Canonical pedagogical content knowledge by CoRes for teaching acid–base chemistry at high school
Clara
Alvarado
*a,
Florentina
Cañada
b,
Andoni
Garritz
c and
Vicente
Mellado
b
aCentro de Ciencias Aplicadas y Desarrollo Tecnológico, Universidad Nacional Autónoma de México, Circuito Exterior S/N, Ciudad Universitaria AP 70-186, C.P. 04510, México, D.F., Mexico. E-mail: clara.alvaradoz@gmail.com
bFacultad de Educación, Universidad de Extremadura, Spain
cFacultad de Química, Universidad Nacional Autónoma de México, Ciudad Universitaria, Avenida Universidad 3000, 04510, México, D.F., Mexico
First published on 22nd May 2015
Abstract
The topic of acid–base chemistry is one of the oldest in general chemistry courses and it has been almost continuously in academic discussion. The central purpose of documenting the knowledge and beliefs of a group of ten Mexican teachers with experience in teaching acid–base chemistry in high school was to know how they design, prepare and organize their classes inside and outside the classroom, from which a set of teaching–learning sequences will be developed, essentially to train new teachers. We decided to document Pedagogical Content Knowledge (PCK), by means of two methodological tools from Loughran, Mulhall and Berry: Content Representation (CoRe), and Pedagogical and Professional experience Repertoire (PaP-eR). In this article, we relate only the first of these tools. It was also important to document concepts, skills and attitudes, so we have made the analysis based on these three items. The main finding was to construct a Canonical PCK including the central concepts of pH and strength in the topic of acid–base chemistry from the main phrases included in the Content Representation answered by those outstanding Mexican teachers. We chose the topic of “acid–base chemistry” because there are numerous examples of its importance related to sustainability (among them acid rain or acidification of oceans), everyday issues (including stomach acidity, antacids, health troubles with the pH of blood and urine), or problems with the longevity of books related to the acidity of paper. Also, it is of fundamental importance because the students often present many alternative conceptions about it.
Introduction
Teachers as a keystone of learning
It is important to remember that teachers, together with students, syllabus, textbooks, laboratories (Mellado, 1998) and now information and communication technologies, are one of the main variables in the teaching and learning of science. Brophy (2001) lists the main tasks teachers should address: instructional goals, content selection and representation, classroom discourse, learning activities, assessment, adjusting instruction to meet the needs of individual students, and managing classrooms in ways that support the instructional program; he says that there is broad agreement among educators that students should learn each subject with understanding of its big ideas (which he also calls powerful ideas and we have called central concepts), and the capability and disposition to apply it in their lives outside of school; he mentions “Content developed with these goals in mind likely to be retained as meaningful learning that is internally coherent, well connected with other meaningful learning, and accessible for application. This is most likely to occur when the content itself is structured around powerful ideas and the development of this content through classroom lessons and learning activities focuses on these ideas and their connections” (Brophy, 2001, p. 10). To help students learn, before starting any class or activity, the teacher should ensure that students know what they will learn and why it is important to learn it, review the subject' powerful ideas and draw attention to the objectives of the activities to be performed and the main steps to prepare. Also, during the development of content in the classroom, the teacher connects with and builds on prior knowledge and experiences of students. To achieve these purposes, the science teachers must possess a thorough knowledge of the topic and diverse professional tools that go beyond those usually studied in college (Shulman, 1987; Fernández et al., 2002).Shulman (1986) proposed and developed Pedagogical Content Knowledge (PCK) as a model for understanding how novice teachers acquire new understanding of the content and how this influences their teaching. PCK is the set of beliefs and knowledge possessed by teachers that can be considered as a bridge between pedagogical aspects and the specific content to be taught, which can be useful for the training and updating of science teachers, which traditionally has focused only on content knowledge. It provides the ability to translate specific contents to a diverse group of students, using multiple strategies, instructional methods and representations, considering the contextual, cultural and social limitations, within the learning environment (Geddis et al., 1993). Current research on the beliefs and practice of teachers is one of the main topics of the research agenda in science education (Mellado et al., 2006; Abell, 2007).
Research question
Productive reasoning in science and engineering often relies on the ability to understand and apply structure–property relationships to explain and predict the behaviour of diverse chemical substances (Cooper et al., 2013; Maeyer and Talanquer, 2013), for example, acid–base chemistry. In science education, particularly chemistry, it is a central learning goal to identify the understanding of the relationships between macroscopic properties and molecular composition and structure. Chemists have developed a complex but rather powerful symbolic and iconic language that serves as a bridge between the macroscopic and the submicroscopic domains. Teachers have to acquire the pedagogical tools to apply this macro-submicron level transposition with their students (Gilbert and Treagust, 2009). Acid–base chemistry is one of the topics in which the interpretation of the properties of substances rests on its molecular structure, the presence of ions in solution, and the mechanistic stage of phenomena.This change between the macroscopic and submicroscopic levels has to be part of the pedagogical reasoning that has to be acquired by teachers in training to be able to explain the relationships between the properties and behaviour of substances and their chemical structure.
Nevertheless one has to be careful with the mixing of levels of chemistry teaching, as Jensen (1998, p. 817) has emphasized: “The most important pedagogical lesson to be extracted is the logical necessity of carefully distinguishing between the molar, molecular, and electrical levels of discourse in chemistry. Unhappily, this is also the point on which most modern textbooks falter, as not only do they generally fail to explicitly point out the existence of these three levels, they normally proceed to randomly mix them together throughout the book”.
Johnstone (1982, 1991) had mentioned that chemists with experience can view their subject matter at three levels: (a) Descriptive and functional: the macro level at which phenomena are experienced, observed, and described; (b) Representational: the symbolic level in which signs are used to represent and communicate concepts and ideas; and, (c) Explanatory: the submicro level at which phenomena are explained.
Talanquer (2011) expressed that the representation of chemical knowledge in this triplet has become paradigmatic in chemistry and science education. However, it sometimes generates confusion and misunderstanding when the people tend to use different terms and concepts for describing the nature and scope of its major components. He (p. 187) characterized the chemistry knowledge that is relevant for teaching into three main “types”:
– Experiences: which includes our descriptive knowledge of chemical substances and processes as acquired in direct (through the senses) or indirect (using instrumentation) ways.
– Models: which includes the descriptive, explanatory, and predictive theoretical models that chemists have developed to make sense of the experienced world. They refer to the theoretical entities, and the underlying assumptions, that are used to describe chemical systems by attributing to them some sort of internal structure, composition, and/or mechanism that serve the purpose of explaining or predicting the various properties of those systems.
– Visualizations: which encompasses the static and dynamic visual signs (from symbols to icons) developed to facilitate qualitative and quantitative thinking and communication about both experiences and models in chemistry. They refer to the chemical symbols and formulas, particulate drawings, mathematical equations, graphs, animations, simulations, physical models, etc., used to visually represent core components of the theoretical model.
He (p. 189) presents a multi-dimensional chemistry knowledge space defined by the different scales/levels (macroscopic, mesoscopic, multi-particle, supramolecular, molecular and subatomic), dimensions (composition/structure, energy and time), and approaches (mathematical, conceptual, contextual, historical) in which each of the three main knowledge types (experiences, models, and visualizations) can be conceptualized. This implies that the meaningful chemistry learning requires students to be able to translate within and across knowledge types, scales, dimensions, and approaches.
Taber (2013) says that although Johnstone's triplet has been extremely appealing to chemical and science educators and very useful in highlighting core components of our chemical knowledge, we need to be careful in its application and interpretation. For example, unfortunately, most chemistry teaching is focused on the submicro-symbolic pair of the triplet and rarely helps students to build bridges to comfortably move between the three levels. This teaching approach often results in confusion and information overload, with negative consequences on student motivation and achievement in the chemistry classroom. He (p. 158) refers to two areas of confusion that can cause this “triplet”: “(1) confusion between two possible foci for the macroscopic: the phenomena studied in chemistry, and the conceptual frameworks developed in chemistry to formalize knowledge about those phenomena; (2) confusion over what is meant by a symbolic ‘level’ – how it fits in an ontology with ‘macroscopic’ and ‘submicroscopic’, and how it relates to notions of there being three different representational levels”.
He (p. 165) says: “… the symbolic knowledge domain cannot be readily separated from the macroscopic and submicroscopic domains as a discrete level of chemical knowledge, as this domain is concerned with representing and communicating the concepts and models developed at those two ‘levels’. The symbolic is inherent in how we think about chemistry; and the processes of learning, teaching and applying chemistry commonly involve re-descriptions into and between components of the specialized symbolic ‘language’ used to describe chemical ideas at the two levels. He summarized these ideas in the drawing shown in Fig. 1.
