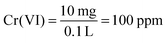Teaching the properties of chromium's oxidation states with a case study method
Zehra
Ozdilek
Uludag University – Faculty of Education, Science Education Department, 16059, Gorukle, Bursa, Turkey. E-mail: zozdilek@uludag.edu.tr; Fax: +90 2242942199; Tel: +90 2242942296
First published on 6th October 2014
Abstract
The purpose of this study was to investigate how a mixed-method case study affects pre-service science teachers' awareness of hexavalent chromium pollution and content knowledge about the properties of chromium's different oxidation states. The study was conducted in Turkey with 55 sophomores during the fall semester of 2013–2014. The students were taught using a case study about chromium's properties, the qualitative and quantitative analysis methods used with chromium compounds, the effects of chromium compounds on human health, and the chemical techniques that can be used to remove hexavalent chromium from wastewater. Open-ended questions were applied to determine the students' pre- and post-knowledge before and after instruction. An open-ended questionnaire and semi-structured interviews showed that the case study had a positive effect on all participants in terms of increasing their awareness of the hazardous effects of hexavalent chromium. Pair sample and independent sample t-test results revealed that the presenting and the audience groups significantly increased their content knowledge after instruction. On the other hand, while there was no statistically significant difference between the groups on verbal questions, there was a difference between the presenting and the audience groups on calculative questions. This finding shows that the mixed-method case instruction might not have affected all subjects in the same way.
Introduction
Chromium is one of the most frequently detected soil and groundwater contaminants due to its widespread use in industrial applications, such as leather tanning, metallurgy, electroplating, and refractory, which have resulted in chromium contamination of natural water (Oktor et al., 2008; Kumar and Riyazuddin, 2011). Chromium usually occurs in two oxidation states, trivalent chromium and hexavalent chromium (represented as Cr(III) and Cr(VI) respectively), which have different toxicities, mobility and bioavailability (Kong and Ni, 2009).In natural systems, Cr(III) is generally precipitated as hydroxide solids or adsorbed onto mineral surfaces as complex ions in soil and aquatic environments. Therefore, Cr(III) has relatively low toxicity and mobility and is also an essential nutrient in low doses. In contrast, most Cr(VI) compounds are highly toxic, soluble, mobile, and carcinogenic (Tirez et al., 2011). However, Szalinska et al. (2010) have suggested that both forms can negatively affect biota.
From an ecotoxicological perspective, the identification and quantification of chromium species has been very important. Consequently, the reduction of Cr(VI) has been widely investigated due to its threat to humans, animals and plants (Dai et al., 2009). Cr removal from certain contaminated groundwater is another important issue in environmental remediation. Cr(VI) is a strong oxidant and reacts quickly with several reducing agents (e.g., Fe0, Fe2+, S2−, and natural organic matter) to form Cr(III). On the other hand, Cr(III) is thermodynamically stable under reducing conditions and is oxidized to Cr(VI) by Mn(III, IV) (hydr)oxides or photo-oxidized by FeOH2+ (Bokare and Choi, 2011). Several treatment techniques have been used to remove Cr(VI) from aqueous waste streams: the electrochemical addition of Fe(II) (Mukhopadhyay et al., 2007), electrocoagulation (Dermentzis et al., 2011), and the activated carbon method (Kobya, 2004). Lai and McNeill (2006) stated that understanding chromium's redox chemistry under such conditions is important when choosing the appropriate treatment method and determining potential health threats.
Thus, an effective way to remove Cr(VI) from groundwater is to reduce it to Cr(III) under neutral to alkaline conditions (Lee and Hering, 2005). In short, hexavalent chromium is a very functional element in terms of its industrial uses, but it is also very harmful to human health. According to EPA, the acceptance limit for Cr(VI) is 0.1 mg l−1 in surface water and 0.05 mg l−1 in drinkable water (Kobya, 2004).
Purpose and research questions
Starting from this highly important environmental issue, the present study attempts to increase pre-service science teachers' knowledge of the properties of two chromium oxidation states and the removal methods for Cr(VI). Teachers are known to play a vital role in affecting and encouraging student interest in environmental issues (Teksoz et al., 2010), as science teachers possess knowledge structures that they communicate to children when they perform science (Bischoff et al., 2010). Therefore, the main aim of the study is to apply a case study method to educate pre-service science teachers about the pollution caused by Cr(VI), which is a great threat to the environment and human health.Theory and practice should be integrated into the contents of teacher education practices. We can assist our pre-service science teachers' knowledge structures by applying various educational strategies based on constructivist theory. One useful method for this purpose is a case study teaching method, in which students apply their content knowledge to solve real-life situations. Thus, case studies are useful for research and teaching that focus on the bridge between theory and practice (Breslin and Buchanan, 2008). Camill (2006) stated that case studies allow students to gain content knowledge, process skills, understand the context and use science in their daily lives. Learners should analyze the case and find possible solutions to the problems (Gallego et al., 2013).
There is much academic literature that argues for the effectiveness of case study methods (Dochy et al., 2003; Camill, 2006; Delpier, 2006; Yadav, 2008; Chaplin, 2009; Healy and Mccutcheon, 2010; Harrison, 2012; Casotti et al., 2013; Gallego et al., 2013). However, it is important to assess students' learning and determine how case studies can be applied in a better way (Garvin, 2004). Avoiding the limited application of case studies in crowded classrooms is a key part of this process (Ayyıldız and Tarhan, 2013). Moreover, few, if any, studies have focused on the effectiveness of using mixed-method cases with small groups in the large classes and then reflecting on the small groups' individual tasks in whole class discussions. Many studies have highlighted the necessity of using discussion methods alongside case study teaching methods. There are common goals in the application of case studies, and objectives must be clearly defined by instructors; this method should develop learners' analytical skills, and student participants must be at a high level (Herreid, 2006). Therefore, the secondary aim is to determine whether mixed-method, case-based instruction is appropriately implemented to meet these goals.
Briefly, due to the reasons discussed above, the study aimed to determine how effective the case method was in teaching the properties of chromium's different oxidation states and alerting participants to chromium pollution with a mixed-method type. The study was guided by the following questions:
(1) Was the case study effective in terms of drawing attention to the environmental pollution caused by chromium?
(2) Was the case study effective in improving content knowledge about chromium?
(3) To what extent was the mixed-method case study effective in achieving the aims of the study?
Theoretical framework
Case studies surfaced as an educational strategy more than 100 years ago at the Harvard Business School. Since then, the various disciplines (e.g., law, education, and medicine) have successfully included case studies in teaching materials (Delpier, 2006). Reynolds (1980); cited in Herreid (1994) classified case study types as (1) decision and dilemma cases, (2) issue cases, and (3) case histories. In decision and dilemma cases, a short introductory paragraph includes historical information to help students understand the situation. A narrative section then presents the problems that the main character faced in the case. This section can be used in issue cases to develop students' analytical skills. It requires students to pay attention to some important questions (e.g., “What is going on here?”), and students must make a decision in a challenging situation. Examples are given with a description, papers, data, and arguing articles without a main character. Case histories usually include stories that present descriptive models of science in action (e.g., Copernican revolution or cold fusion).There are various implementation types for case studies, i.e., analysis actions and resulting student outcomes, and these types provide a wide range of mix combinations to achieve a particular teaching and learning objective (Scott, 2007). In his studies, Herreid (2005, 2006, 2011) stated that cases can be presented in the classroom using seven basic methods:
(1) The lecture method presents the information in a context based on one of the method's advantages. The instructor acts as storyteller, and students are passive recipients of information.
