy is the one in which the book is on a higher shelf. The “books on shelves” question was chosen because our experience suggested that most students would be able to respond to it correctly. We hoped to use the “books on shelves” question to draw out some correct ideas about potential energy. This familiar scenario was also to serve as a reconciliatory task to build confidence in their emerging conception of potential energy.
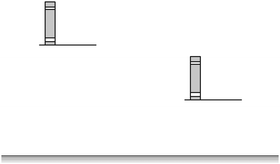 | ||
| Fig. 4 The “books on shelves” question. Students were asked to identify which configuration has a greater potential energy, and to justify their response. | ||
Student discussions as they responded to this series of questions provide some major contrasts to their discussions of the “ions” question. Immediately after reading the “space shuttle” question, Carla (from Interview Group 1) responds:
Carla: “I would say as it moves from point A to point B the potential energy would increase because the kinetic energy would decrease because it would be moving slower as it gets farther away because the gravitational pull of earth would be less.”
Not only does Carla correctly identify the position where the system has greater potential energy, she does so confidently with a clear and organized justification for her answer. Her justification is not entirely correct–the space shuttle is not moving more slowly because the gravitational pull of the Earth is less, but rather because force of the Earth has a component opposite the motion of the shuttle throughout the trip from A to B. Her behavior, however, is quite different from her initial reaction to the “ions” question.” For the “ions” question, Carla immediately second-guessed her initial correct response and then she and Natalie engaged in a lengthy and scattered discussion attempting to invoke numerous concepts to justify their incorrect response. For the “space shuttle” question, Carla and Natalie again discuss how to properly justify their answer choice, but this discussion remains focused around ideas of distance, kinetic energy, and potential energy. During this discussion, Natalie once again expresses some frustration about what potential energy means, but states:
Natalie: “I remember the example where if you hold a pencil really high up in the air it has the potential to fall and the higher the pencil is up in the air, the higher the potential energy.”
Carla: “Right because potential energy is related to its position. Kinetic energy is related to the motion. So, like I always know like they used to say like a swing set example. So when you're like at the top of the swing set, the second that you're not moving there is potential energy and then as soon as you start to move, it changes into kinetic energy. Does that make sense? So like kinetic energy is related to the movement and potential energy is related to its position, right?”
Natalie and Carla have a productive conversation in which they engage in sense-making and acknowledge a connection between potential energy and position – an idea that never emerged during their discussion of the “ions” question. Throughout the discussion of the “ions” question, Natalie had consistently questioned what is meant by potential energy, and this continues when she is presented with the “space shuttle” question. The “space shuttle” question, however, leads Natalie to invoke correct ideas that she remembers about potential energy in ways that the “ions” question did not. The two students settle on a reasonably correct response to the “space shuttle” question.
Natalie and Carla are next presented with the “books on shelves” question. Again, they immediately correctly identify which configuration has a greater potential energy. Carla provides the following rationale: “So if it would fall off the shelf, there would be more kinetic energy because it's higher above the ground. So therefore, there is more potential energy.”
At this point Natalie and Carla are asked to return to the “ions” question. They now recognize that their original response is inconsistent with the principles they have just described. Carla summarizes:
Carla: “If we're thinking about it the same way we thought about these two [the “space shuttle” and “books on shelves” questions], then this would have more potential energy because of the distance between them.”
Although they have arrived at the idea that potential energy is related to position, they do exhibit signs of reluctance to change their answer because they were satisfied with some of their initial justification, particularly those ideas related to stability.
Carla: “I feel like it makes sense when you look at it one way, but when you look at it another way, it doesn't. Because when we were working on this problem [initial attempt at “ions” question]. This [their initial justification] made sense, but then now after doing these problems [the “space shuttle” and “books on shelves” questions] and coming back to it an applying the same logic, it doesn't quite fit, but I don't think our answer is completely wrong either. So I'm just trying to figure out like…”
Natalie: “I think our reasoning wasn't incorrect, but perhaps our understanding of potential energy wasn't correct.”
Natalie and Carla struggle to resolve their initial response to the “ions” question with their newly gained understanding of potential energy.
Natalie: “Where if something is stable, then maybe there isn't a lot of potential for energy to happen because it's happy where it is. Like, the book on the ground versus a book on the shelf is not going to move because it's more stable.”
