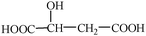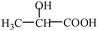Probing high school students' cognitive structures and key areas of learning difficulties on ethanoic acid using the flow map method
Qing
Zhou
*,
Tingting
Wang
and
Qi
Zheng
School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi’an, China. E-mail: zhouq@snnu.edu.cn; Fax: +86 29 8153 0829; Tel: +86 29 8153 0829
First published on 1st May 2015
Abstract
The purpose of this study was primarily to explore high school students' cognitive structures and to identify their learning difficulties on ethanoic acid through the flow map method. The subjects of this study were 30 grade 1 students from Dong Yuan Road Senior High School in Xi’an, China. The interviews were conducted a week after the students had received instruction on ethanoic acid. The interview narratives were then transcribed into flow maps. Analysis of the flow maps showed that there existed a difference between the individual students’ cognitive structure on ethanoic acid, higher academic achievers constructed more enriched cognitive structures and had higher scores for cognitive structural variables than lower academic achievers. Furthermore, the results of the correlation analysis of the students' cognitive structure outcomes, academic achievement and the information processing modes revealed that there was a positive correlation between two of them to some extent; in general, the higher academic achievers tended to have higher cognitive structure outcomes and employed high-order information processing modes. Analysis of the content of the flow maps of all the participants indicated that most of the students knew the main facts about ethanoic acid, but the details were not complete, and the misconceptions were mainly related to the molecular constitution and structure of ethanoic acid as well as to the esterification reaction, while the knowledge of the physical properties and the acidity of ethanoic acid seemed to have been mastered relatively well, as for the applications of ethanoic acid, students only mentioned that it can be used as condiment.
Introduction
Many research studies have clearly demonstrated the importance of cognitive structures in subsequent learning as the building blocks of meaningful learning and retention of instructional materials (Snow and Lohman, 1990). Cognitive structure is a hypothetical construct indicating the organization of concepts in learners' long-term memories and the relationships between them (Shavelson, 1974). Some researchers also use other terms, such as structural knowledge (Jonassen et al., 1993) and knowledge structure (Champagne et al., 1981; Nakiboglu, 2008) to describe cognitive structure. Ausubel (1963, p. 217) highlighted the significance of this hypothetical construct as the principal factor in the accumulation of knowledge: “If existing cognitive structure is clear, stable, and suitably organized, it facilitated the learning and retention of new subject matter. If it is unstable, ambiguous, disorganized, or chaotically organized; it inhibits learning and retention.”In the paradigm of constructivism, knowledge cannot be directly transmitted but must be actively constructed by an individual leaner (Bonder, 1986; Fosnot, 1996). While, the pre-knowledge of a specific domain in each learner's memory is always different, and is the dominant determination for reconstruction and information processing of the incoming stimuli (Taber, 2009; Nakiboglu, 2008). So, knowing what a learner knows is important for science instruction. Traditional paper-and-pencil tests are widely used to assess students' existing knowledge in chemical education, but instructors cannot get more information about the relationships between the concepts in their memory, as well as how they construct and organize the knowledge through them, so the exploration of learners' cognitive structures can be another important indicator in assessing what learners know (Tsai, 2001). Exploring the learners' cognitive structures can not only help educators to know the knowledge structures, pre-knowledge and misconceptions in students' minds, but also helps teachers to gain knowledge of the students' mental representations and the information processing modes the students employ to acquire and organize knowledge. As a result of this, instructors can identify knowledge gaps, relate new materials to existing slots or anchors within the learners' cognitive structures and arrange teaching strategies in an appropriate way (Jonassen, 1987; Ifenthaler et al., 2009). The diagnosis of cognitive structures can act as a “topographic map” to identify key areas of learning difficulties and facilitate instructional interventions (Snow, 1989).