(2) Whole class discussion, a classic teaching method for case studies, is conducted by the course instructor to gauge students' ideas.
(3) The small-group method is especially effective in stimulating diverse opinions and improving the expression of ideas. Students groups work exclusively with the instructor to solve problems, usually obtain information over several class periods with opportunities to research the topic.
(4) Individual cases ask students to write a dialogue between two opposing views on a controversial topic (e.g., global warming). The students must state their own opinion and reasons for it at the end of the dialogue.
(5) In the direct case method, the instructor gives a brief scenario with a series of questions to the whole class. Students work individually to find the answers to questions with single-correct answers instead of open-ended questions with multiple possible answers. The instructor then begins a class discussion on their responses in the next lesson.
(6) The interrupted method is defined as the best technique. The instructor presents students with a problem that real researchers face. Students work in small groups for about 15 min and then report their thoughts on problem-solving approaches. The instructor provides additional information about the problem, as suggested by the real scientists. The instructor then presents additional problems and asks learners to find solutions with brainstorm discussions. Students also write reports. Interrupted method case studies are applied in one class period rather than over several days.
(7) In the mixed-method instruction, the instructor starts cases working with small student groups and finishes with general whole class discussions by including other types of case studies. Among different approaches for teaching case studies, the mixed-method type can be effective in a large class size. In the study, it was thought that the mixed-method instruction could be combined with a series of techniques (e.g., small group, individual and interrupted case methods).
Much research has focused on the benefits of case studies in helping students gain content knowledge. For example, Camill (2006) stated that students gain processing and application skills, along with more content and context, through interrupted type case studies and inquiry-based labs. Case studies serve as a capstone experience to show students why the concepts they just learned are critical for understanding contemporary ecological and environmental issues.
Cassimjee (2007) evaluated students' perceptions of the use of case-based teaching and group work in a first year nursing program. Although the researcher did not evaluate how case studies directly affected learning outcomes, the students' perceptions showed that the students enjoyed working in groups and learned from these groups. The result showed that students believed that their learning in group-based teaching was effective.
Chaplin (2009) investigated how case studies affected student learning gains in an introductory biology course. She used case studies by assigning homework in order for students to work on part of the textbook chapter, subsequent to a brief introduction to the topic at the end of one class period. Then, the students discussed the answers to the questions posed in the case study during the next class period. The researcher concluded that the case-based instruction was effective and beneficial for academic performance, due to improvement on the pre- and post-exam scores. In addition, over the course of the semester, students in the case-based course showed more improvement in correctly answering application-analysis questions than did students in the lecture-based classroom.
Murray-Nseula (2011) conducted a study in an effort to determine the effects of the case based teaching on student perception and performance during the 2007, 2008, and 2009 academic years respectively in an undergraduate level genetics course. He administered six case studies; acquired from the National Center for Case Study Teaching in Science (NCCSTS) Case Study collection website (http://sciencecases.lib.buffalo.edu/cs/) introduced by the University of Buffalo, with small group, interrupted, mixed method. The researcher used mixed method consisted of lecture, directed and whole class discussion methods. The researcher found that case-based teaching improved students' perceptions and indirect performance outcomes in a genetics course. The researchers also stated that students mostly participated in the interrupted case format within small groups. Therefore, their analytical and critical thinking skills improved as a result of this implementation.
Ayyıldız and Tarhan (2013) examined how effectively case studies on gases, liquids, and solids aided the understanding of 52 first-year undergraduate students enrolled in a general chemistry course in the Department of Science Teaching in the School of Education. While 25 students were instructed with case study method as the experimental group, 27 students were taught with the traditional chemistry curriculum as the control group. Results showed that students in the experimental group learned the concepts more meaningfully than those in the control group, and the former had fewer misconceptions after the implementation.
More recently, Ozdilek (2014) examined 16 pre-service science teachers' views about implementing a case study and asked which skills they improved as a result of using the method in the summer term of 2013. When teaching students about chromium's properties, teachers used a mix of individual and interrupted methods. In the study, students worked individually, as very few students were enrolled in the course. All students had individual computers and an Internet connection in the classroom. Participants responded in writing to open-ended questions and completed semi-structured interviews to provide qualitative data. The responses indicate that much more attention is given to an enhanced understanding of the studied phenomena than to other learner benefits. They also improved their interests and motivation to the subject as a result of using case study teaching.
Previous research emphasized that case studies have many benefits in terms of understanding the subject matter. Although there are many techniques that can be applied to cases in class, the large class size is a limitation for implementation. The current study, therefore, investigates the effectiveness of mixed-method case studies.
Method
The study was conducted in a 6 h class during the fall semester of 2013–2014. There was only one second-year class pre-service science teacher at our university at that time. Therefore, the students who completed a particular task and presented in class were assigned to the presenting group, and the students who did not complete the task and just participated in the class discussions were assigned to the audience group in the same class. Student achievement levels for presenting and audience groups were measured with specific questions for each group using both qualitative and quantitative analysis.Participants
The participants in this study were 55 sophomores who attended an undergraduate analytical chemistry class in the Department of Science Education of a large state university in northwest Turkey. Participants were identified with a convenience sampling method. This sample included 41 female and 14 male pre-service science teachers. The mean age of students was 20.6 years.The participants in the study group studied the analytical chemistry topics within the General Chemistry III course. Unlike General Chemistry I and II, laboratory is not a separate course in the second year. The course included two 45 min lectures and two 45 min practice periods each week. Over the course of the semester, the students had also taken a series of traditional lectures on the subject, including methods for identifying qualitative and quantitative analysis (e.g., gravimetric analysis, volumetric analysis, and instrumental analysis).
The practice period included solving questions and doing research homework related to the theoretical topics in addition to laboratory experiments. Acid–base titrations and gravimetric analysis experiments were done in the laboratory. The case study was implemented in the last two weeks of the term. It was thought that the participants would be able to readily use their previous knowledge to answer the questions in the case. The participants did not have any previous case based teaching experience.
Activity design
The case was developed by the researcher around an issue and implemented in class using mixed-method instruction. In general, the instructor starts with students working in small groups and finishes with general discussions with the whole class (Herreid, 2005). In this particular approach, the instructor provided students with a case scenario by showing them selections of the movie Erin Brockovich (DeVito et al., 2000), an adaptation of a true story. Ten groups were formed consisting of five to seven students according to their preferences at the beginning of the term. The setting has remained the same to preserve the integrity of the work groups for the implementation. The instructor then gave different assignments to each group to implement the case study in a classroom environment, as can be seen in Table 1.| Groups | Task of the groups |
|---|---|
| 1 and 10 |
Provide information about chromium's properties.
Is chromium a helpful or harmful element? Please briefly explain why you think it is helpful or harmful. |
| 2 and 9 | How can you find out whether chromium exists in the water where you live? How can you determine the amount of chromium that is in the drinking water? |
| 3–5 | What methods can be used to remove Cr(VI)? |
| 6–8 | A question needs problem solving with the spectrophotometric analysis method. |
The case study was implemented in class as follows:
The first part of the movie was shown to whole class to introduce them to chromium. The instructor then gave different assignments to each group that dealt with different aspects of chromium. The students worked in groups over the next several days to find answers to the questions required of them. At the beginning of the next class, each group presented their conclusions to the other groups and the class instructor. The instructor collected the group papers and then ran a discussion on the topic dealing with the group's assignment. At the end of the each group presentation, the instructor discussed the answers again. The instructor used Fendorf's “Surface reactions of chromium in soils and waters” (1995) as a basis for the discussion section. The cycle was repeated with the other parts of the movie, and each group started with their questions. The presenting and audience groups were taught by the same researcher. Appendix 1 presents the case study implemented in the classroom. Appendix 2 shows additional teaching notes.