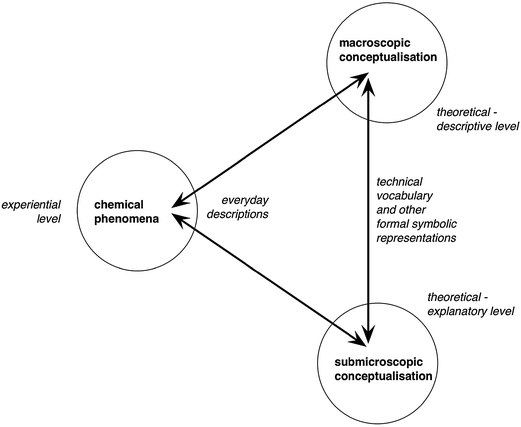 | ||
| Fig. 1 Learning chemistry involves re-descriptions (represented by the arrows) between the everyday language of direct experience and formal representations of the conceptualization of the subject at two distinct levels Reproduced from Taber (2013, p. 165). | ||
The research question of this work was: is it possible to document the structure–property relationship of pH and strength concepts of acid–base chemistry shown by ten outstanding Mexican teachers in a Canonical PCK and construct from it teaching/learning sequences for training new teachers on this topic?
The Pedagogical Content Knowledge, its portrayal and documentation
Shulman (1986, 1987) considered PCK as part of the knowledge base for teaching, which describes the ability of teachers to help students understand a specific topic, and considered that the key factors of PCK were (a) using representations of knowledge on the subject and (b) understanding specific learning difficulties, and the conceptions and preconceptions of students. Similar ideas are mentioned by Loughran et al. (2012).Park and Oliver (2008) say that teachers develop their PCK through a relationship that is in the dynamics of knowledge acquisition, new applications of that knowledge and reflection on its application in practice. This assertion also supports the idea that teachers do not simply receive knowledge that others create to teach, but produce knowledge for teaching through their own experiences. This characteristic is essential to view teachers as professionals.
The Magnusson et al. (1999) model of five components, complemented with the ideas on the first component developed by Friedrichsen et al. (2011), was considered as the PCK model of this work. They conceptualize the PCK as a “mixture” and transformation of several types of teacher knowledge and they argue that effective teaching requires the integration of knowledge from various domains, as a product of knowledge of subject matter, pedagogy and context. These components are: orientation toward science teaching; Knowledge and beliefs about science curriculum; Knowledge of students′ understanding of science; Knowledge of assessment in science; Knowledge of instructional strategies.
In PCK Summit,† a consensus definition of personal PCK was proposed: it is the “personal attribute of a teacher, considered both a knowledge base and an action. It is the knowledge of, reasoning behind, planning for, and enactment of teaching a particular subject in a particular way for particular reasons to particular students to enhance students' outcome” (Carlson and Gess-Newsome, 2013). The four times that the word “particular” appears in this definition is a double-edged sword. On one hand, it means that PCK must be constructed specifically every time a given teacher, with some objectives, has to lecture on a precise topic to a certain set of students with definite background and learning characteristics. But, on the other hand, it represents a superb challenge, since PCK is an academic construct that represents an intriguing idea, rooted in the belief that teaching requires much more than delivering content knowledge to students, and which includes the aims involved and the best ways to represent and evaluate that knowledge.
The authors of this paper believe, as Smith and Banilower (2012), in PCK Summit, that there are two types of PCK: the “personal” PCK (substantiated by personal experience and beliefs/orientations) and the “canonical” one (substantiated by systematic research and that can be shared and applied by many teachers). All teachers have personal PCK, mainly tacit, but after a full discussion of a collective Content Representation (CoRe), all teachers participating may acquire a “Canonical PCK”. Our set of ten interviewed teachers is one of the outstanding teachers that seem to exhibit an integrative PCK. They “know” instructional strategies, student difficulties, curriculum requirements, assessment methods and the Subject Matter Knowledge required so their students can learn.
The concept of PCK has been receiving a reformulation and re-examination during and after the PCK Summit. One of the leaders of the meeting, Julie Gess-Newsome, has revealed an innovated vision on the construct, in one book (Berry et al., 2015), in which she presents a “Model of Teacher Profession Knowledge and Skill”, shown in Fig. 2. The model identifies the overarching role of teacher professional knowledge and situates PCK within that model, including all of the complexity of teaching and learning.
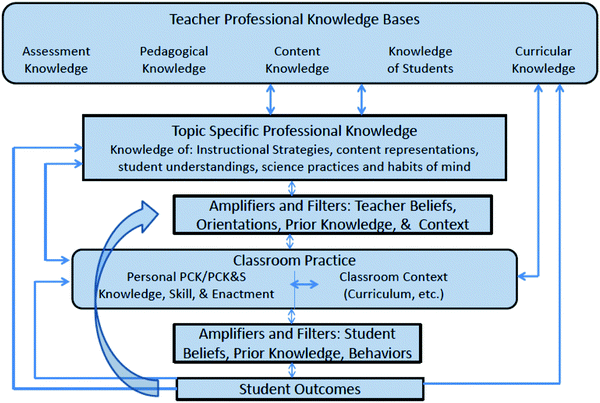 | ||
| Fig. 2 Model of Teacher Professional Knowledge and Skill including PCK and influences on classroom practice and students outcomes. | ||
In this Model, teacher affection is recognized as making a contribution to teacher knowledge, skill, and practice. These beliefs and orientations act as amplifiers or filters to teacher learning and mediate teacher actions. Unique to this model, PCK is defined as both a knowledge base used in planning for and the delivery of topic-specific instruction in a very specific classroom context, and as a skill when involved in the act of teaching. Finally, student outcomes are explicit in this model, considering that student learning is not an automatic product of instruction.
Some of the main ideas have also been formulated after the Summit, in the ESERA Conference-2013 (Carlson and Gess-Newsome, 2013; Carlson et al., 2013). In this last reference, the summary reveals in relation to a two-year intervention that combined the implementation of educative curriculum materials with a transformative professional development program that “positively influenced teachers' pedagogical content knowledge (in both components, the content knowledge and the pedagogical knowledge) as intended and had additional positive effects on both knowledge”.
Placing PCK and CoRes at the centre of science teacher's educational programs both in-service and in-training enables them to become professional science teachers (Gess-Newsome and Lederman (1999) and Kind (2009). De Jong (2002, p. 366)) cited that “Prospective chemistry teacher education and professional development should pay more attention to improving the topic knowledge of primary and secondary teachers, and develop teacher training programs aimed at PCK at the university level”. Similar ideas were cited by Van Driel et al. (2002). Abell (2008) highlights the use of PCK to guide programs for preparing future elementary teachers and for new alternative certification programs for middle and high school science and mathematics teachers, such as in the United States of America, where the National Science Foundation has recognized the value of the PCK as a paradigm for research on teacher learning. The PCK research leads us to better understand the kinds of knowledge that experienced teachers use when they plan and carry out instruction, which in turn helps us to define goals for programs and courses in science teacher preparation or professional development. Science teacher education would benefit from utilizing PCK more actively, that is, helping novice and experienced teachers to understand how knowing about PCK may help their practice development and improvement.
Content Representation (CoRe)
We document PCK by means of two methodological tools proposed by Loughran et al. (2004): Content Representation (CoRe), and Pedagogical and Professional experience Repertoire (PaP-eR). In this paper, we relate the first of these tools. The CoRe framework was chosen because it has given good results in other investigations by one of the authors (Garritz and Trinidad, 2006; Reyes and Garritz, 2006; Garritz et al., 2007, 2008, 2013; Padilla et al., 2008; Espinosa et al., 2011; Padilla and Garritz, 2014).For Kind (2009, p. 194), “a CoRe is a detailed description tabulating the ‘big ideas’ or concepts relating to a topic being taught against points such as what exactly students have to learn about each big idea; their possible difficulties with each concept; why it's important for them to know these concepts; how these concepts fit in with others; and any knowledge the teacher holds that connects the big ideas in this CoRe to others”. She says (p. 199) that the CoRe “offers, in my opinion, the most useful technique devised to date for eliciting and recording PCK directly from teachers. This method is clearly focused on the knowledge and tools for teachers, and a CoRe provides a powerful resource to record the work of an outstanding teacher, useful to exemplify good practice”. This was the main reason why the CoRe played a very important role in the development of our Teaching-Learning Sequences about acid–base chemistry from Canonical PCK of high school teachers (Alvarado, 2012). The groups of Rollnick et al. (2008), Hume and Berry (2011), Bertram and Loughran (2012) and Williams (2012) also use this methodology. However, this method is not unproblematic: the daunting task of completing it for some teachers, for example, the lack of confidence in their abilities, and the long time required to complete it.