Carla: “Well actually, our reasoning with the stable state, kind of makes sense, but we just have to think about it in the opposite way.”
Natalie: “Fig. 2 has more potential energy.”
Ultimately for this question, Natalie and Carla are confident that Fig. 1b represents a system with greater potential energy. This suggests that working through two gravitational examples – the “space shuttle” and the “books on shelves” questions –helped Carla and Natalie develop a deeper understanding of potential energy that they could apply to the “ions” question. Not only that, but in this conversation, Carla and Natalie demonstrate that they are able to extend their ideas about potential energy to reach novel conclusions. They draw on ideas from the “books on shelves” question to spontaneously generate the idea that greater stability implies less potential energy.
The experience of Natalie and Carla was not unique. Three of the five interview groups initially responded incorrectly to the “ions” question (choosing the configuration where the distance between the ions was smaller to have greater potential energy). Each of these three groups was then given the “space shuttle” and the “books on shelves” questions. The groups not only responded correctly to the two follow-up questions, but with each group, as was illustrated with Carla and Natalie, a relationship between distance and potential energy emerged from the discussion in such a way that allowed students to apply it to the atomic-level system. Following the interview process, Jerry, a student from Interview Group 3, observed that “the whole thing is just one idea.”
The results from the semi-structured group interviews suggested that students readily recognize the relationship between potential energy at the macro-scale and at the atomic-molecular scale when presented with appropriate scaffolding. The group interview participants engaged in exemplary levels of sense-making as they worked through the materials. This may have been due in part to the presence of an interviewer in the room with them – they may have framed the interview experience differently than they would a typical classroom experience. In the next section, we describe whether the effects of an intervention similar to the sequence of tasks presented in interviews were replicable at the scale of an entire class.
An interdisciplinary instructional sequence in the classroom
The results from the “Chemistry Energy Survey” suggest that students are not leaving General Chemistry I with an understanding of how distance between particles is related to the energy of the system. Additionally, the results of the Energy Pretest suggest that the correct ideas about gravitational potential energy that students demonstrate might serve as a foundation for scaffolded instruction in chemistry. Therefore an intervention was designed, deployed, and assessed in one section of Introductory Chemistry, a course largely populated with students having little chemistry or physics background (serving as a General Chemistry prerequisite for many students). Like the interview process, the classroom intervention was designed to provide students with scaffolded instruction that allowed them to make the appropriate connections between potential energy at the macroscale and the atomic-molecular level.When students arrived in class, they spent the 50 minute lecture period working through a tutorial developed in the style of Tutorials in Introductory Physics (McDermott and Shaffer, 2002). The tutorial was designed to reemphasize the major points from the video, but also to provide some conceptual depth, without requiring students to have any physics knowledge beyond what was presented to them. Students were guided through questions that required them to recognize that potential energy for a system of attracting objects does not increase or decrease in the same way as the strength of the attraction (i.e., as two attracting objects move farther apart, the magnitude of the force they exert on one another decreases while the potential energy of the system increases). They also were introduced to the functional form for electrostatic potential energy (U = kq1q2/R). They responded to questions that required them to justify the negative sign in the function for attracted objects, and made use of graphs of potential energy as a function of the distance between two interacting objects. While the tutorial began by having students consider macroscopic objects, it ultimately presented students with some electrostatically attracted systems in the context of chemistry, including oppositely charged ions and also two polar molecules experiencing an intermolecular attraction. Here students were led to consider not only the relative distance between the particles and its relation to the potential energy of the system, but also how the resultant changes in energy of the system might be related to energy changes of the surroundings.
To explore the depth to which students had learned potential energy concepts, two groups of students (Groups 4 and 5) who had completed Introductory Chemistry (including the pre-class video and tutorial) the previous semester as a prerequisite for General Chemistry I were included as participants in the semi-structured group interviews. These interviews took place more than a full semester after the students had completed the tutorial in Introductory Chemistry. None of the students in either group had taken any university-level physics. At the time of the interview, all of the students were enrolled in a section of General Chemistry I taught by a different instructor, who was not familiar with the tutorial materials or the approach to potential energy that students had been exposed to in Introductory Chemistry. During the interview, when presented with the “ions” question (before seeing either the “space shuttle” question or the “books on shelves” question), both groups of students immediately and confidently responded that Fig. 1b represented a greater potential energy. In Interview Group 4, Richard and Trisha provide the following justifications for their selection:
Richard: “It has more potential in Fig. 1b. The further away, the more potential it has to come together. Like if it were a rubber band, it has more potential to…”
{He uses his hands to mimic stretching and holding a rubber band at some distance.}
Richard: “…you know what I mean. It has more potential in Fig. 1b.”