Measurement tools
Answered with paper and pencil, the open-ended questions asked the students to determine their knowledge before and after instruction.Please briefly indicate to what extent you have focused on chromium pollution and its hazardous effects on human health due to the classroom implementation.
Data analysis
To investigate the first research question, open-ended and interview responses were analyzed using a content analysis approach. For the second and third research questions, the researcher and a subject area expert collaboratively evaluated pre- and post-tests to ensure inter-rater reliability. A rubric was prepared to evaluate the results of open-ended questions. Each question was evaluated using a scale of 0 to 3 (0 = completely wrong or unanswered, 1 = partial understanding with the wrong answer, 2 = partial understanding, 3 = scientific understanding). Consequently, each question was scored with 0 to 3 points for each open-ended question. Appendix 3 presents the grading rubric for student responses and a sample of student responses. Pair sample and independent sample t-tests were used to investigate the differences between groups in terms of achievement levels on pre- and post-test scores.Results
Results are organized according to research questions.Findings on the case study's effectiveness in highlighting the environmental pollution caused by Cr(VI) for pre-service science teachers
All students stated that they were not aware of the threats of Cr(VI) to human health before the case study implementation. Students felt that the case scenario was beneficial in introducing them to chromium pollution.Some participants focused on the necessity of environment protection in the interview. Participant 4 (P4) thought we should not use hazardous elements in the environment in any way. P1 and P8 offered similar comments:
In my opinion, we should not use Cr(VI) in any industrial production process. We should find other alternatives instead of the element (P4).
I am aware that we must protect our environment when we use such hazardous chemicals in production processes. We must take the necessary measures to remove the hazardous effects of chemicals (P1).
In my opinion, we should not use dangerous chemicals in industrial production. I think our environment is more important than the national economy. I will be a more sensitive person now. Otherwise, we will lose our health and our environment (P8).
In contrast, some participants specifically focused on removal techniques for Cr(VI). They thought hazardous materials could be used in the industrial process as long as necessary procedures were applied. P3 and P5 stated the following:
We learned that Cr(VI) is a very hazardous element for human health. But if we can use it for industrial applications, we should develop easy and cheap chemical removal techniques (P3).
There are always dangerous materials and elements in our environment. The important thing is that we must use chemicals consciously and not damage our environment (P5).
I have learned that Cr(VI) is a very hazardous element. At the same time, it is useful for much industrial production. Having chemistry knowledge, I wonder if I can find other removal techniques myself (P10).
Some pre-service science teachers also mentioned the positive effect of case studies on environmental awareness. P2 specifically suggested that case studies could be a method to ensure raising awareness in children. P6 stated that because the case method presented real-life situations, the method would be more effective than others, saying:
I can use the case study teaching method to increase the environmental awareness of my students in my instruction. I think that if we trained the next generation, there would be less environmental pollution (P2).
My main goal will be to train my students as people who have high awareness of the environment. When we hear environmental issues, we do not show adequate interest. We should use real-life experiences to increase environmental awareness…The case study method fits well for this purpose (P6).
Two participants mentioned that case studies are a bridge between theory and practice. They stated that they could use their theoretical chemistry knowledge to solve real problems. Their illustrative comments appear below:
It was very interesting to use our knowledge about oxidation to solve a daily life problem. Generally we solve many questions, do experiments and homework within our chemistry lessons. But we rarely use our experiences in our daily lives. We saw that we can solve very important problems by using easy chemical experiences and knowledge, such as the oxidation of Cr(VI) to Cr(III) (P7).
We can use the knowledge gained in chemistry courses in a similar way to that in implemented case studies—to solve environmental and other types of daily life problems (P9).
Findings on the case study method's effectiveness for content knowledge
Pair sample and independent sample t-tests were performed to identify differences in content knowledge on chromium's properties, the qualitative and quantitative analysis methods, chromium's oxidation states, and the spectrophotometric analysis between and within the presenting and audience groups. Tables 2 and 3 display pair sample and independent sample t-test results, respectively.| Task | Group | N | Means | SD | df | t | p | d |
|---|---|---|---|---|---|---|---|---|
| PG: presenting group, AG: audience group | ||||||||
| Properties of chromium | Pre-test of PG | 12 | 0.92 | 0.67 | 11 | 4.304 | 0.001 | 1.72 |
| Post-test of PG | 12 | 2.25 | 0.86 | |||||
| Pre-test of AG | 43 | 0.67 | 0.61 | 42 | −9.675 | 0.000 | 1.83 | |
| Post-test of AG | 43 | 2.16 | 0.97 | |||||
| Qualitative and quantitative analysis methods | Pre-test of PG | 13 | 0.54 | 0.52 | 12 | −6.278 | 0.000 | 2.12 |
| Post-test of PG | 13 | 1.84 | 0.69 | |||||
| Pre-test of AG | 42 | 0.52 | 0.50 | 41 | −5.383 | 0.000 | 1.62 | |
| Post-test of AG | 42 | 1.74 | 0.94 | |||||
| Chromium's oxidation states | Pre-test of PG | 16 | 0.25 | 0.45 | 15 | −13.330 | 0.000 | 4.06 |
| Post-test of PG | 16 | 2.06 | 0.44 | |||||
| Pre-test of AG | 39 | 0.38 | 0.49 | 38 | −5.350 | 0.000 | 1.20 | |
| Post-test of AG | 39 | 1.23 | 0.87 | |||||
| Spectrophotometric analysis | Pre-test of PG | 14 | 0.36 | 0.49 | 13 | −8.039 | 0.000 | 3.48 |
| Post-test of PG | 14 | 2.21 | 0.57 | |||||
| Pre-test of AG | 41 | 0.44 | 0.50 | 40 | 19.893 | 0.000 | 1.14 | |
| Post-test of AG | 41 | 1.32 | 0.96 | |||||
| Task | Test | N | Means | SD | df | t | p | d |
|---|---|---|---|---|---|---|---|---|
| Properties of chromium | Pre-test of PG | 12 | 0.92 | 0.67 | 53 | 1.197 | 0.484 | — |
| Pre-test of AG | 43 | 0.67 | 0.61 | |||||
| Post-test of PG | 12 | 2.25 | 0.87 | 53 | 0.280 | 0.568 | — | |
| Post-test of AG | 43 | 2.16 | 0.97 | |||||
| Qualitative and quantitative analysis methods | Pre-test of PG | 13 | 0.54 | 0.52 | 53 | 0.091 | 0.841 | — |
| Pre-test of AG | 42 | 0.52 | 0.50 | |||||
| Post-test of PG | 13 | 1.84 | 0.69 | 53 | 0.383 | 0.085 | — | |
| Post-test of AG | 42 | 1.74 | 0.94 | |||||
| Chromium's oxidation states | Pre-test of PG | 16 | 0.25 | 0.45 | 53 | −0.944 | 0.035* | 0.27 |
| Pre-test of AG | 39 | 0.38 | 0.49 | |||||
| Post-test of PG | 16 | 2.06 | 0.44 | 53 | 3.613 | 0.000* | 1.20 | |
| Post -test of AG | 39 | 1.23 | 0.87 | |||||
| Spectrophotometric analysis | Pre-test of PG | 14 | 0.36 | 0.49 | 53 | −0.528 | 0.227 | — |
| Pre-test of AG | 41 | 0.44 | 0.50 | |||||
| Post-test of PG | 14 | 2.21 | 0.57 | 53 | 3.286 | 0.001* | 1.12 | |
| Post-test of AG | 41 | 1.32 | 0.96 | |||||
Pair sample t-test results revealed that there was a statistically significant difference between pre- and post-test results in the PG (t11 = 4.304, p < 0.05, d = 1.72) and AG (t42 = −9.675, p < 0.05, d = 1.83). Cohen's d showed that there was a large effect size on the mean differences between PG and AG (d > 0.08). This shows that there was a considerable difference between the pre- and post-test scores of both the PG and AG.