Acid–base chemistry. Its importance
A major topic in the High School of Science is the acid–base chemistry, but the students often have difficulties (as well as alternative conceptions) in learning this subject (Kolb, 1978; Jensen, 1980; de Vos and Pilot, 2001). De Vos and Pilot have characterized the addition of topics to the syllabus of acid–base chemistry as sequential layers of a stratum type construction: “This structure, like a sedimentary rock, shows a number of successive layers of concepts, each with its own history” (p. 494), which causes a lot of confusion in students. Furió-Más et al. (2005) say “macroscopic and microscopic conceptual models involved in the explanation of acid–base processes are mixed in textbooks and by teachers” (p. 1337). These authors find a juxtaposition of the macroscopic and microscopic model in the teachers' interviews: “The majority of teachers interviewed present a linear, cumulative view in which they consider an acid substance to be the acid particle (overlapping macro–micro) or that the Brønsted–Lowry theory is an extended version of the Arrhenius theory” (p. 1353).We chose “Acid–base chemistry” as the specific topic of analysis for PCK, because there are numerous examples of its importance in various phenomena, as well as its multiple applications in school laboratories, research and industry. Thus, for example, in the human body: alkaline urine may indicate kidney or urinary tract infection, while highly acidic urine may be the manifestation of emphysema or diabetes; at the stomach level, an intense chemical treatment of food is caused by the action of gastric juice, which contains hydrochloric acid, responsible for the stomach pH being less than 2, and which prevents microbiological contamination and favours the action of proteolytic enzymes of gastric juice; human blood is a complex aqueous medium buffered at pH 7.2 to 7.4, any small change in pH results in a severe pathologic response and eventually in death; when performing intense muscular effort muscular acidosis occurs due to the accumulation of lactic acid, this process is accompanied by severe pain.
The important issue of acidification of seawater is an emerging problem for humanity (Kerr, 2010; Alvarado et al., 2011). On the other hand, one of the most important characteristics of paper is the degree of acidity of the paper and the print media, because too low a pH causes the inks to dry out and corrode the metal plates of the press, an excessive alkalinity can cause immiscibility between ink (fat medium) and water, leading to other printing problems. Finally, composting is a bio-oxidative degradation process of organic waste that requires aeration and certain other conditions, such as temperature, humidity and pH, which favour the action of microorganisms; in foods the pH control is critical, it is a parameter of degree of conservation of the food.
In high school programs in chemistry, around 15 to 20 hours are spent in teaching acid–base chemistry. The Science–Technology–Society (STS) approach is promoted in most of them by its critical reflection on social, environmental and economic impact, for example: blood as a damping system, the study of heartburn, fertilizers as strategic chemicals, acid–base behaviour of materials for domestic use (such as vinegar, coffee and toilet plunger); the understanding of the formation of acid rain and its polluting effect.
As an example of the development of this subject matter, we present the First Unit – Soil. Source of nutrients for plants – of Chemistry 2 course, of Colegio de Ciencias y Humanidades (CCH – UNAM), in which the topic ‘how important is to know the soil acidity?’ is treated in 4 hours:
(a) The fundamental items are Arrhenius acid, base and salt, concepts, formula and name; ion (hydrogen and hydroxide ions) and dissociation concept; neutralization reactions concept and representation.
(b) The expected learning outcomes are to: increase the skills in finding relevant information for analysis and synthesis; increase the capacity to formulate hypotheses, and skills to observe and develop experimental tasks; differentiate acids and bases using its properties; recognize the pH as a measure of the acidic, basic or neutral character of a substance or its solutions; establish that the neutralization reaction is the result of the combination of acids and bases; interpret acids and bases according to Arrhenius; increase oral and written communication to substantiate findings and conclusions.
(c) Suggested strategies: bibliographic research on the properties of acids and bases, Arrhenius' model, the scale and measuring the pH and importance of pH soil for assimilation of nutrients; collectively design an experiment to determine the acidity of a soil sample; laboratory activity to determine the characteristic properties of acids and bases such as colour with indicators, electric conductivity and behaviour when interacting with metals and carbonates; measure pH using paper or a potentiometer; group analysis of the researched and performed in the laboratory for differentiating acids and bases, correct use of the pH scale and explanation of neutralization; prepare a report of the experimental activity; group discussion based on the literature research and experimental activities to highlight the importance of knowing the pH soil for crop selection, reforestation, choosing fertilizers and plant nutrition.
Finally, a central purpose of documenting the knowledge, skills, beliefs, etc. of ten outstanding Mexican teachers, with experience in teaching acid–base chemistry in high school, was to know how they design, prepare and organize their classes inside and outside the classroom to construct Canonical PCK, and thus teaching–learning sequences (Alvarado, 2012) to train new teachers on the topic. These sequences will be reported in another paper.
As will be seen in the Methodology section, we establish a relation between the five components of PCK of Magnusson et al. (1999) and concepts, skills and attitudes required for the students′ understanding and proper handling of acid–base chemistry.
Methodology
We describe the procedure to capture and analyze Mexican teachers' PCK-CoRe on acid–base chemistry at the high school level, developed between 2009 and 2011.The sample (participants)
The sample of ten high school teachers was selected on the basis that they may be considered a very select group of teachers, mostly with long experience in teaching, including the topic of acid–base chemistry; most of them are considered outstanding by their peers and pupils. Their recruitment was done with a personal interview in which all of them agreed consensually to be treated as anonymous. Although they work in different schools, mainly in Mexico City, they often coincide in seminars and academic events. All expressed availability and a positive attitude towards collaboration with this project. It has been said (Kind, 2012) that the factors combining to produce effective PCK are Subject Matter Knowledge, classroom experience and positive emotional attributes; we are sure that the ten teachers have acquired these three factors.All were linked to the National University of Mexico (UNAM is the acronym in Spanish for “Universidad Nacional Autónoma de México”), either as students or teachers in service, whether at the School of Chemistry, or at the high school of UNAM: National Preparatory School (ENP is the acronym in Spanish) or the College of Sciences and Humanities (CCH Spanish acronym) or other schools (two of them are now working at private schools and a third at a university in the United States of America). In Table 1 the detailed background of the teachers is described.
| Teachers | Age (years) | Academic degree | Level of courses taught | Teaching experience (years) | Teaching experience in acid–base chemistry (years) |
|---|---|---|---|---|---|
| T1 | 40 | BS Chemistry and Master Education | High school | 13 | 5 |
| T2 | 43 | PhD Chemistry |
High school
Undergraduate |
23 | 23 |
| T3 | 46 | MSc Chemistry |
Secondary
High school |
20 | 20 |
| T4 | 73 | MSc Chemistry |
High school
Undergraduate |
39 | 39 |
| T5 | 64 | MSc Chemistry | High school | 35 | 35 |
| T6 | 63 | PhD Analytical Chemistry |
High school
Undergraduate Master |
34 | 34 |
| T7 | 38 | BS Chemical Engineering and Master Education | High school | 8 | 8 |
| T8 | 45 | PhD Chemistry |
High school
Undergraduate |
22 | 22 |
| T9 | 34 | BS Chemical Engineering and PhD in Education | High school | 9 | 9 |
| T10 | 47 | BS Chemistry and Master of Education | High school | 20 | 20 |
Capturing and documenting Content Representation (CoRe)
In order to investigate if the original matrix of the questionnaire (Appendix 1, Table 6) proposed by Loughran et al. (2004) was applicable or satisfied our needs, a pilot study was conducted with 64 high school teachers, during two days in an eight-hour period in a workshop about Pedagogical Chemistry Knowledge at a university in Mexico City. The teachers were asked to form groups of two or three of them to develop the CoRe on a topic chosen by them, not necessarily acid–base chemistry. They gave back the responses to it via Internet two weeks later.As a result of the analysis of this study, we modified the original questionnaire. Upon analyzing the answers of the teachers, it was considered that some modifications were worthwhile. For example, they did not differentiate the two first questions (1. What you intend the students to learn about this idea? 2. Why it is important for students to know this?). Or they were confused with the third question (3. What else you know about this idea – that you do not intend students to know yet?). That was why the first two questions were joined in a single one, the third was eliminated and new questions were incorporated: about historical aspects (Question 3 on Appendix 2, Table 7), on aspects of daily life (Question 4 on Appendix 2, Table 7); and about concepts, skills and attitudes that have a bearing on the teaching (Question 6 on Appendix 2, Table 7) of the central concepts. The modified questionnaire (Appendix 2, Table 7) was then applied to the group of ten teachers reported in this paper. None of these ten teachers participated in the workshop.