In recent decades, many researchers have tried to use various methodological techniques and analytical frameworks to conduct research on exploring students' thinking modes and cognitive structure (Taber, 2009), and the major issues in representing people's cognitive structures have focused on how to represent the cognitive structure quantitatively and through visual formats. For this purpose, numerous researchers have explored several methods for identifying people's cognitive structures, such as word association (Shavelson, 1972; Gunstone, 1980), concept maps (Novak, 1990; Novak and Gowin, 1984) and flow maps (Anderson and Demetrius, 1993). Some researchers have proposed that there are three major aspects in describing cognitive structures: the concepts or ideas acquired, the connections between the concepts and the information processing strategies (Tsai and Huang, 2002). In order to indicate these three aspects of cognitive structures, five different variables of cognitive structure (Extent, Correctness, Integration, Availability and Analysis of information processing strategies) are included. Tsai and Huang (2002) introduced and compared free word association, controlled word association, tree construction, concept maps and flow maps for data collection, data analysis and assessment of the validity of the methods, the research results indicated that the flow map offers relatively more information for the analysis of the variables about cognitive structures than the other methods and among all of these methods it is the only method that can describe all the variables of a cognitive structure. On the other hand, a flow map is created from the free expressions of the learners in a non-directive way, and it is a useful way to capture both the sequential and interrelated relationships of people's thoughts by probing learners' cognitive structures (Anderson and Demetrius, 1993). The use of the flow map method is also in accordance with current neuroscience models about human cognition and information processing (Anderson, 1997).
Since it was first introduced by Anderson and Demetrius (1993), the flow map has been used in numerous studies (Bischoff, 1999, 2002; Tsai, 2000, 2001; Tsai and Huang, 2001; Dhindsa and Anderson, 2004; Bischoff et al., 2010; Oskay and Dinçol, 2011; Oskay et al., 2012) as a tool to access and study the knowledge structures of science learners, and many researchers have asserted that the flow map is a particularly effective analytical tool for gaining insight into an individual's knowledge structures because it enables researchers to identify an individual's ideas or concepts, the connections between the concepts and the information processing modes used in organizing the concepts. Therefore, in this study flow maps were used to determine the students' cognitive structures.
In general, chemistry knowledge is abstract, strongly theoretical and logically structured. When faced with a lot of chemical symbols, complicated chemical reactions and theoretical chemical principles, students always have difficulties in learning chemistry (Yujuan Li, 2006; Ling Zhu and Houxiong Wang, 2011). In order to gain a better understanding of students' chemical learning difficulties, lots of educators have devoted their time to identifying the difficulties in chemistry learning (Shanshan and Hualin, 2013; Leilei, 2014). The academic achievement scores provided by traditional paper-and-pencil tests, enable teachers to identify the students who have understood the information well and those who have not, but these tests cannot detect the specific difficulties that students have had in acquiring this knowledge. Whereas, by probing students' cognitive structures about a specific subject, teachers gain information on the acquired knowledge and misconceptions in students' minds, and also gain information on the methods students have employed to construct and organize the concepts, as a result, teachers can arrange their teaching strategies in a more appropriate way to promote students' learning outcomes (Jonassen, 1987; Tsai, 2001; Ifenthaler et al., 2009). Organic chemistry is an important content in the high school chemistry curriculum; it has a highly integrated knowledge structure and a closely logical content. However, some chemistry teachers have found that some problems arise when students are first taught organic chemistry, and studies (Yuqun, 2000; Ling and Houxiong, 2011; Xialin, 2014) about students' difficulties in learning organic chemistry have shown that these difficulties arise mainly because of the following three aspects: multitudinous organic substances are difficult to remember, the molecular structure of organic substances are too complicated and organic reactions are difficult to understand. In addition, students find it difficult to apply suitable learning methods, which are completely different from the ones