Trisha: “This is a plus and this is a minus. Wait…”
Richard: “That has something to do with it.”
{A few minutes pass as they discuss the size and magnitude of the ions.}
Richard: “Fig. 1b has more potential energy because in Fig. 1b, they are father apart which would cause the surroundings to lose energy to the system, therefore, having more potential energy.”
In Interview Group 5, Ronald and Amanda take a slightly different approach:
Ronald: “This one has… Fig. 1b… the farther two object are…”
Amanda: “…away, the greater their potential.”
Ronald: “Fig. 1b. The farther two objects are from each other, the greater the potential energy they will contain… assuming they aren't moving.”
Interviewer: “Why does farther apart mean greater potential energy?”
Amanda: “Because kinetic energy is the energy… that is by moving. So, the farther it is the more it has to gain kinetic energy. I remember talking about it as the potential to gain kinetic energy.”
Ronald: “It has to do with two objects being attracted because they're opposites.”
Amanda: “Yeah, so it has the potential to gain energy of movement.”
Ronald: “Greater potential energy equates to greater kinetic energy because…”
Amanda: “You have two attractive forces.”
The responses of these two groups suggest that students had not simply memorized a rule that greater distance implies greater potential energy. Richard initially references the distance, but he immediately connects this to a rubber band, a relevant macroscopic illustration of potential energy that had not been presented in the tutorial materials. Richard accounts for the differences in potential energy of the two systems as a result of some contribution of energy from the surroundings, recognizing that the energy of a system is not necessarily constant, as many students appear to believe (Lindsey et al., 2009). (The energy of this system may or may not actually be changing, as the kinetic energy of these two scenarios was intentionally left ambiguous.) Ronald and Amanda take the approach that potential energy is the potential to gain kinetic energy. They recognize that the farther apart two attracted objects are, the more kinetic energy they could potentially gain. Furthermore, both groups explicitly mention the fact that the two ions are oppositely charged. Although these students had only been exposed to attractive cases in the course of the tutorial materials, their responses suggest that they realize the attractive nature of the scenario is relevant to their answer. In fact, on the “water molecules” question, Ronald notes, “If the hydrogens were flipped {pointing at one of the water molecules – indicating a repulsive case between two water molecules}, then it would be different.”
Of the five interview groups (four of which were selected from the same section of General Chemistry I, while the remaining group was near the end of General Chemistry II instruction), only the two groups of students who had been exposed to the tutorial materials in introductory chemistry were able to correctly answer the initial “ions” question without the scaffolding provided by the “space shuttle” and the “books on shelves” questions. Furthermore, many ideas that commonly arose in other interviews are absent in the discussions of the former Introductory Chemistry students. Neither group gives any indication that they believe that bond breaking releases energy, nor do they attempt to conflate greater stability, attraction or closeness with greater potential energy. These had all been common incorrect justifications provided by students who had not worked through the tutorial materials. In fact, interview responses and reasoning patterns demonstrated by the group nearing the completion of General Chemistry II were strikingly similar to those finishing General Chemistry I (who had not taken Introductory Chemistry), suggesting that simply having greater exposure to chemistry instruction is not an alternative remedy for bridging this gap in understanding potential energy. While the former Introductory Chemistry students could successfully complete the initial group interview tasks (in contrast to their General Chemistry I peers), further research is required to unambiguously link their success to their experiences with the Introductory Chemistry tutorial materials, and not to other factors (for instance, discussions outside of class, repeated exposure to the topics, or instruction from another source).