It is important to point out that the questionnaires Appendix 1 (Table 6) and Appendix 2 (Table 7) were applied in Spanish in México. The corresponding translations to Spanish and English languages were revised by a Scottish academic colleague. He has been living in Mexico for fifteen years.
The authors of this study started by interviewing each of the participants, explaining to them the way in which the CoRe had to be filled out and the aims of the study. After that, the questionnaire of Appendix 2 (Table 7) was sent to them via e-mail, in order for the ten participant teachers in this project, to reflect on their answers; they were asked to complete it within three to four weeks. They had the prerogative to reformulate their answers as they wished, as long as they resorted to their own knowledge, and beliefs, preferably without consulting books, articles, etc. In order to answer the questionnaire, they first had to define the concepts or ideas considered central to the subject matter; and then answer the eight questions for each one of the central concepts.
Later all teachers' responses were transcribed to each of the survey questions, to know what they thought together about the importance of teaching the topic, its historical evolution, and so on. Of special interest were the central concepts cited by the teachers and which were cited most frequently. It will be seen that our ten participants mentioned a huge set of central concepts (28), meaning that it was difficult to arrive at a set of eight central concepts that constitute the central concepts of the Canonical PCK. Then we proceeded to concentrate information about each of the central concepts mentioned at least twice, noting that some of the information was closely linked, the concepts were regrouped and reduced to eight concepts, as in the case of the pH and relative strength of acids and bases. These eight concepts were considered for the final analysis and report of the captured information. In this analysis, the comments of the ten teachers with respect to the eight central concepts were included, although not all teachers cited them as such.
Initially, we went ahead by getting an idea of the type of education mastered by each teacher. However, given the purpose of the project, it was not important to get the teaching profile of each of them, but instead the knowledge, strategies, skills, attitudes, etc., which together favored the design and development of the teaching–learning sequence of acid–base chemistry (Alvarado, 2012).
This classification of the three items (concepts, skills and attitudes) was employed to construct Canonical PCK of the set of ten teachers. The reasons are that:
(1) Concepts are the basis on which to program the teaching/learning activities, giving a unique way to attain the planned objectives. The teacher must centre the classroom activities on the concepts that contribute most of the explanation of the topic on these conceptual contents;
(2) It is also important to develop the skills to acquire, understand and communicate information on the topic;
(3) Attitudes are basic to know the teachers′ limitations in making their own teaching work and in their aspirations to improve the learning of the students. Furthermore, it is necessary to know teachers' opinions about the habits and attitudes of their students.
As mentioned above, we rely on Magnusson et al. (1999) model of five components of PCK to analyze the responses of ten teachers, and considering that the phenomena and processes involving acidic and basic solutions offer an excellent opportunity for the teacher to help students develop concepts, skills and attitudes, required for the proper understanding and handling of the topic. We propose the structure of Table 2 to categorize information Canonical CPK teachers, because like Coll et al. (1995), we believe that learning is an integrated process in which concepts, skills and attitudes are learned together; each depends on each other. Including the three types of contents in a didactic proposal tries to break with the usual practice of teaching focusing solely on memorization and repetition of facts and data, to promote instead the understanding of concepts, mastery of certain procedures and the behaviour with certain values. We think that considering skills and attitudes at the same level as concepts emphasizes that they should be the object of teaching and learning in school. It amounts to accept the principle that everything that can be learned by students can and should be taught by teachers. We link them as follows:
| 1. Concepts |
| 1.1 Historical aspects |
| 1.2 Importance of learning |
| 1.3 Relationship with the daily environment |
| 1.4 Knowledge required for learning |
| 1.5 Difficulties in the teaching–learning process |
| 1.6 Representations and resources to motivate students |
| 1.7 Assessment |
| 2. Skills |
| 2.1 Logical skills |
| 2.2 Math skills |
| 2.3 Experimental skills |
| 2.4 Communication and dissemination skills |
| 3. Attitudes |
| 3.1 Related to teachers |
| 3.2 With regard to students |
• Orientation toward science teaching. It considers a set of beliefs about how science should be learned and coupled with certain instructional strategies, for example, the use of Historical aspects and the Relations with the daily environment.
• Knowledge and beliefs about the science curriculum. A component that includes knowledge of and the ability to articulate goals and objectives, as well as the vertical position of their topic within the progression of student learning, that we denominate: Importance of learning.
• Knowledge of students' understanding of science. It includes teachers' knowledge of prerequisite ideas and skills that students will need to learn a topic, and the areas that the student will find difficult to learn. In our proposal, we refer to it as Knowledge required for learning, Difficulties in the teaching–learning process, and Skills. We categorize the skills into four types: Logical, Math, Experimental and Communication and dissemination skills.
• Knowledge of assessment in science. It includes teachers' knowledge of which parts of student learning are the most important to assess in a certain content area and the way in which a teacher assesses certain aspects of student learning specific to a topic area. We call it Assessment.
• Knowledge of instructional strategies. It includes strategies for teaching the subject of science and it recognizes that certain strategies are connected with certain goals. Magnusson et al. claim that teachers' use of strategies is influenced by their beliefs about the teacher's role in student learning. We named it: Representations and resources to motivate students.
As other educators, we include the teachers' attitudes in our scheme because they are the filter through which new knowledge is integrated into their practice and conceptual framework. We considered two types of attitudes: Related to teachers and With regard to students.
So the analysis of the responses to each central concept was performed by classifying the teachers' comments, according to the three items (concepts, skills and attitudes) with the sub items of the scheme shown in Table 2.
The eight CoRe questions of Appendix 2 are related to the three items of Table 2.
As was also analyzed by Bertram and Loughran (2012), in terms of the validity and reliability of the CoRes' study, there are perhaps two areas of weakness. One might be the dominant use of interviews as the major form of data collection —that is, much of the data relies on self-report. The other weakness of the study might be in the researcher's interpretation and analysis of the data. To ameliorate these weaknesses, the four authors of this paper each independently reviewed the information, which was afterwards discussed, analyzed and finally categorized by its location in the structure of Table 2.
Results and discussion
This section presents the results and analysis of the information on Pedagogical Knowledge of acid–base chemistry, at the high school level.Documentation of the CoRes
The understanding of the term «central concepts» is important because they are at the core of understanding and teaching the subject of science; they are the topics that belong to the disciplinary knowledge which the teacher usually uses for planning the teaching of the topic. The clue is that those concepts sharply reflect the most important contents of the subject, maybe including some of its precedents.Initially teachers cited 28 central concepts; we proceeded to concentrate information of those central concepts cited at least twice; we noted that some of them were closely linked to each other, as in the case of the Neutralization/neutralization reaction, to which we added titration feasibility. After regrouping, Table 3 presents the eight central concepts of the topic of acid–base chemistry, considered definitive and consensual for the development of the CoRe documentation. Only one teacher selected the eighth central concept, but it was included because of the importance of the thermodynamic point of view of the topic.
| Teachers | Central Concepts | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | pH/relative strength of acids and bases | X | X | X | X | X | X | X | X | ||
| 5 | Concept of acids and bases/distinctive properties of substances/substance/reactivity | X | X | X | X | X | |||||
| 4 | Neutralization/neutralization reaction/feasibility titration | X | X | X | X | ||||||
| 4 | Concentration | X | X | X | X | ||||||
| 3 | Define acids and bases according to the Brønsted–Lowry model/acid–base reaction in aqueous dissociation as particle exchange H+ | X | X | X | |||||||
| 2 | Acids and bases in terms of Arrhenius | X | X | ||||||||
| 2 | Water auto-ionization (and pH) | X | X | ||||||||
| 1 | Acid–base equilibrium/constant of acidity | X |
Over the eight surveyed questions of CoRe related to the central concepts that each teacher selected, the three questions that involved more extensive responses were: No. 1, regarding the importance of learning, No. 2 with respect to the background content and skills students must possess to properly understand each concept, and No. 6, on the concepts, skills and attitude learning that influence each of the concepts.
Regarding the difficulties encountered during the capture and documentation of the Content Representation, two main problems emerged:
Table 3 shows the eight consensual central concepts most often mentioned by the ten teachers. However, it was difficult to discriminate this information from the additional central concepts that teachers cited.