required for the study of inorganic chemistry, for the study of organic chemistry which requires the understanding of intricate chemical symbols, the mastering of multitudinous isomers and judging the unpredictable chemical products of organic reactions (Grove and Bretz, 2010; Yuqun, 2000). Therefore, it is necessary and significant to explore students' learning characteristics and their mode of thinking about organic chemistry to help instructors to understand the development of students' minds and to arrange instruction strategies in an appropriate way (Xiaojie, 2011; Xialin, 2014). In this study, we chose ethanoic acid as a focal concept to research students' cognitive structure mainly for the following reasons. Firstly, the study of ethanoic acid is a key content of the compulsory high school chemistry course in China, it is a typical organic compound that students studying organic chemistry are taught about, a typical representative of oxygenated derivatives of hydrocarbon and the main ingredient of vinegar, which students are very familiar with in daily life. In addition, the functional group of ethanoic acid is carboxyl, which is a very important functional group that is involved in abundant organic reactions, and the reaction of carboxyl is one of the core reactions in organic chemistry (Jiane, 2009; Jianjun et al., 2012). Secondly, understanding students' thinking modes and learning difficulties when they are first taught organic chemistry is very important for teachers to enable them to arrange subsequent teaching strategies (Yuqun, 2000). Chemistry teachers have found that students tend to have some misconceptions whilst learning about ethanoic acid, such as the nature of the esterification reaction and the experimental operations of the reaction between ethanoic acid and ethanol (Jiane, 2009). Therefore, exploring students' cognitive structure characteristics and learning difficulties about ethanoic acid can provide educators with information about the typical cognitive characteristics and information processing modes of organic chemistry, according to which teachers can design more appropriate teaching strategies for organic chemistry to optimize students' cognitive structures and to enhance their meaningful learning.
Many researchers have ascertained the importance of exploring learners' cognitive structures in science education, and have tried to use several ways to represent learners' cognitive structures (Anderson and Demetrius, 1993; Tsai, 2001; Tsai and Huang, 2002; Taber, 2009; Nakiboglu, 2008). The flow map method is an effective way of representing learners' cognitive structures both in quantitative terms and through visual formats (Anderson and Demetrius, 1993; Bischoff, 1999, 2002; Tsai, 2001; Tsai and Huang, 2002; Bischoff et al., 2010). Although, we found that the flow map method has been used in numerous studies to explore students' or teachers' cognitive structures within a specific knowledge domain, such as the atomic model (Tsai, 2001), biological reproduction (Tsai and Huang, 2001), oxidation and reduction reaction (Bischoff et al., 2010), hybridization and bonds (Oskay and Dinçol, 2011) and so on, and researchers could get more information about the subjects' cognitive structures through this method, which helped researchers understand the individual characteristics of the subjects' cognitive structures very well. None of them studied the characteristics of subjects' cognitive structure about organic chemistry, which is very important for chemistry education. On the other hand, some studies have been carried out on the teaching and learning of organic chemistry in China, and these studies have focussed mainly on learning difficulties and the corresponding teaching strategies (Yuqun, 2000; Xialin, 2014), teaching design (Jianjun et al., 2012), students' cognitive structure within organic chemistry learning (Xiaojie, 2011) and so on. But the method used to explore students' cognitive structures and learning difficulties in these studies was mainly the traditional paper-and-pencil test, and methods such as, word association and flow map, were rarely used because the study of the cognitive structure is relatively recent. Therefore, in this study we wanted to employ the flow map method to probe students' cognitive structures about ethanoic acid, in order to study the characteristics of Chinese students' cognitive structures about organic chemistry. Our research questions mainly contained the following three aspects: first, what was the pattern of the students' cognitive structures after instruction and were there any differences between the students' individual cognitive structures about ethanoic acid? Second, what information processing modes did the students tend to use whilst learning about ethanoic acid? Third, what difficulties did the students' experience when learning about ethanoic acid?