Extension of student understanding for repulsive cases
Four of the five groups had time to participate in a final question during the interview. In this question, students were asked a variant of the “water molecules” question (Fig. 2) in which one of the water molecules has been flipped such that they repel one another. All interview participants recognized that this scenario represented something different from the attractive cases they had previously analyzed. As a result, they did not conclude that potential energy increased for the repulsive scenario, as evidenced by this exchange between Roxanne and Jeremy (Interview Group 2):Roxanne: “If you pull these two apart, [potential energy] is going to decrease, because it's a repulsion force, but the repulsion force isn't going to be as effective if distance is greater. So the energy there should decrease.”
Jeremy: “They're the same charge so they're going to repel each other. There isn't much attractive force between them. So, when they get farther apart, the PE would decrease versus increase. The closer together they are… maybe think about it like this, it's almost like you have a spring that you compress together and it obviously wants to push back so it's kind of like that. So there's a lot of potential energy when you compress a spring and then all that potential energy gets released when you finally release that spring and it can finally spring forward.”
These two students realize that this scenario is different from the attractive case, and they are also able to extend their understanding of potential energy to come to a correct conclusion: the potential energy of the system actually decreases with increasing distance between the repelling particles. Jeremy does so by drawing on a macroscopic example (a spring) that had not previously been invoked in the interview materials. Although it is unclear whether these students may be conflating potential energy and force in this situation, it is clear that they treat this situation differently from the attractive case and are able to use a macroscopic example from personal experience to help describe the energy of the molecular-scale interaction. This suggests that students can apply the understanding they gain from working through the scaffolded question sequence to novel situations.
Conclusions
In this paper, we have demonstrated that traditional college chemistry instruction is not sufficient to allow students to develop a functional understanding of potential energy in chemical systems. Without guidance, students do not make the connections that most General Chemistry textbooks assume they will make. Results from data collected near the end of the first semester of General Chemistry, including written problems and semi-structured group interviews, reveal that students do not spontaneously connect ideas about gravitational and other macro-scale forms of potential energy to electrostatic potential energy at the micro-scale, which is most relevant for many applications in chemistry. While others have previously commented on this mismatch (Cooper et al., 2014; Lindsey, 2014) and have suggested a need for instruction that explicitly draws these connections for students (Becker and Cooper, 2014) we are the first to document students' reasoning as they encounter and resolve this mismatch.Rather than seeking to engage in a whole-course reform as others have done (Cooper et al., 2012; Dreyfus et al., 2014a, 2014b; Redish et al., 2014), we have set out to explore if a series of scaffolded questions can help students develop an understanding of potential energy that spans disciplines. An in-class intervention designed to target common difficulties appears to aid students in responding correctly to questions about the potential energy changes of a system of attracting charges. Results from small-group interviews provide insights as to why this intervention may help, by demonstrating that a brief and targeted intervention can aid students in bridging the gap between the generally correct ideas they hold about gravitational potential energy in near-Earth systems and the potential energy of Coulombic interactions of atomic-molecular level systems. These scaffolded questions appear to provide students with an obvious link to existing knowledge. There is also evidence that students can be directed away from common difficulties, specifically the commonly-reported idea that energy is stored in chemical bonds. The semi-structured group interviews further demonstrate that after engaging with questions designed to help them link gravitational and electrostatic energy, students were able to correctly analyze the changes in potential energy of repulsive systems, despite the fact that they had so far only considered attractive systems. Following exposure to the intervention materials, students also begin to spontaneously draw connections between electrostatic potential energy and other forms of potential energy at the macroscale (such as the potential energy stored in a spring) even though these connections were not explicitly prompted by the intervention.
This investigation has targeted a fairly narrow concept that is nonetheless critical to the development of a functional understanding of energy; namely, the idea that potential energy increases as the distance between attracting objects increases. We have developed a sequence of questions that allow students to make interdisciplinary connections regarding potential energy and appears to improve their understanding of this idea, which is relevant for a wide range of chemistry topics that are related to the relative positions of electrostatically interacting particles, including intermolecular forces, phase changes, and chemical bonding. These results will inform the refinement of future curricular materials, yet it remains to be seen how the improvement in student understanding of potential energy that results from our intervention will impact student learning of these and other chemistry topics.