The classification of the statements of teachers in terms of each of the eight consensual central concepts (pH/relative strength of acids and bases; and so on…) and each indicator of the concepts, skills and attitude items, established in Table 3, was problematic. The reason is that the other central concepts could correspond to two or more sub-contents and, for example T7 mentioned “Acids and bases allow connections with the experiences of students and their immediate environment”. Then, where to classify this concept? In Importance of learning or in Relationship with the daily environment? In this case, the authors chose the second, trying to be consistent without repeating the concepts.
We have restricted this paper to report one (pH and strength of acids and bases) of the eight central concepts collected, because it was the central concept the teachers cited more frequently (see Table 3); also due to the large amount of information in their CoRes it is impractical to include more than one in this paper.
Global CoRe results
The fundamental items considered in the First Unit (Soil. Source of nutrients for plants) of the Chemistry 2 course, of the Colegio de Ciencias y Humanidades (CCH – UNAM): “Arrhenius acid, base and salt, concepts, formula and name; ion (hydrogen and hydroxide ions) and dissociation concepts; neutralization reactions' concept and representation”, were all cited by the Mexican teachers when answering the questionnaire; even the items “Arrhenius model”, “hydrogen and hydroxide ions”, “dissociation” and “neutralization”, are mentioned within the 28 central concepts cited originally by the teachers.The citations mentioned by them coincided with the suggestions in “The expected learning outcomes” and “Suggested strategies”, in the First Unit of the program, excepting “Experimental activities to highlight the importance of knowing the pH soil for crop selection, reforestation, choosing fertilizers and plant nutrition”, which were not mentioned. Even though establishing the teaching profile of each of the ten teachers was not one of the main purposes of this research, they could be classified into three main groups, considering the information provided by them with respect to acid–base chemistry (see Table 4). We use the letter “T” followed by a subscript with the number of the teacher.
| Group | Teachers | Teaching orientation |
|---|---|---|
| A | T1, T3, T6, T7, T8 and T10 |
Mastery of the subject (Pedagogical Content Knowledge and Subject Matter Knowledge).
Also, information about Science–Technology–Society, and nature of science. Often reference to skills and attitude items. |
| B | T2 and T9 |
In general, abundant information about Science–Technology–Society, nature of science and conceptual contents.
Scarce reference to skills and attitude items, and to the students. |
| C | T4 and T5 |
Conceptual content the most important.
Only strong emphasis on the conceptual content of the discipline. |
With respect to the teaching and professional profile of teachers, we can say that six teachers (T1, T3, T6, T7, T8 and T10, those of group A in Table 4) were centred on students and in their learning difficulties, with diversified teaching activities; the other four (T2, T4, T5, and T9, those of groups B and C in Table 4), were centred on the teacher and the disciplinary content, with a transmissive approach while teaching.
Even with these different teaching orientations it was possible – in our case – to integrate a Canonical PCK. The question remains as to what extent these differences or similarities among the teachers' teaching and professional profiles influences the integration of the Canonical PCK, as pointed out by a reviewer of this paper.
Regarding the possibility of being able to infer from the responses, those teachers who have participated in graduate studies in Pedagogy (as they are called in Mexico) or Didactics (as they are known in Spain), it becomes a complicated issue to make a very strict distinction. For example, both teachers T7 and T8 showed innovative ideas and possess good command of the topic, however, the first one was studying a Masters in the field of Education at the time when he was answering the questionnaire, while the second had no formal studies in this area.
From the analysis of the responses, a big difference can be seen between an expert and an experienced teacher in a particular topic, although both terms are often considered synonymous. This large difference can be seen, for example, between teachers T5 and T6: both of similar age and teaching experience, but with a very contrasting expertise to address the issue. To identify more clearly what the differences are, the analysis could be extended by saying that T5 responses were very short and only addressed 44% of the selected indicators of analysis of the central concepts (provided little information on Historical aspects, Knowledge required for teaching/learning, and Procedures and resources to motivate students, also on Skills and Attitudes); T6 was one of the two teachers whose responses were more extensive and addressed prominently all indicators. T5 was very oriented to disciplinary knowledge; T6 is recognized as an innovative and renowned teacher in teacher's education programs.
Table 5 shows the number of the eight central concepts in which the teacher gave information regarding the concepts, skills and attitude items; thus, for example, T1 addressed historical aspects on six of the eight selected central concepts, T2 only on two and T3 on five.
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Concepts | ||||||||||
| Historical aspects | 6 | 2 | 5 | 1 | — | 6 | 7 | 3 | 3 | 5 |
| Importance of learning | 4 | 4 | 6 | 2 | 4 | 7 | 4 | 4 | 5 | 4 |
| His connection with the daily environment | 4 | 2 | 3 | 2 | 3 | 2 | 5 | 4 | 3 | 3 |
| Knowledge required for learning | 6 | 3 | 7 | 1 | 1 | 4 | 2 | 1 | 4 | 5 |
| Difficulties in the teaching–learning process | 5 | 4 | 4 | 2 | 3 | 6 | 4 | 4 | 6 | 3 |
| Representations and resources to motivate students | 4 | 3 | 4 | 2 | 1 | 3 | 4 | 3 | 5 | 5 |
| Assessment | 4 | 3 | 4 | — | 2 | 5 | 1 | 3 | 4 | 3 |
| Skills | ||||||||||
| Logical skills | 4 | 1 | — | — | — | 1 | 3 | 3 | 1 | 1 |
| Math skills | 4 | 2 | 3 | 1 | 1 | 4 | 5 | 4 | 1 | 6 |
| Experimental skills | 4 | 1 | 4 | 1 | — | 6 | 2 | 3 | 2 | 3 |
| Communication and dissemination skills | 4 | 1 | 4 | — | 1 | 4 | 2 | 3 | 2 | 5 |
| Attitudes | ||||||||||
| With respect to the teachers | 4 | — | 1 | — | 1 | — | 2 | 2 | 1 | 1 |
| With respect to the students | 4 | — | 5 | — | — | 3 | — | 2 | 2 | |
| Important science ideas/concepts | |||
|---|---|---|---|
| Big Idea 1 | Big Idea 2 | etc. | |
| 1. What you intend the students to learn about this idea. | |||
| 2. Why it is important for students to know this. | |||
| 3. What else you know about this idea (that you do not intend students to know yet). | |||
| 4. Difficulties/limitations connected with teaching this idea. | |||
| 5. Knowledge about students' thinking which influences your teaching of this idea. | |||
| 6. Other factors that influence your teaching of this idea. | |||
| 7. Teaching procedures (and particular reasons for using these to engage with this idea). | |||
| 8. Specific ways of ascertaining students' understanding or confusion around this idea (include a likely range of responses). | |||
| Pedagogical knowledge of expert teachers in the subject of “acid–base chemistry” at high school level. | |
|---|---|
| This questionnaire has been designed with the purpose of documenting the knowledge that teachers have experienced on the subject of “Acid–base chemistry” guiding students to understand the subject in a way personally meaningful to them. The information you provide will help us to implement the teaching–learning sequences that contribute to the training and retraining of teachers of high school in the area, enriching the delivery of content in the classroom and avoiding, in many cases, the monotony of the lectures. We sincerely appreciate your cooperation. | |
| Name | |
| Age | |
| Academic degree | |
| Level at which you lecture | |
| Global Teaching Experience (years) | |
| Teaching experience in the subject of acid–base chemistry | |
| i. How relevant is the topic of acid–base chemistry in a high school course? | |
| ii. What content and skills students should have before entering the school to understand the issue properly? | |
| Place in the three to five rows of the right the name of the central concepts (CC) on the topic of “acid–base chemistry”. We understand by those central concepts in the “core” of understanding and teaching the subject, are the most important concepts that are part of disciplinary knowledge in which you divide or split the teaching of the topic, including perhaps some of its precedents. Please answer as widely as possible, for each of the core concepts (CC), the following questions: | |||||
|---|---|---|---|---|---|
| CC1 | CC2 | CC3 | CC4 | CC5 | |
| 1. What do you intend the students to learn about this concept and why is it important for students to learn it? | |||||
| 2. What content and skills students should have as background just entering the school to properly understand the concept? | |||||
| 3. What knowledge you know about the history of this concept? And what historical aspects are important for teaching it? | |||||
| 4. In particular, what aspects of daily life are important in teaching this concept? | |||||
| 5. What are the difficulties connected to the teaching and learning of this concept? | |||||
| 6. What knowledge about the concepts, skills and attitude items of students influence your teaching of this concept? | |||||
| 7. What representations and resources (analogies, metaphors, examples, videos, demonstrations, simulations, practical activities, etc.) are used for students to motivate and be committed to the concept? | |||||
| 8. What specific forms used to assess understanding or confusion from students about the concept? | |||||
| iii. Comments and/or contributions to the teaching/learning of the subject and the central concepts mentioned by you. | |||||
The Canonical PCK on pH and strength concepts that can be constructed from the ten personal CoRes of this study
The research question of this study was settled in the Introduction section as: is it possible to document the structure–property relationship of the pH and strength concepts of acid–base chemistry shown by ten outstanding Mexican teachers in a canonical PCK and construct with it teaching/learning sequences made for training new teachers on this topic?The authors have selected the main quotes of the ten selected teachers to construct some notes of a consensual PCK that can be considered as a canonical one. It has been organized by means of the three types of content included in Table 2. The first section has to do with concepts, the second with skills and the third with attitudes.