An independent sample t-test was also run to determine the differences between the pre-test and post-test scores of the PG and AG. Table 3 displays these results. The independent sample t-test results showed that there was not a statistically significant difference between the PG and AG in the pre-test (t53 = 1.197, p > 0.05) and post-test (t53 = 0.280, p > 0.05) results. These results indicated that students who just attended the class discussions increased their scores as much as those who completed the task relating to chromium's properties.
Fig. 1 shows that while the majority of the students received the scores of 0 (25% on PG and 39.5% on AG) and 1 (58.3% on PG and 53.5% on AG) before the instruction, there are a few students who received the scores of 0 and 1 in the PG (respectively 8.3% and 0%) and AG (11.6% and 4.7%) after the instruction. While, 16.7% of the PG and 7% of the AG received a score of 2 before the instruction, the percentage of the students who received that score increased to 50% on PG and 39.5% on AG after the implementation. Interestingly, none of the students had received a score of 3 before the instruction; 41.7% (PG) and 44.7% (AG) received a score of 3 after the instruction. This result revealed that students' incorrect and incomplete knowledge was greatly decreased. This is expected as the number of students who correctly answered the questions increased.
The answers in the category 0 were usually “I don't know”, “I have no idea”, “no response” and the category 1 answers were “chromium is a useful metal”, “it is used in the medical field”, “a nonmetal with different oxidation states”. On the other hand, the answers such as “I know that it is a heavy metal with different oxidation states”, “it is a harmful element for human health”, and “it can be both useful and harmful” were coded as the category 2. Prior to the study, none of the students were able to give completely correct answers to the questions. In the post-test, however, almost half of the students both in PG and AG received a score of 2. These students could explain all of the features of Cr(VI) but could not classify it as Cr(VI). While some of them could explain the properties of either Cr(III) or Cr(VI), the others just discussed the useful or harmful effects of the element. About 41.7% of the students in PG and 44.7% in AG answered the question correctly as can be seen in Appendix 3.
Table 2 shows that there is a difference in the knowledge of chromium's determination methods between the PG (t12 = −6.278, p < 0.05, d = 2.12) and the AG (t41 = −5.383, p < 0.05, d = 1.62) with a large effect size (d > 0.08) after the case study implementation.
As can be seen in Table 3, there is not a significant difference between the PG and AG in terms of prior knowledge of chromium's determination methods (t53 = 0.091, p > 0.05). After the implementation, both groups equally learned the determination methods, as there was not a significant difference between each group's post-test results (t53 = 0.383, p > 0.05).
Fig. 2 shows that students in both PG and AG received varying scores. 46.2% of PG and 47.6% of AG received a score of 0 and 53.8% PG and 52.4% AG received a score of 1. After the implementation, the majority of students increased their scores from 0 and 1 to 2 (PG = 53.8% and AG = 35.7%) and 3 (PG = 15.4% and AG = 23.8%).
 | ||
| Fig. 2 Percentages of pre- and post-test scores of PG and AG on qualitative and quantitative analysis methods. | ||
Before the instruction they responded to the question as “I don't know”, “No comment” or “I have no idea”, or the majority of them stated that “with gravimetric methods, volumetric and instrumental analysis methods”. The majority of the answers related to spectrophotometric methods and were coded as 1.
After the instruction, the answers such as “with spectrophotometric methods” or “the 1,5-diphenylcarbazide method” were coded as 2. The answers including both of the methods were coded as 3.
Table 3 shows that there was a significant difference between the pre-test scores of the PG and AG (t53 = −0.944, p < 0.05, d = 0.27). Although the pre-test scores of the AG were higher than those of PG with a small size effect (0.2 < d < 0.5). Interestingly, it was found that the PG's post-test scores were higher than those of the AG (t53 = 3.613, p < 0.05, d = 1.20) with a large size effect (0.8 < d) after the case study implementation. These results showed that both experimental and control groups increased their knowledge of chromium's oxidation states. However, after the implementation, the students who investigated the removal techniques for chromium were more successful in terms of retaining oxidation knowledge.
While PG and AG' scores on pre-test were mainly 0 and 1, post-test scores of the groups were mainly 2. 12.5% of the students in PG were able to get a score of 3 and 81.2% get a score of 2. However, none of the students on AG were able to get a score of 3. See Fig. 3.
Before the implementation the students in both groups responded to the question as “I have no idea, some of the answers were like “we can reduce Cr(VI) at the high temperature to Cr(III)”. These answers were coded as 1. An illustrative answer of the PG and AG students after the instruction is shown below:
In order to avoid chromium polluting drinking water, it can be reduced to Cr(III) by passing through acidic soil and using Fe according to the equation:
| CrO42− + 3Fe3− → Cr3+ + Fe(OH)3 |
Only 12.5% of the PG students were able to answer the question on level 3 by using the reaction equation with the correct explanation:
| CrO42− + 3Fe2+ + 4H2O + 4OH− → 3Fe(OH)3 + Cr(OH)3 |
Table 3 shows that the mean pre-test score for the PG was 0.36, and the mean pre-test score for the AG was 0.44. However, there was not a significant difference between the groups' prior knowledge of the spectrophotometric analysis of chromium in wastewater samples. On the other hand, there was a statistically significant difference, as the PG received significantly higher scores (t53 = 3.286, p < 0.05, d = 1.12) with a large size effect.
As seen in Fig. 4 the majority of the students got a score of 0 on PG (64.3%) and AG (56.1%) and a score of 1 (PG = 35.7%) and (AG = 43.9%) on pre-test. Post-test scores of PG and AG groups were mostly 2 (64.3% and 51.2% respectively).
It was observed that the majority of students did not answer the question before the instruction. However, they were able to calculate the unknown solution concentrations correctly. They had difficulties in drawing graphics with the variables they found and with calculating the unknown concentration by using the slope of the graph equation.
Findings on how effectively mixed-method case studies facilitate higher learner achievement levels
As can be seen in Table 2 PG and AG increased their content knowledge about Cr(VI) at a statistically significant level after the case study implementation. This result revealed that mixed-method, case-based instruction has been effective in terms of increasing content knowledge when compared prior knowledge levels. The frequencies of the scores as shown in (Fig. 1–4) indicate that they mostly increased their scores from 0 and 1 to 2 and 3 as a result of the case study implementation.As presented in Table 2, the PG increased their mean score for knowledge of chromium's properties from 0.92 to 2.25, and the AG increased their score from 0.67 to 2.16. Both showed above average scores, considering their maximum scores would be 3.00. In addition, Table 3 shows that there is no significant difference between the post-test scores of both groups.