Acknowledgements
Thanks are due to Ed Bittner and Alandra Kahl for allowing us access to their classes to administer surveys and recruit interview participants. Thanks are also due to the Office of Academic Affairs at Penn State Greater Allegheny, for financial support for the project.References
- Ausubel D. P., (1968), Educational Psychology: A Cognitive View, New York: Holt, Reinhart, and Winston, Inc.
- Barker V. and Millar R., (2000), Students' reasoning about basic chemical thermodynamics and chemical bonding: what changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 22(11), 1171–1200.
- Becker N. and Cooper M. M., (2014), College chemistry students' understanding of potential energy in the context of atomic-molecular interactions, J. Res. Sci. Teach..
- Boo H. K., (1998), Students' understandings of chemical bonds and the energetics of chemical reactions, J. Res. Sci. Teach., 35(5), 569–581.
- Brewe E., (2011), Energy as a substancelike quantity that flows: theoretical considerations and pedagogical consequences, Phys. Rev. Spec. Top. – Phys. Educ. Res., 7(2), 020106.
- Brown T. L., LeMay H. E., Bursten B. E., Murphy C. J. and Woodward P. M., (2012), Chemistry: The Central Science, 12th edn, Boston: Prentice-Hall.
- Carson E. M. and Watson J. R., (2002), Undergraduage students' understanding of entropy and Gibbs free energy, Univ. Chem. Educ., 6, 4–12.
- Chang J., (1999), Teachers college students' conceptions about evaporation, condensation, and boiling, Sci. Educ., 83(5), 511–526.
- Chini J. J., Carmichael A., Rebello N. S. and Puntambekar S., (2009), Does the Teaching/Learning Interview Provide an Accurate Snapshot of Classroom Learning? in Sabella M., Henderson C. and Singh C. (ed.), Proceedings of the 2009 Physics Education Research Conference, Ann Arbor, MI: AIP Conference Proceedings, vol. 1179, pp. 113–116.
- Cooper M. M. and Klymkowsky M. W., (2013), The trouble with chemical energy: why understanding bond energies requires an interdisciplinary systems approach, CBE Life Sci. Educ., 12(2), 306–312.
- Cooper M. M., Underwood S. M., Hilley C. Z. and Klymkowsky M. W., (2012), Development and Assessment of a Molecular Structure and Properties Learning Progression, J. Chem. Educ., 89(11), 1351–1357.
- Cooper M. M., Klymkowsky M. W. and Becker N., (2014), Energy in Chemical Systems: An Integrated Approach, in Chen R. F., Eisenkraft A., Fortus D., Krajcik J., Neumann K., Nordine J. and Scheff A. (ed.), Teaching and Learning of Energy in K-12 Education, Cham: Springer International Publishing, pp. 301–316.
- Corbin J. and Strauss A., (2008), Basics of Qualitative Research: Techniques and procedures for developing grounded theory, 3rd edn, Thousand Oaks: Sage Publications.
- Dreyfus B. W., Geller B., Gouvea J., Sawtelle V., Turpen C. and Redish E. F., (2013), Negative energy: why interdisciplinary physics requires multiple ontologies, in Engelhardt P. V., Churukian A. and Jones D. (ed.), Proceedings of the Physics Education Research Conference, Portland, OR.
- Dreyfus B. W., Gouvea J., Geller B. D., Sawtelle V., Turpen C. and Redish E. F., (2014a), Chemical energy in an introductory physics course for the life sciences, Am. J. Phys., 82(5), 403–411.
- Dreyfus B. W., Sawtelle V., Turpen C., Gouvea J. and Redish E. F., (2014b), Students' reasoning about “high-energy bonds” and ATP: a vision of interdisciplinary education, Phys. Rev. Spec. Top. – Phys. Educ. Res., 10(1), 010115.
- Dreyfus B. W., Redish E. F. and Watkins J., (2012), Student views of macroscopic and microscopic energy in physics and biology, in Rebello N. S., Engelhardt P. V. and Singh C. (ed.), Proceedings of the 2011 Physics Education Research Conference, AIP Conference Proceedings, Omaha, NE, vol. 1413, pp. 179–182.
- Ebenezer J. and Fraser D., (2001), First year chemical engineering students' conceptions of energy in solution processes: phenomenographic categories for common knowledge construction, Sci. Educ., 85(5), 509–535.