Concepts
The teachers mentioned mainly the following: (a) Bergman related the acid and base strength to the amount of each reagent, and Kirwan related it to the rate of reaction between acids and bases; (b) Arrhenius (1884) proposed one of the first models of acids and bases, which classifies an important group of substances through the transfer of protons or hydroxyl ions, and proposed in 1887 the concept of acid and base strength as an absolute concept; (c) Sørensen (1909) introduced the concept of pH as a way to simplify the management of concentrations, as a new way to measure the acidity of substances and he presented the concept of pH introducing a logarithmic scale; (d) Brønsted and Lowry independently published the acid–base concept in 1923, focusing on the exchange of protons; they conceived acid and base strength as a relative property.
With respect to the use of different models for considering acid and base behaviour, our study shows the same results as the nine teachers of Drechsler and van Driel (2008): “although all teachers recognized some of the students' difficulties as confusion between models, only a few chose to emphasize the different models of acids and bases” (p. 611); these authors also mention that the existence of those models gives teachers a good opportunity to discuss the use of them to explain phenomena in a historical perspective (p. 612). Some features of its chemical composition determine whether a substance is an acid or a base – and this depends on the model adopted.
Concentration is one of the parameters that determine the degree of acidity or alkalinity of a substance. The concept of concentration must be clear before the definition of pH, which includes it. Knowledge of the concentration of a solution allows the calculation of the number of elementary chemical entities that exist in any sample. Its conceptual understanding and skills are required for managing the acid or basic character of a solution and its stoichiometric calculations. It is the basis for understanding the concept of chemical equilibrium.
“pH” is one of the most famous terms of chemistry among students. The incorrectness of some alternative conceptions of students in relation to pH must be addressed: It is only a way to write the proton concentration of any solution (not only acid ones), it does not have to do with “degree of acidity”, “strength”, or “intensity of a chemical reaction”. It does not refer to “the injury” caused by its “burning”, or the color of the solution (students relate it to phenolphthalein).
The pH allows students to differentiate between the chemical force of a material (measured as the degree of dissociation) and the chemical character of that material; it is useful to discuss the dissociation of water and determine the relationship between the concentrations of H3O+ and OH− in aqueous solutions, which leads to the pH scale and determine the acidity or alkalinity of a solution.
The concept of pH facilitates the handling of the concept of acid–base chemistry and is a parameter that is frequently used in the everyday context – pH of a shampoo, for example.
The Science–Technology–Society dimension has to be considered in aspects such as:
• In the everyday environment, the strength can be related to the care that must be taken when handling strong acids or bases to prevent accidents that could be fatal: the reactions between strong acids and strong bases can be violent and release a significant amount of heat.
• It is important for students to recognize that there are different types of acids and bases, some are stronger than others, and that the effect of the substance depends on both the relative strength and the concentration in solution; they must understand that, for example, it is not the same to ingest sulphuric acid or ascorbic acid.
• Changes in the pH of water by pollutants should be controlled in activities such as agriculture, medicine, cleaning, and others.
• It is important that students recognize the importance of pH control in chemical reactions, including those that occur in living beings.
This issue is also emphasized by Furió-Más et al. (2005) because those authors have found that “half of the teachers interviewed have not taken into account STS relationships” (p. 1347) and the same for “70% of the textbooks analyzed” (p. 1353).
In particular, for the proper understanding of the concept of pH, it is important to have a good idea of the amount of substance, as the student must understand that weighing a substance is an indirect way of counting the number of elementary entities in it.
The teachers recognize a lot of difficulties, mainly for learning. Some of them are the following:
• There is no full differentiation between the terms acidity and pH.
• The strength term is also used with different meanings.
• Students consider that the strength of the substances is absolute and is not taken as a relative property.
• They generally think of a unique pH scale, and that neither the temperature nor the solvent has an influence.
• Students used as synonymous strength and concentration, the strength of the acid must be related to the acidity constant.
• Understanding the concept of pH and acidity is complicated because pH varies inversely with the concentration of hydronium ions.
• The concept terms of one of the two main models of acids and bases are carelessly handled.
In relation to the teaching and learning difficulties on the topic, several Mexican teachers referred to the importance of knowing the conceptions of their students, for example: (a) The concentration of a solution is a highly abstract concept that requires understanding of the concept “amount of substance”, which has had serious problems in its handling and understanding: its definition is fuzzy, inaccurate and polysemic, because it has different meanings, for example; it is used to refer to a portion of substance, or as a single mass unit; or associated with the Avogadro's number; or considered as a counting unit; amount of substance and mass are handled as equivalent concepts. All of these meanings promote numerous alternative conceptions. (b) One of the main difficulties in teaching acids and bases lies in alternative conceptions that students possess through their relationship with daily life. (c) If a teacher does not know the students' alternative conceptions, these can be strengthened through his language or examples that he uses in the classroom. (d) Students bring from their experience and prior education, an idea of what an acid is, but the idea of what constitutes a base is not so well known. They have an idea of pH, but the concept is not developed qualitatively nor quantitatively; knowing what students know or think about the subject will help us find a starting point to rebuild the concepts: knowledge of acidic or basic substances of daily living, pH, etc.
Therefore, it is necessary to conduct experiments or demonstrations to determine and check the acidity/alkalinity of various household materials (milk, saliva, soda, etc.). Use can be made of indicators extracted from natural products that can be obtained at home, such as purple cabbage or a red flower, and what colour is observed with them in acid–base chemistry. In that way, arbitrary classification systems are created based on identifying similarities and differences between a distinctive property; everyday materials such as acids or bases can be classified according to their pH and contrasted with student's previous hypothesis; also recreational activities (games where simple calculations are made) and classroom experiences can motivate students.
Experiments can be performed in which the amount of acid/base present in a commercial product is determined. Environmental (acid rain) and metabolic (acidosis) phenomena in which the acids and bases play a major role can be analyzed. Other examples are: add chunks of marble to lemon water, reduce the alkalinity of a detergent by squeezing half of an orange in it; observe the action of products with sulphamic acid as a remover of limescale in sinks, for example.
Calculations can be made to determine the pH; the concepts of acid, base and pH that the student possesses can be discussed; exercises can be performed to represent a solution microscopically by adding an acid, in terms of concentration of H+; the students can develop work proposals; films, models, demonstrations, computer animations and simulations can be used.
The assessment of acid–base chemistry can be made by means of a set of actions such as, for example: quantification of the amount of acid present in a kitchen substance; explaining what happens to the concentration of H+ when adding a base or an acid to the solution; problem solving from a question posed by the students; development of posters for research on applications or phenomena in which the pH is relevant (industrial, environmental, etc.).
Skills
There were a few logical, mathematical and experimental skills chosen by the teachers as important for students to manage. There is an overemphasis on mathematical skills in this first central idea.Also, there are several general short comings observed in the profile of students coming from the secondary level: low reasoning ability; deficiencies in learning key concepts; dependence on what the teacher tells them or what they read in the textbook, without themselves being able to acquire or enrich their learning; low reading habit; poor or no skill in written or oral expression; bad spelling; deficiencies in the development of the formal logical thinking, and so on.
• Using mathematical relationships to calculate pH and understanding its relationship to the concentration of H+ ions, as well as handling very large quantities using exponents.
• Manage logarithmic functions such as the expression of pH of a solution; calculating and having a qualitative interpretation of the meaning of the numerical value and its implications. Because of the difficulty in explaining logarithmic variations, students find it difficult to relate the pH value with exponentially increasing amounts.
• Interpretation of what a logarithm is; students only know that in their calculator there is a key with the “log” name.
• Students have a lack of knowledge of stoichiometric calculations, logarithms and buffer solutions. Drechsler and Schmidt (2005) found the same problem.