As can be seen in Table 2, the PG increased their scores on chromium's qualitative and quantitative analysis methods from 0.54 to 1.84 and the AG increased their scores from 0.52 to 1.74, which were above average. However, as can be seen in Table 3, there is no statistically significant difference between the post-test mean scores of each group.
For the question about chromium's oxidation states, Table 2 shows that the PG increased their scores from 0.25 to 2.06, which was above the average level. The AG increased their scores from 0.38 to 1.23, which was below the average level. As can be seen in Table 3, there is a significance difference between the groups' post-test mean scores.
Table 2 shows that while the PG increased their scores from 0.36 to 2.21, which was above the average; the AG increased their mean scores from 0.44 to 1.32, which was below the average level. Similarly, as can be seen in Table 3, there was a significant difference between the groups' post-test mean scores.
Briefly, it can be said that the achievement level of the students in PG and AG and the response rates on the scientific understanding of students have greatly increased as a result of the case study implementation. On the other hand, while there is no significant difference between the PG and AG on the properties of chromium and qualitative and quantitative analysis methods questions, the PG students were more successful on the question related to the oxidation states of chromium and spectrophotometric analysis methods as a result of the intervention. Moreover, the percentages of the PG students' responses on the scientific and partial understanding categories were higher than those of the AG students. These results show that while the mixed method is effective, it was not equally effective in improving students' knowledge in all aspects of chromium related questions.
Conclusions and discussion
Although a number of studies report increased learning gains for students in case-study classrooms versus those enduring a lecture-based format, none provide quantitative proof of the effectiveness of a specific method used in case-based instruction. The main goal of the study was to create a high-quality, case-based course to draw attention to an environmental issue involving the element of Cr(VI). The other goal of the study was to implement a case-based scenario with the most appropriate method for instruction.The mixed-method case study that I used is described in detail in previous sections. As Bowe et al. (2009) described for the case study presentation, the case study, which was based on a real-life scenario, provided supporting data and documents to be analyzed. Additionally, open-ended questions were presented to invite possible solutions. This case was presented to groups rather than individuals due to the large class size. Students worked in groups to complete their assignments and to brainstorm in the classroom environment.
Based on the results, the instruction very much achieved its goals, as both groups improved their knowledge on the topic compared with their prior knowledge levels. This result is consistent with the findings of Camill (2006), Boubouka et al. (2008), Chaplin (2009), Jalgaonkar et al. (2012),Casotti et al. (2013), who found that case-based instruction increases content knowledge. The findings are also consistent with Camill (2006), who found that case-based instruction ensures understanding of the contexts in which knowledge is relevant. Cassimjee (2007) mentioned that case studies help students understand theoretical issues and make decisions about real-life situations. Case studies also assist in the integration of different aspects of knowledge, thus allowing for a wider range of issues to be discussed. I can thus say that case studies can be used to apply theoretical and analytical chemistry subjects to practical pursuits by integrating many subjects.
It was also concluded that the case study teaching method has effectively highlighted the threats of chromium for participating students. Some learners stated that they could use case studies to increase their students' environmental awareness in the future. This result is consistent with the suggestion of Teksoz et al. (2010) that teachers can affect their students' interest in environmental issues. There appeared to be different views on chromium use. While some learners believed that chromium compounds should not be used in any way, some learners thought that chromium could be used if necessary techniques were used to remove chromium. Case studies should cause discussion on multiple levels of abstraction—with no single right answer (Merseth, 1990 cited in Colburn and Tillotson, 1998) and should help students with problem solving skills and discussion (Chaplin, 2009; Celik et al., 2012). Individuals thus may use rational and sensible approaches to solve the problems in a different way. As Ding (2004) stated, case studies that include environmental issues ensure that learners learn how to observe, investigate, analyze, discuss, practice, think, and summarize. In addition, in this study some learners specifically expressed that case-based instruction ensured applying science to daily real-life problems. Similar findings were reported in the study of Yoon et al. (2006)—instructors should obviously decide which technique is most appropriate for the implementation at hand, as some types of case-based instruction will likely be more effective for learning than others (Herreid, 2011). In this study, using a case study with mixed-method instruction was not completely effective in increasing all groups' content knowledge at equal levels. Interestingly, the differences appeared only on the questions that included calculations. These calculations such as questions, balancing chemical equations on redox reactions correspond to the symbolic level of chemistry which includes chemical formulae, equations, ionic drawings, and so on (Johnstone, 1991; Gabel, 1993). Literature shows that students have difficulties in understanding symbolic representations; for instance, they handle symbols as algebraic entities without clear interpretations from a macro–micro perspective (Doymuş et al., 2009; Thadison, 2011; Dori and Kaberman, 2012). Moreover, students usually are not aware of the relationships among the three levels of chemistry (macroscopic, microscopic, and symbolic). As a result, they may encounter difficulties or develop misconceptions related to core chemistry concepts (Nakiboglu and Yildirir, 2010). In this study the audience group students were taught the redox equations of chromium and spectrophotometric analysis through traditional methods. On the other hand, the presenting group investigated, solved the problems, discussed, and expressed the same subjects to their classmates. Savec et al. (2006) stated that many students have difficulties in understanding the different representation levels in chemistry. Compared to the experienced students, naive learners have difficulty in navigating between different representations to solve problems. Students are further expected to connect the symbolic representations to the macroscopic level on the topic they studied (Stief and Wilensky, 2003). The results of this study suggest that the presenting group students are more successful than the audience group students.
According to the results, it appears that mixed-method, case-based instruction is more effective for verbal issues in a large size class. It is recommended that this method will be useful when applying these types of cases for consideration. Interrupted small group methods can be more effective when the whole class investigates all sections of the case studies to gain content knowledge. It should be noted that there is a limitation in the study due to the small sample size. In the study, a total of 55 students were divided into smaller groups to address specific single tasks. Therefore, a generalization cannot be made to a general population, these results can only be evaluated within the context of the study.
Appendix 1. Case study: hexavalent chromium
Part 1: A strange situation
Erin, the movie's heroine, has luckily started working at a lawyer's office. Erin notices something strange in a real estate case, as there are medical and blood sample reports in the case file. She then wants to investigate the issue and goes to the house of Donna Jensen, the plaintiff on the case. Donna says that although they did not put their house up for sale, the Pacific Gas & Electric Company wants to buy it. She added that she does not want to sell her house, as she and her husband have fallen ill. Erin asks her why her blood tests are in the case documents. She says the company pays for the doctor's visits and covers all of her family members' hospital expenses. Erin is surprised and asks “why” again.Donna answers, “because of chromium. All of the things have started with chromium.”
Questions
1. What are the properties of chromium?
2. Is chromium a helpful or harmful element? Please explain briefly why you think that it is helpful or harmful.
Part 2: How can we understand whether wastewater consists of Cr(VI)?
After the meeting with Donna Jensen, Erin looks at Pacific Gas & Electric Company's buildings and goes to a university to get information about chromium.The professor asks, “What kind of chromium is it?”
Erin responds, “There is more than one type?”
The professor answers that there is a straight-up chromium, which makes good things for the body, Chromium(III), which is fairly benign, and Cr(VI), or hexavalent chromium, which depending on its amount can be very harmful.
Erin is surprised and asks, “Harmful? How?…What would it get?”
The professor says depending on the amount of exposure any kind of health problem (e.g., chronic headache, nosebleed, shortness of breath, pulmonary failure, heart failure, reproductive system disorders, bone and organ failure), plus of course any type of cancer…
Erin says, “So that stuff kills people?”