- Feynman R., Leighton R. and Sands M., (1963), The Feynman Lectures on Physics, 2nd edn, New York: Addison-Wesley.
- Galley W. C., (2004), Exothermic Bond Breaking: A Persistent Misconception, J. Chem. Educ., 81(4), 523–525.
- Goldring H. and Osborne J., (1994), Students' difficulties with energy and related concepts, Phys. Educ., 29, 28–32.
- Greenbowe T. and Meltzer D., (2003), Student learning of thermochemical concepts in the context of solution calorimetry, Int. J. Sci. Educ., 25(7), 779–800.
- Hadfield L. C. and Wieman C. E., (2010), First Law of Thermodynamics, J. Chem. Educ., 87(7), 750–755.
- Heron P. R. L., (2003a), Emperical Investigations of learning and teaching, part II: Developing reserach-based instructional materials, in Redish E. F. and Vincentini M. (ed.), Proceedings of the International School of Physics “Enrico Fermi” Course CLVI: Research on Physics Education, Varenna, Italy: IOS Press, pp. 351–365.
- Heron P. R. L., (2003b), Empirical investigations of learning and teaching part I: Examining and interpreding student thinking, in Redish E. F. and Vincentini M. (ed.), Proceedings of the International School of Physics “Enrico Fermi” Course CLVI: Research on Physics Education, Varenna, Italy: IOS Press, pp. 341–350.
- Jewett J. W., (2008a), Energy and the Confused Student II: Systems, Phys. Teach., 46(2), 81–86.
- Jewett J. W., (2008b), Energy and the Confused Student III: Language, Phys. Teach., 46(3), 149–153.
- Kryjevskaia M., Stetzer M. R. and Grosz N., (2014), Answer first: applying the heuristic-analytic theory of reasoning to examine student intuitive thinking in the context of physics, Phys. Rev. Spec. Top. – Phys. Educ. Res., 10(2), 020109.
- Lancor R. A., (2012), Using Student-Generated Analogies to Investigate Conceptions of Energy: A Multidisciplinary Study, Int. J. Sci. Educ., 36(1), 1–23.
- Lindsey B. A., (2014), Student reasoning about electrostatic and gravitational potential energy: an exploratory study with interdisciplinary consequences, Phys. Rev. Spec. Top. – Phys. Educ. Res., 10(1), 013101.
- Lindsey B. A., Heron P. R. L. and Shaffer P. S., (2009), Student ability to apply the concepts of work and energy to extended systems, Am. J. Phys., 77(11), 999–1009.
- Lindsey B. A., Heron P. R. L. and Shaffer P. S., (2012), Student understanding of energy: difficulties related to systems, Am. J. Phys., 80(2), 154–163.
- Loverude M. E., (2005), Student Understanding of Gravitational Potential Energy and the Motion of Bodies in a Gravitational Field, in Heron P., McCullough L. and Marx J. (ed.), Proceedings of the 2004 Physics Education Research Conference, AIP Conference Proceedings, Sacramento, CA, vol. 790, pp. 77–80.
- McDermott L. C., (1991), Millikan Lecture 1990: What we teach and what is learned—Closing the gap, Am. J. Phys., 59(4), 301–315.
- McDermott L. C., (2001), Oersted Medal Lecture 2001: “Physics Education Research—The Key to Student Learning,” Am. J. Phys., 69(11), 1127–1137.
- McDermott L. C., (2014), Melba Newell Phillips Medal Lecture 2013: discipline-Based Education Research—A View From Physics, Am. J. Phys., 82(8), 729–741.
- McDermott L. C. and Shaffer P. S., (2002), Tutorials in Introductory Physics, Upper Saddle River, NJ: Prentice-Hall.
- Nilsson T. and Niedderer H., (2014), Undergraduate students' conceptions of enthalpy, enthalpy change and related concepts, Chem. Educ. Res. Pract., 15, 336–353.
- NRC, (2012), A Framework for K–12 Science Education: Practices. Crosscutting Concepts, and Core Ideas, Washington, DC, Retrieved from http://www.nap.edu/openbook.php?.
- Ortiz L. G., Heron P. R. L. and Shaffer P. S., (2005), Student understanding of static equilibrium: predicting and accounting for balancing, Am. J. Phys., 73(6), 545–553.