Should perform exercises on the calculation of pH and contrast them experimentally.
They must be able to prepare solutions of given concentrations, including the preparation of dilutions.
Students should be able to choose appropriate indicators for a particular neutralization reaction and demonstrate, for example, the various turning points of the indicators.
They must differentiate between an acid–base reaction and a neutralization reaction, by doing experimental activities.
The use of new technologies for searching, capturing, recording and reporting data is overemphasized, for example, during experimental activities.
Attitudes
Teachers do not promote reasoning and analysis of concepts, instead they favor rote learning, repetition and replacing values in formulas, as in the case of formula pH = −log[H+].
High school teachers of chemistry attach little importance to the details associated with the study of the strength of acids and bases, even sometimes this topic is not addressed in the classroom.
Many teachers did not attach great importance to the review of the topic, do not explain clearly the differences between acids and bases of different strength, or how they interact with each other.
The students favor rote learning of the values of the pH scale, without going into the mathematical expression of pH and its qualitative explanation. Thus, they are interested only in the results of applying the formula without thinking about its implications.
Concluding remarks pointing to the future
The main contribution of this study is the documentation of PCK, in particular of CoRe, of ten Mexican chemistry teachers with experience in teaching the acid–base chemistry, at the high school level; a difficult subject for students due to its high level of abstraction, that has not received the attention it deserves in the literature (De Jong et al., 2002; Drechsler and van Driel, 2008). With the analysis of the consensual answers of Mexican teachers, in the frame of a convenient concept–skill–attitude scheme, a canonical PCK has been constructed within generalities on acids and bases, pH and strength concepts. The objective is to use this canonical PCK for training new high school teachers and to develop some Teaching/Learning Sequences on the topic. We hope that the information provided by means of the CoRe of the ten Mexican teachers, under the scheme concepts–skills–attitudes, becomes an important contribution to the teaching of acid–base chemistry.Considering that “A CoRe provides a powerful resource to record the work of an outstanding teacher, useful to exemplify good practice” (Kind, 2009), because the CoRe provides a vision of how teachers approach the teaching of a certain topic to a specific group of students, it provides the reasons linking how, why and what of teaching that content; it is recommended to introduce CoRes as a way of describing current practice, and/or using completed CoRes as an exemplary material to promote reflective practical skills and offering a means of acknowledging changes in PCK through application of classroom experience (Hume and Berry, 2011, 2013; Bertram and Loughran, 2012; Williams, 2012; Williams et al., 2012).
The answer to our research question is “Yes, is it possible to document the knowledge and beliefs of pH and strength concepts shown by ten outstanding Mexican teachers in a canonical PCK”. The construction from it of teaching/learning sequences for training new teachers on this topic will be treated in another paper. As a closing remark we repeat two questions that Abell (2008, p. 1412) posed to PCK researchers as future challenges: The first one is: “What is the relation of PCK (in terms of quality and quantity) to teacher practice?” The corollary question of the same work is: “What is the relation of PCK to student learning?” The last question was also mentioned as a next-ten-years interest of PCK researchers in the “PCK Summit”. We conclude that the next step to be taken is to evidence that PCK influence students' outcomes. As, Kind (2009, p. 198) emphasizes, “There is strong evidence that PCK is a useful concept and tool for describing and contributing to our understanding of teachers' professional practices”. Now we may explore students' outcome as a main topic and its relation with PCK of individual teachers: how do peculiarities of a given teacher's pedagogy impact students?
Also in a recent work, Bertram and Loughran (2012) pointed out that PCK has been attractive to researchers' construct but “remained closeted in the world of academia” (p. 1027). Now they have shown that the two Loughran et al. (2004) instruments, CoRe, and PaP-eRs, are a meaningful methodology for teachers to examine their PCK progress:
“… gave [teachers] a stronger feel for their own professional development … and [enabled them] to explore in more detail the underpinnings of their teaching” (p. 1030).
So a foreseeable conclusion is that PCK portrayal must be used in the near future to evidence the kind of student outcomes when a given teacher (with specific CoRe and PaP-eRs) takes action in the classroom, and as a methodology for assessing and scaffolding the progress of PCK of teachers in training and in service (Hume and Berry, 2011).
Appendix 1
Appendix 2
Acknowledgements
We thank the English revision by Dr Ricardo R. Boullosa, and Dr N. C. Bruce, colleagues in the UNAM.References
- Abell S. K., (2007), Research on Science Teaching Knowledge, in Abell S. K. and Lederman N. G. (ed.), Handbook of Research on Science Education, Mahwah, NJ, USA: Erlbaum, pp. 1105–1149.
- Abell S. K., (2008), PCK twenty years later: does pedagogical content knowledge remain a useful idea? Int. J. Sci. Educ., 30, 1405–1416.
- Alvarado C., (2012), Secuencias de enseñanza-aprendizaje sobre acidez y basicidad, a partir del Conocimiento Didáctico del Contenido de profesores de Bachillerato con experiencia docente. [Teaching/Learning Sequences on acid-base chemistry from Pedagogical Content Knowledge of High School Teachers with Teaching Experience], Doctoral dissertation, Universidad de Extremadura, Spain.
- Alvarado C., Garritz A., Guerra-Santos G. V., Sosa A. M. and de Teresa C., (2011), Enseñanza y aprendizaje de ácidos y bases en contexto: acidificación de los océanos [Teaching and Learning of Acids and Bases in Context: Ocean Acidification], EducacióQuímica (EduQ), 3(9), 4–12.
- Berry A., Friedrichsen P. and Loughran J. (ed.), (2015), Re-examining Pedagogical Content Knowledge in Science Education, London: Routledge Press.
- Bertram A. and Loughran J., (2012), Science Teachers' Views on CoRes and PaP-eRs as a Framework for Articulating and Developing Pedagogical Content Knowledge, Res. Sci. Educ., 42(6), 1027–1047.
- Brophy J., (2001), Introduction. Generic Guidelines for Good Teaching, in Brophy J. (ed.), Subject-specific instructional methods and activities, Advances in Research on Teaching, Amsterdam: JAI, vol. 8, pp. 1–23.
- Carlson J. and Gess-Newsome J., (2013), The PCK Summit Consensus Model and Definition of Pedagogical Content Knowledge, The Symposium “Reports from the Pedagogical Content Knowledge (PCK) Summit, ESERA Conference 2013”, Nicosia, Cyprus, September.
- Carlson J., Gess-Newsome J., Gardner A. and Taylor J. A., (2013), A Framework for Developing Pedagogical Content Knowledge: The Role of Transformative Professional Development and Educative Curriculum Materials, ESERA Conference 2013, Nicosia, Cyprus, September.
- Coll C., Pozo J. I., Sarabia B. and Valls E., (1995), Los contenidos en la Reforma. Enseñanza y aprendizaje de conceptos, procedimientos y actitudes. Tercera reimpresión, Madrid: Aula XXI, Santillana.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An Investigation of College Chemistry Students' Understanding of Structure–Property Relationships, J. Res. Sci. Teach., 50(6), 699–721.
- De Jong O., (2002), Developing Teachers and Chemical Education, in Gilbert J., De Jong O., Justi R. Treagust D. and Van Driel J. (ed.), Chemical Education: Towards Research-based Practice. (Preface to part E), Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 365–367.
- De Jong O., Veal W. R. and van Driel J. H., (2002), Exploring Chemistry Teachers' Knowledge Base, in Gilbert J. K. et al. (ed.), Chemical Education: Towards Research-based Practice, Dordrecht, The Netherlands: Kluwer Academic Publishers, ch. 16, pp. 369–390.
- De Vos W. and Pilot A., (2001), Acids and Bases in Layers: The Stratal Structure of an Ancient Topic, J. Chem. Educ., 87(1), 102–106.
- Drechsler M. and Schmidt H. J., (2005), Textbooks' and teachers' understanding of acid-base models used in chemistry teaching, Chem. Educ. Res. Prac., 6(1), 19–35.
- Drechsler M. and van Driel J. H., (2008), Experienced Teachers' Pedagogical Content Knowledge of Teaching Acid–base Chemistry, Res. Sci. Educ., 38(5), 611–631.
- Espinosa J. S., Labastida D. V., Padilla K. and Garritz A., (2011), Pedagogical Content Knowledge of Inquiry: An Instrument to Assess It and Its Application to High School In-Service Science Teachers, US-China Education Review, 8(5), 599–614.