Professor: “Definitely, highly toxic and carcinogenic.”
Erin: “So what is it used for?”
Professor: “It's anti-rust. Water used for cooling heated engines in factories. Chromium is added to water to prevent corrosion.”
Erin: “How can I learn what kind they are using in Hinkley?”
Questions
1. What are the quantitative analysis methods for chromium?
2. What are the qualitative analysis methods for chromium?
Part 3: Does the water where you live contain Cr(VI)?
Erin goes to the California water quality control board and examines the clean-up and abatement orders. She realizes that the amount of chromium is higher than the acceptable level, and that local people might be sick for that reason. Erin goes to Donna's house and warns her that the company uses Cr(VI) and tells her she should not believe the drinkable water is safe, as the company claimed. Erin tells Donna that they might be sick for that reason. Donna says, “No, the doctor said there isn't any relationship among our illnesses”. Erin says, “But the company covers the doctor visits”. Donna realizes the truth. Erin goes to collect wastewater samples from around the plant.Questions
1. Erin brings you wastewater samples to analyze, as you are an analytical chemist at a government laboratory. Please complete the analysis procedure below and write a results report for Erin about your decision about whether the wastewater is harmful.
1. Prepare 100 ppm 100 mL Cr(VI) solution.
2. Take 5 mL, 15 mL, 25 mL, and 35 mL samples from the stock solution, prepare new 50 mL solutions from each sample, and calculate the solutions' absorbencies at 435 nm.
3. Then fill in the table below.
| Solution | Concentration (ppm) | Absorbance (A) |
|---|---|---|
| 100 mL 0.0283 g K2Cr2O7 stock solution | 0.439 | |
| Sample of 5 mL stock solution to adjust 50 mL | 0.046 | |
| Sample of 15 mL stock solution to adjust 50 mL | 0.125 | |
| Sample of 25 mL stock solution to 50 mL | 0.232 | |
| Sample of 35 mL stock solution to 50 mL | 0.308 | |
| Unknown Cr(VI) concentration | X ppm | 0.096 |
Part 4: How can we remove Cr(VI) from wastewater?
Erin goes to meet with local people and learns that they have many types of cancer (e.g., gastrointestinal, lung and skin cancer).In subsequent research, Erin learns that the firm did not develop any measures to remove Cr(VI) from wastewater for 14 years. She explains the situation to the lawyer, saying that Cr(VI) is the most likely the reason for the local people's illnesses.
Questions
If you were the firm's executive director, which removal methods would you use?
Appendix 2. Teaching notes
Part 1: A strange situation
At the beginning of the class, the instructor showed the first part of the movie to all participants. The movie was stopped after Donna says “because of the chromium”. At that moment, Groups 1 and 10 presented their assignments about chromium's properties, uses, and human health effects to the rest of the class. The instructor then gave additional information about chromium using the paper of Fendorf (1995), which was translated into Turkish, as shown below.Redox reactive metals, however, often do have different degrees of toxicity depending on the specific metal oxidation state. Chromium, one of the metal ions that persist in the environment as either Cr(III) or Cr(VI). Cr(VI) is toxic to both plants and animals, being a strong oxidizing agent, corrosive, and a potential carcinogen. In contrast, the trivalent species is not toxic to plants and is necessary in animal nutrition. It is therefore essential to determine both the total amount of Cr in a system and its oxidation state (p. 55).
The instructor then started a discussion about whether students have decided if chromium is or is not a helpful element.
Part 2: How can we understand whether wastewater contains Cr(VI)?
The movie was stopped after Erin says, “How can I learn what kind of chromium is used in Hinkley?” and Groups 2 and 9 presented their task to the entire class. Their assignment was about the qualitative and quantitative analysis methods used to determine the amount of chromium.The instructor then gave examples of one of chromium's qualitative analysis methods (i.e., the 1,5-diphenylcarbazide method) and presented a video that included the instructor conducting the chosen procedure with a research assistant in a chemistry department lab. The hexavalent chromium then reacted with 1,5-diphenylcarbazide to form 1,5-diphenylcarbazone. The amount of red color formed with hexavalent chromium is directly proportional to the amount of chromium present in the sample (Fig. 5).
In addition, the UV-visible spectrophotometer method was introduced for the quantitative determination of chromium(VI), and the instructor presented a related video to students, as shown below (Fig. 6).
Part 3: Does the water where you live contain Cr(VI)?
The groups should solve the problem as written below:Solution:
ppm = mg L−1 ppm
Weigh out 0.0283 g K2Cr2O7. Place the K2Cr2O7 into a 100 mL volumetric flask. Add a small volume of distilled, pH 2 deionized water to dissolve the salt. Fill the flask to the 100 mL line. As you calculate, you prepare a 100 ppm Cr(VI) solution. According to the stock solution formula, you calculate the sample solutions' concentrations, as shown in the table.
| Solution | Concentration (ppm) | Absorbance (A) |
|---|---|---|
| 100 mL 0.0283 g K2Cr2O7 stock solution | 100 ppm | 0.439 |
| Sample of 5 mL stock solution to adjust 50 mL |
M1V1 = M2V2
100 × 5 = ppm × 50 = 10 ppm |
0.046 |
| Sample of 15 mL stock solution to adjust 50 mL |
M1V1 = M2V2
100 × 15 = ppm × 50 = 30 ppm |
0.125 |
| Sample of 25 mL stock solution to 50 mL |
M1V1 = M2V2
100 × 25 = ppm × 50 = 50 ppm |
0.232 |
| Sample of 35 mL stock solution to 50 mL |
M1V1 = M2V2
100 × 35 = ppm × 50 = 70 ppm |
0.308 |
| Unknown Cr(VI) concentration | X ppm | 0.096 |
Plot a graph of C values on the X axis and A values on the Y axis, as shown below:
Calculate the slope of the graph equation using Excel software (y = 0.0042x − 0.0009).
Use the A value of the unknown sample to calculate the concentration (A = 0.096).
Y = 0.0045x − 0.0009
0.096 = 0.0045x − 0.0009
X = 21.53 ppm
You decide that the wastewater sample is highly toxic according to EPA standards (X > 0.1 mg l−1).
Part 4: How we can remove Cr(VI) from wastewater?
In this step, the groups present their assignments to their classmates about which methods can be used to remove Cr(VI). The instructor then presents students Treatment of Hexavalent Chromium video relating to electrocoagulation which can be found on the Youtube homepage (http://www.youtube.com).Following this, the instructor provides information on chromium using the paper of Dermentzis et al. (2011), which is translated into Turkish, as shown below:
Electrocoagulation is a process consisting of creating metallic hydroxide flocks inside the wastewater by electrodissolution of soluble anodes made of aluminum or iron. The electrochemical oxidation of iron anodes produces ferrous, Fe2+ and ferric, Fe3+ ions. The Fe2+ ions can reduce Cr(VI) to Cr(III) in alkaline to neutral medium, while they are oxidized to Fe3+ ions according to the reaction (p. 414).
| CrO42− + 3Fe2+ + 4H2O + 4OH− → 3Fe(OH)3 + Cr(OH)3 |
The followings are the major reactions taking place in the EC cell (Gao et al., 2005, p. 118).