- Papadouris N. and Constantinou C. P., (2010), A Philosophically Informed Teaching Proposal on the Topic of Energy for Students Aged 11–14, Sci. Educ., 20(10), 961–979.
- Quinn H. R., (2014), A Physicist's Musings on Teaching About Energy, in Chen R. F., Eisenkraft A., Fortus D., Krajcik J., Neumann K., Nordine J. and Scheff A. (ed.), Teaching and Learning of Energy in K-12 Education, Cham: Springer International Publishing, pp. 15–36.
- Redish E. F., (2014), Oersted lecture 2013: How should we think about how our students think? Am. J. Phys., 82(6), 537–551.
- Redish E. F., Bauer C., Carleton K. L., Cooke T. J., Cooper M., Crouch C. H., Zia R. K. P., et al., (2014), NEXUS/Physics: an interdisciplinary repurposing of physics for biologists, Am. J. Phys., 82(5), 368–377.
- Scherr R., (2009), Video analysis for insight and coding: examples from tutorials in introductory physics, Phys. Rev. Spec. Top. – Phys. Educ. Res., 5(2), 020106.
- Scherr R., Shaffer P. and Vokos S., (2002), The challenge of changing deeply held student beliefs about the relativity of simultaneity, Am. J. Phys., 70(12), 1238–1248, http://scitation.aip.org/content/aapt/journal/ajp/70/12/10.1119/1.1509420.
- Scherr R. E., Close H. G., Close E. W., Flood V. J., McKagan S. B., Robertson A. D., Vokos S., et al., (2013), Negotiating energy dynamics through embodied action in a materially structured environment, Phys. Rev. Spec. Top. – Phys. Educ. Res., 9(2), 020105.
- Shaffer P. S. and McDermott L. C., (1992), Research as a guide for curriculum development: an example from introductory electricity. Part II: design of instructional strategies, Am. J. Phys., 60(11), 1003–1013.
- Sreenivasulu B. and Subramaniam R., (2013), University Students' Understanding of Chemical Thermodynamics, Int. J. Sci. Educ., 35(4), 601–635.
- Stephanik B. M. and Shaffer P. S., (2012), Examining student ability to interpret and use potential energy diagrams for classical systems, in Rebello N. S., Engelhardt P. V. and Singh C. (ed), Proceedings of the 2011 Physics Education Research Conference, AIP Conference Proceedings, Omaha, NE, 1314, 1413, pp. 367–370.
- Taber K. S., (2001a), Building the structural concepts of chemisry: some considerations from educational research, Chem. Educ.: Res. Pract. Eur., 2(2), 123–158.
- Taber K. S., (2001b), The Mismatch between Assumed Prior Knowledge and the Learner's Conceptions: a typology of learning impediments, Educ. Stud., 27(2), 159–171.
- Taber K. S., (2003), Understanding ionisation energy: physical, chemical and alternative conceptions, Chem. Educ. Res. Pract., 4(2), 149–169, http://pubs.rsc.org/en/content/articlehtml/2003/rp/b3rp90010j.
- Taber K. S., (2009), College Students' Conceptions of Chemical Stability: the widespread adoption of a heuristic rule out of context and beyond its range of application, Int. J. Sci. Educ., 31(10), 1333–1358.
- Tan K.-C. D. and Treagust D., (1999), Evaluating Students' Understanding of Chemical Bonding, Sch. Sci. Rev., 81(294), 75–83.
- Teichert M. A. and Stacy A. M., (2002), Promoting understanding of chemical bonding and spontaneity through student explanation and integration of ideas, J. Res. Sci. Teach., 39(6), 464–496.
- Tro N. J., (2012), Chemistry in focus: A Molecular View of Our World, 5th edn, Belmont, CA: Cengage Learning.
- Wosilait K., Heron P. R. L., Shaffer P. S. and McDermott L. C., (1999), Addressing student difficulties in applying a wave model to the interference and diffraction of light, Am. J. Phys., 67(7), S5–S15.
- Zumdahl S. S. and Zumdahl S. A., (2013), Chemistry, 9th edn, Belmont, CA: Cengage Learning.
| This journal is © The Royal Society of Chemistry 2015 |