- Fernández L., Gil D., Carrascosa J., Cachapuz A. and Praia J., (2002), Visiones deformadas de la ciencia transmitidas por la enseñanza: Una revisión bibliográfica [Distorted visions of science transmitted by teaching: a bibliographic review], Enseñanza de las Ciencias, 20(3), 477–488.
- Friedrichsen P., van Driel J. H. and Abell S. K., (2011), Taking a Closer Look at Science Teaching Orientations, Sci. Educ., 95(2), 358–376.
- Furió-Más C., Calatayud M. L., Guisasola J. and Furió-Gómez C., (2005), How are the Concepts and Theories of Acid–Base Reactions Presented? Chemistry in Textbooks and as Presented by Teachers, Int. J. Sci. Educ., 27(11), 1337–1358.
- Garritz A. and Trinidad R., (2006), El conocimiento pedagógico de la estructura corpuscular de la materia [Pedagogical Knowledge of the Particulate Structure of Matter], Educación Química, 17, 236–263.
- Garritz A., Porro S., Rembado F. M. and Trinidad R., (2007), Latin-American Teachers' Pedagogical Content Knowledge of the Particulate Nature of Matter. J. Sci. Educ., 8(2), 79–84.
- Garritz A., Nieto E., Padilla K., Reyes F. and Trinidad R., (2008), Conocimiento didáctico del contenido en química. Lo que todo profesor debería poseer [Chemistry Pedagogical Knowledge. What Every Teacher Should Possess], Campo Abierto, 27(1), 153–177, Publication of School of Education of the University of Extremadura, Spain.
- Garritz A., Alvarado C., Cañada F. and Mellado V., (2013), PCK by CoRes and PaP-eRs for Teaching Acids and Bases at High School, presented at the NARST-2013 Conference, at Río Grande, Puerto Rico, April, 49 pages.
- Geddis A. N., Onslow B., Beynon C. and Oesch J., (1993), Transforming Content Knowledge: Learning to Teach about Isotopes, Sci. Educ., 77(6), 575–591.
- Gess-Newsome J. and Lederman N. G. (ed.) (1999), Examining Pedagogical Content Knowledge. The Construct and its Implications for Science Education, Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Gilbert J. K. and Treagust D. (ed.), (2009), Multiple representations in chemical education, The Netherlands: Springer.
- Hume A. and Berry A., (2011), Constructing CoRes—a Strategy for Building PCK in Pre-service Science Teacher Education, Res. Sci. Educ., 41(3), 341–355.
- Hume A. and Berry A., (2013), Enhancing the Practicum Experience for Pre-service Chemistry Teachers Through Collaborative CoRe Design with Mentor Teachers, Res. Sci. Educ., 43(5), 2107–2136.
- Jensen W. B., (1980), The Lewis Acid-Base Concepts: An overview, Acid-base concepts: seventeenth to nineteenth centuries' and 2 ‘Modern acid-base definitions, New York: Wiley, ch. 1.
- Jensen W. B., (1998), Logic, History, and the Chemistry Textbook. I. Does Chemistry Have a Logical Structure? II. Can We Unmuddle the Chemistry Textbook? J. Chem. Educ., 75(7), 817–828.
- Johnstone A. H., (1982), Macro- and microchemistry. [Notes and correspondence], Sch. Sci. Rev., 64(227), 377–379.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Kerr R., (2010), Ocean acidification unprecedented, Unsetling Science, 328, 1500–1501.
- Kind V., (2009), Pedagogical content knowledge in science education: perspectives and potential for progress, Stud. Sci. Educ., 45(2), 169–204.
- Kind V., (2012), Extended Paper for the PCK Summit. It can be downloaded from http://pcksummit.bscs.org/sites/default/files/Kind_EP1.pdf, last retrieval on May 17th, 2014.
- Kolb D., (1978), Acids and bases, J. Chem. Educ., 55(7), 459–464.
- Loughran J., Mulhall P. and Berry A., (2004), In Search of Pedagogical Content Knowledge in Science: Developing Ways of Articulating and Documenting Professional Practice, J. Res. Sci. Teach., 41(4), 370–391.
- Loughran J., Berry A. and Mulhall P., (2012), Understanding and developing science teachers' pedagogical content knowledge, Rotterdam, The Netherlands: Sense Publishers, 2nd edn, p. 232.
- Maeyer J. and Talanquer V., (2013), Making Predictions About Chemical Reactivity: Assumptions and Heuristics, J. Res. Sci. Teach., 50(6), 748–767.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources, and development of the PCK for science teaching, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge, Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 95–132.
- Mellado V., (1998), Preservice teacher' classroom practice and their conceptions of the nature of science, in Fraser B. J. and Tobin K. (ed.), International Handbook of Science Education, Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 1093–1110.
- Mellado V., Ruiz C., Bermejo M. and Jiménez R., (2006), Contributions from the philosophy of science to education of science teachers, Sci. Educ., 15(5), 419–445.
- Morán-Oviedo P., (2010), Aproximaciones teórico-metodológicas en torno a uso del portafolio como estrategia de evaluación del alumno en la práctica docente. Experiencias en un curso de Laboratorio de Didáctica en la docencia universitaria [Theoretical-Methodological approaches about the use of portfolio as teaching practice for the student assessment. Experiences in a Didactic Laboratory course in university teaching], Perfiles Educativos, XXXII(129), 102–128.
- Padilla K. and Garritz A., (2014), Stoichiometry's PCK of University Chemistry Professors, in Sunal D. W., Sunal C. S., Wright E. L., Mason C. L., Zollman D. A. (ed.), Research Based Undergraduate Science Teaching, Research in Science Education, Charlotte, NC, USA: Information Age Publishers, ch. 16, part IV, vol. 6, pp. 499–523.
- Padilla K., Ponce-de-León A. M., Rembado F. M. and Garritz A., (2008), Undergraduate Professors' Pedagogical Content Knowledge: the case of ‘amount of substance”, Int. J. Sci. Educ., 30(10), 1389–1404.
- Park S. and Oliver J. S., (2008), Revisiting the Conceptualization of Pedagogical Content Knowledge(PCK): PCK as Conceptual Tool to Understand Teachers as Professionals, Res. Sci. Educ., 38(3), 261–284.
- Reyes C. F. and Garritz A., (2006), Conocimiento pedagógico del concepto de ‘reacción química' en profesores universitarios mexicanos [Pedagogical Knowledge of Mexican Professors on the ‘chemical reaction' concept], Rev. Mex. Ing. Educ., 11(3), 1175–1205.
- Rollnick M., Bennett J., Rhemtula M., Dharsey N. and Ndlovu T., (2008), The Place of Subject Matter Knowledge in Pedagogical Content Knowledge: A case study of South African teachers teaching the amount of substance and chemical equilibrium, Int. J. Sci. Educ., 30(10), 1365–1387.
- Shulman L., (1986), Those who understand. Knowledge growth in teaching, Educ. Res., 15(2), 4–14.
- Shulman L., (1987), Knowledge and teaching: Foundations of the New Reform, Harvard Educ. Rev., 57(1), 1–22.
- Smith S. and Banilower E., (2012), Extended Paper for PCK Summit, can be downloaded from http://pcksummit.bscs.org/sites/default/files/Smith Banilower EP.pdf.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195.
- Van Driel J., De Jong O. and Verloop N., (2002), The development of preservice chemistry teachers' pedagogical content knowledge, Sci. Educ., 86, 572–590.
- Williams J., (2012), Using CoRes to Develop the Pedagogical Content Knowledge (PCK) of Early Career Science and Technology Teachers, J. Technol. Educ., 24(1), 34–53.
- Williams J., Eames C., Hume A. and Lockley J., (2012), Promoting pedagogical content knowledge development for early career secondary teachers in science and technology using content representations, Res. Sci. Technol. Educ., 30(3), 327–343.
Footnote |
| † The PCK Summit was held from 20 to 25 October 2012 at the “Cheyenne Mountain Resort“ in Colorado Springs, Colorado State, United States. The National Science Foundation provided funding to bring together experts on PCK from eight countries (Germany, Australia, Korea, United States, Great Britain, Holland, Mexico and South Africa). The page of the summit of Pedagogical Content Knowledge can be visited in the URL http://pcksummit.bscs.org/ URL where the initial Keynote lecture by Lee S. Shulman can be seen and the documents made available and discussion presentations on the six sub-themes developed: 1: Content Knowledge and PCK; 2: Beliefs, Teaching Orientation, and PCK; 3: Nature of PCK; 4: PCK Models and Assessment Implications; 5: Assessment of PCK; 6: Research Findings on PCK. |
| This journal is © The Royal Society of Chemistry 2015 |