Anode (oxidation):
| Fe ↔ Fe2+ + 2e− |
| Cr6+ + 3Fe2+ ↔ Cr3+ + 3Fe3+ |
Cathode (reduction):
| 2H2O + 2e− ↔ H2 + 2OH− |
Co-precipitation:
| Cr3+ + 3OH− ↔ Cr(OH)3 |
| Fe3+ + 3OH− ↔ Fe(OH)3 |
| Fe2+ + 2OH− ↔ Fe(OH)2 |
Appendix 3. Questions and coding scheme
| Question | Code | Criteria | Examples of student responses |
|---|---|---|---|
| (1) Is chromium a helpful or harmful element? Please briefly explain why you think it is helpful or harmful. | SU | Identify each aspect of chromium regarding its uses, different oxidation state properties, and human health effects according to EPA standards. | We cannot say that chromium is a harmful or helpful element. It depends on its oxidation states. Cr(VI) is used in many industrial applications (e.g., leather tanning and metallurgy). However, according to EPA, it is highly toxic and carcinogenic if it is found at levels above 0.1 mg l−1 in surface water and 0.05 mg l−1 in drinkable water. However, Cr(III) has relatively low toxicity and mobility, and it is also an essential nutrient in low doses. |
| PU | Answers for just one aspect, i.e., harmful or helpful effects or an explanation of only one oxidation state, Cr(III) or Cr(VI). | Chromium(VI) is a very harmful element, as it is carcinogenic and highly toxic. | |
| PU/WA | Confusing the properties of Cr(III) and Cr(VI). | Cr(III) and Cr(VI) are highly toxic for plants and animals, as strong, corrosive oxidizing agents and potential carcinogens. | |
| UA/CW | Wrong answers about chromium's usage or human health effects. | No comment | |
| (2) What are chromium's qualitative and quantitative analysis methods? | SU | Identifies chromium's qualitative and quantitative analysis methods. | Identifies the 1,5-diphenylcarbazide method to form 1,5-diphenylcarbazone for the quantitative analysis method and the UV-visible spectrophotometer method for the quantitative determination analysis of chromium(VI). |
| PU | Correctly identifies either the qualitative or quantitative analysis methods of chromium. | The UV-visible spectrophotometer method is used for the quantitative determination analysis of chromium(VI). | |
| PU/WA | Confusing qualitative and quantitative analysis methods of chromium and a partial truth. | The 1,5-diphenylcarbazide method to form 1,5-diphenylcarbazone is used for quantitative and qualitative analysis of chromium. | |
| UA/CW | Wrong answers about the qualitative and quantitative analysis methods. | No comment or gravimetric analysis methods | |
| (3) A question that needs to be solved with the spectrophotometric analysis method | SU | Being able to correctly solve the question in its entirety and decide if chromium is harmful or not. |
All of the processes in Part 3 of Appendix 2 are correct, and the student finds the x concentration at X = 1.83 ppm.
His/her decision should be that the wastewater sample is highly toxic according to EPA standards (X > 0,1 mg l−1). |
| PU | Being able to solve the problem partially but unable to decide if chromium is harmful or not. | All of the processes in Part 3 of Appendix 2 are correct, but the student cannot decide if chromium is harmful or not. | |
| PU/WA | Although some parts of problem are correct, there are also calculative errors. | All of the processes explained in Part 3 of Appendix 2 are partially correct, but the student cannot find the x concentration at x = 1.83 ppm. | |
| UA/CW | Completely wrong or unanswered | I have no idea | |
| (4) Please explain one of the methods that can be used to remove Cr(VI) from wastewater. | SU | Being able to explain the electrocoagulation method using chemical equations that indicate the oxidation and reduction processes. |
Electrocoagulation is a process that creates metallic hydroxide floks inside the wastewater through the electrodissolution of soluble aluminum or iron anodes.
All of the chemical equations in Part 3 of Appendix 2 are correct. |
| PU | Being able to explain the electrocoagulation method without chemical equations. | Electrocoagulation is a process that creates metallic hydroxide floks inside the wastewater through the electrodissolution of soluble aluminum or iron anodes. | |
| PU/WA | Being able to explain the electrocoagulation method but confusing the chemical equations. | Electrocoagulation is a process that creates metallic hydroxide floks inside the wastewater through the electrodissolution of soluble aluminum or iron anodes. | |
| UA/CW | Completely wrong or unanswered | No comment | |
Acknowledgements
The author would like to thank Dr. Ali Kara who suggested the idea of working with chromium's oxidation states and lent his analytical chemistry laboratory to film the videos. I especially thank: Dr Muhlis Özkan, Dr Salih Çepni, Dr Keith S. Taber, and the two reviewers of this paper, for valuable comments and suggestions. Dr Mehmet Aydeniz, Dr I. Taci Cangul and Dr Sehnaz Baktaci-Goktalay are thanked for the critical reading of the manuscript. I would also like to extend my thanks to assistants Nuray Dinibutun and Aysu Alan who made the experiments regarding chromium's qualitative and quantitative analysis methods.References
- Ayyıldız, Y. and Tarhan L., (2013), Case study applications in chemistry lesson: Gases, liquids, and solids, Chem. Educ. Res. Pract., 14, 408–420.
- Bischoff P. J., Avery L., Golden C. F. and French P., (2010), An analysis of knowledge structure, diversity and diagnostic abilities among pre-service science teachers within the domain of oxidation and reduction chemistry, J. Sci. Teacher Educ., 21, 411-429.
- Bokare A. D. and Choi W., (2011), Advanced oxidation process based on the Cr(III)/Cr(VI) redox cycle, Environ. Sci. Technol., 45, 9332–9338.
- Boubouka M., Verginis I. and Grigoriadou M., (2008), Comparing the implementation of the case study method in the classroom and from distance using a course management system. IADIS International Conference on Cognition and Exploratory Learning in Digital Age (CELDA 2008), 285–292.
- Bowe C. M., Voss J. and Aretz T., (2009), Case method teaching: an effective approach to integrate the basic and clinical sciences in the preclinical medical curriculum, Med. Teach., 31, 834–841.
- Breslin M. and Buchanan R., (2008), On the case study method of research and teaching in design, Des. Issues, 24(1), 36–40.
- Camill P., (2006), Case studies add value to a diverse teaching portfolio in science courses, J. Coll. Sci. Teach., 36(2), 31–37.
- Casotti G., Beneski J. T. and Knabb M. T., (2013), Teaching physiology online: successful use of case studies in a graduate course, Adv. Physiol. Educ., 37, 65–69.
- Cassimjee R., (2007), An Evaluation of students' perceptions of the use of case-based teaching and group work in a first-year nursing programme, South African Journal of Higher Education, 21(3), 412–428.
- Celik S., Cevik Y. D. and Haslaman T., (2012), Reflections of prospective teachers regarding case-based learning, Turkish Online Journal of Qualitative Inquiry, 3(4), 68–82.
- Chaplin S., (2009), Assessment of the impact of case studies on student learning gains in an introductory biology course, J. Coll. Sci. Teach., 39, 72–79.
- Colburn A. and Tillotson J. W., (1998), A case study for use in science methods courses, J. Sci. Teacher Educ., 9(2), 153–164.
- Dai R., Liu J.,Yu C., Sun R., Lan Y. and Mao. J. D., (2009), A comparative study of oxidation of Cr(III) in aqueous ions, complex ions and insoluble compounds by manganese-bearing mineral (birnessite), Chemosphere, 76, 536–541.
- Delpier T., (2006), Cases 101: learning to teach with cases, Nurs. Educ. Perspect., 27(4), 204–209.
- Dermentzis K., Christoforidis A., Valsamidou E., Lazaridou A. and Kokkinos N., (2011), Removal of hexavalent chromium from electroplating vaste water by electrocoagulation with iron electrodes, Global NEST J., 13(4), 412–418.
- DeVito D., Shamberg M., Sher S., Grant S., Soderbergh S., Newman T., Roberts J., Universal Studios Home Video (Firm), (2000), Erin Brockovich, Universal City, CA: Universal Studios.
- Ding L., (2004), Practicing and researching the environmental case study teaching method in middle schools, Chinese Educ. Soci., 37(4), 75–80.
- Dochy F., Segers M., VandenBossche P. and Gijbels D., (2003), Effects of problem-based learning: a meta-analysis, Learn. Instr., 13(5), 533–568.
- Dori Y. J. and Kaberman Z., (2012), Assessing high school chemistry students' modeling sub-skills in a computerized molecular modeling learning environment, Instr. Sci., 40, 69–91.
- Doymuş A., Şimşek Ü. and Karaçöp A., (2009), The effects of computer animations and cooperative learning methods in micro, macro and symbolic level learning of states of matter, Eurasian Journal of Educational Research, 36, 109–128.
- Fendorf S. E., (1995), Surface reactions of chromium in soils and waters, Geoderma, 67, 55–71.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptional understanding, J. Chem. Educ., 70(3), 193–194.
- Gallego A., Fortunato M. S., Rossi S. L., Korol S. E. and Moretton J. A., (2013), Case method in the teaching of food safety, J. Food Sci. Educ., 12, 42–47.
- Garvin D., (2004), Participant-centered learning and the case method: A Case study teacher in action, Harvard Business School, http://Garvin,2004/multimedia/pcl/pcl_1/start.html, retrieved 10th January 2014.
- Gao P., Chen X., Shen F. and Chen G., (2005), Removal of chromium(VI) from wastewater by combined electrocoagulation–electroflotation without a filter, Sep. Purif. Technol., 43, 117–123.
- Harrison E., (2012), How to develop well-written case studies the essential elements, Nurs. Educ., 37(2), 67–70.
- Healy M. and Mccutcheon M., (2010), Teaching with case studies: An empirical investigation of accounting lecturers' experiences, Accounting Education: An International Journal, 19(6), 555–567.
- Herreid C. F., (1994), Case studies in science–A novel method of science education, J. Coll. Sci. Teach., 23(4), 221–229.
- Herreid C. F., (2005), Using case studies to teach science, Action Bioscience.org, retrieved 24th February 2014 from http://www.actionbioscience.org/education/herreid.html.
- Herreid C. F., (2006), Start with a story: the case study method of teaching college science, Arlington, VA: NSTA Press.
- Herreid C. F., (2011), Case study teaching, New Directions for Teaching and Learning, 128, 32–40.
- Jalgaonkar S. V., Sarkate P. V. and Tripathi R. K., (2012), Students' perception about small group teaching techniques: Role play method and case based learning in pharmacology, Educ. Med. J., 4(2), 13–18.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Instr., 7(1), 75–83.
- Kobya, M (2004), Removal of Cr(VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: kinetic and equilibrium studies, Biosource Technology, 91(3), 317–321.
- Kong F. and Ni Y., (2009), Determination of Cr(VI) concentration in diluted samples based on the paper test strip method, Water Sci. Technol., 60(12), 3083–3089.
- Kumar A. R. and Riyazuddin P., (2011), Chromium speciation in a contaminated groundwater redox processes and temporal variability, Environ. Monit. Assess., 176, 647–662.
- Lai H. and McNeill L. S., (2006), Chromium redox chemistry in drinking water systems, J. Environ. Eng., 132(8), 842–851.
- Lee G. and Hering J. G., (2005), Oxidative dissolution of chromium (III) hydroxide at pH 9, 3, and 2 with product inhibition at pH 2, Environ. Sci. Technol., 39(13), 4921–4928.
- Merseth K. K., (1990), Case studies and teacher education, Teacher Education Quarterly, 17(1), 53–62.
- Mukhopadhyay B., Sundquist J. and Schmitz R. J., (2007), Removal of Cr(VI) from Cr-contaminated groundwater through electrochemical addition of Fe(II), J. Environ. Manage., 82, 66–76.
- Murray-Nseula M., (2011), Incorporating case studies into an undergraduate genetics course, Journal of the Scholarship of Teaching and Learning, 11(3), 75–85.
- Nakiboglu C. and Yildirir H. E., (2010), Analysis of Turkish high school chemistry textbooks and teacher-generated questions about gas laws, Int J. Sci. Math. Educ., 9, 1047–1071.
- Oktor K., Yılmaz S., Turker G. and Erkus E., (2008), Speciative determination of Cr(III) and Cr(VI) in dyeing waste water of Dil Creek discharge to Izmit Gulf (Izmit-Kocaeli, Turkey) by ICP-AES, Environ. Monit. Assess., 141, 97–103.
- Ozdilek Z., (2014), Learners' views about using case study teaching method in an undergraduate level analytical chemistry course, Journal of Baltic Science Education, 13(5), in press.
- Random.org, (2014), http://www.random.org, accessed December 17, 2013.
- Reynolds, J. I., (1980), Case types and purposes, in Reynolds R. I. (ed.) Case method in management development: Guide for effective use, Geneva, Switzerland: Management Development Series No. 17, International Labour Office.
- Savec V. F., Vrtačnik M., Gilbert J. K. and Peklaj C., (2006), In-service and pre-Service teachers' optionion on the use of models in teaching chemistry. Acta Chim. Slov., 53, 381–390.
- Scott N., (2007), An evaluation of the effects of using case method on student learning outcomes in a tourism strategic planning course, Journal of Teaching in Travel & Tourism, 7(2), 21–34.
- Stief M. and Wilensky U., (2003), Connected chemistry incorporating interactive simulations into the chemistry classroom, J. Sci. Educ. Technol., 12(3), 285–302.
- Szalinska E., Dominik J., Vignati D. A. L., Bobrowski A. and Bas B., (2010), Seasonal transport pattern of chromium (III and VI) in a stream receiving wastewater from tanneries, Appl. Geochem., 25, 116–122.
- Taber K. S., (2013), Non-random thoughts about research, Chem. Educ. Res. Pract., 14, 359–362.
- Teksoz G., Sahin E. and Ertepinar H., (2010), A new vision for chemistry education students: Environmental education. International J. Environ. Sci. Educ., 5(2), 131–149.
- Thadison F. C., (2011), Investigating Macroscopic, Submicroscopic, and Symbolic Connections in a College-Level General Chemistry Laboratory. ProQuest LLC, PhD dissertation, The University of Southern Mississippi.
- Tirez K., Silversmit G., Bleux N., Adriaensens E., Roekens E., Servaes K., Vanhoof C., Vincze L. and Berghmans P., (2011), Determination of hexavalent chromium in ambient air: A story of method induced Cr(III) oxidation, Atmos. Environ., 45, 5332–5341.
- Treatment of hexavalent chromium (2013), http://https://www.youtube.com/watch?v=YjC744L7xlg, retrieved 5th October 2013.
- Yadav A., (2008), What works for them? Pre-service teachers' perceptions of their learning from video case, Action in Teacher Education, 29(4), 27–38.
- Yoon S., Pedretti E., Bencze L., Hewitt J., Perris K. and Van Oostveen R., (2006), Exploring the use of cases and case methods in influencing elementary pre-service science teachers' self-efficacy beliefs, J. Sci. Teacher Educ., 17, 15–35.
| This journal is © The Royal Society of Chemistry 2015 |