Methodology
This study was carried out in Dong Yuan Road Senior High School in Xi'an. Before the interviews were conducted, the researchers informed the students that the aim of this study was to determine what they had learned about ethanoic acid and to try to find if there were any ambiguous concepts in learning about ethanoic acid. We told the students that we would ask them three questions and that they should just answer as much as they liked. And we also told them that the information we received relating to their knowledge structures about ethanoic acid could help teachers to design appropriate teaching strategies to promote effective teaching and meaningful learning, and the data offered by them may be published in the future but would be anonymous. And we promised that the outcomes of their interviews and the scores obtained from the paper-and-pencil test would not be discussed with anybody other than themselves. All the participants of our study volunteered and wanted to talk to the postgraduates who conducted the interviews. In addition, the flow map method requires that the participants are not disturbed by the researchers during the interviews, so the interview questions were presented in a non-directive way. And the researchers told participants that they could tell the researchers what they knew about ethanoic acid and express their ideas freely, the answers to the questions were respected and would not be judged to be right or wrong. The interviews were carried out in a friendly atmosphere. The study was approved by both Dong Yuan Road Senior High School and Shaanxi Normal University, and was carried out with the informed consent of the participants.Subjects
The subjects of the study consisted of 30 senior grade 1 students (15 years old) from a class of Dong Yuan Road Senior High School in Xi'an, the capital city of Shaanxi province in China. After a week of instruction about ethanoic acid, 30 students (16 boys and 14 girls) were selected, taking into consideration their different academic achievement levels and their skill at expressing their ideas, for further interviews. Among them, 10 students had high academic achievements, 10 students had medium academic achievements and the other 10 had relatively low academic achievements.Data collection
First of all, it should be noted that both interviews and paper-and-pencil tests in this study were carried out in Chinese, which is the language of instruction used in the school. The interview questions and the test paper reported in this paper were translated into English by two researchers together, refined by a postgraduate whose major was English, and are included in the appendix. The original Chinese version of the test is available from the author.The interviews were conducted a week after the instruction on ethanoic acid, then the interview narratives were transcribed into flow maps according to the process for the creation of flow maps developed by Anderson and Demetrius (1993) for further analyses.
In addition, the students' test scores for a paper-and-pencil test about ethanoic acid were also collected for subsequent correlation analyses. The reliability of the test was analyzed using SPSS 21.0 (Statistical Product and Service Solutions); the value of Cronbach's Alpha was 0.89, which indicated that the internal consistency coefficient of the test was good. To validate the test, a university professor of chemistry education, two doctors of chemistry education and a high school chemistry teacher with more than 10 years of teaching experience analyzed the test in detail together, they agreed that the test covered the comprehensive knowledge of ethanoic acid, that the level of difficulty was moderate for students who had been taught about ethanoic acid for the first time, and that the test had a good content validity. Therefore, the scores derived from the paper-and-pencil test were reliable in the study.
Flow map method
In the study, flow maps were used to determine the individual cognitive structure of 30 students about the subject of ethanoic acid. In order to elicit the learners' cognitive structures about ethanoic acid, non-directive questions were asked by the researchers, as follows:1. Could you tell me the main facts about ethanoic acid?
2. Could you tell me more about the facts that you have mentioned?
3. Could you tell me the relationships between the facts that you have already told me?
In order to obtain students' descriptions about ethanoic acid which were as complete as possible, after a student's answers to these questions had been tape-recorded, each student was asked to listen to his/her ideas immediately, so that he/she could recall additional information that had not been mentioned previously. This is called a “meta-listening” technique (Tsai, 1998, 2000, 2001). After all the interviews had been tape-recorded, each student's interview narrative was transformed into the format of a “flow map” according to the process for the creation of flow maps developed by Anderson and Demetrius (1993). When drawing the flow maps, the following rules were used. First, each completed thought uttered by the respondent was entered as a statement in the flow map and linked by an arrow to the next uttered statement; second, compound sentences were separated into component clauses, and each clause entered in sequence as a separate statement; third, after all the statements had been entered in sequence, statements containing recurrent thoughts or cross-references were identified and recurrent arrows were drawn linking the related statements, when recurrent thoughts occurred, the arrows were linked to the earliest statement where the thought had been expressed in the sequence (Anderson, 1991; Anderson and Demetrius, 1993).
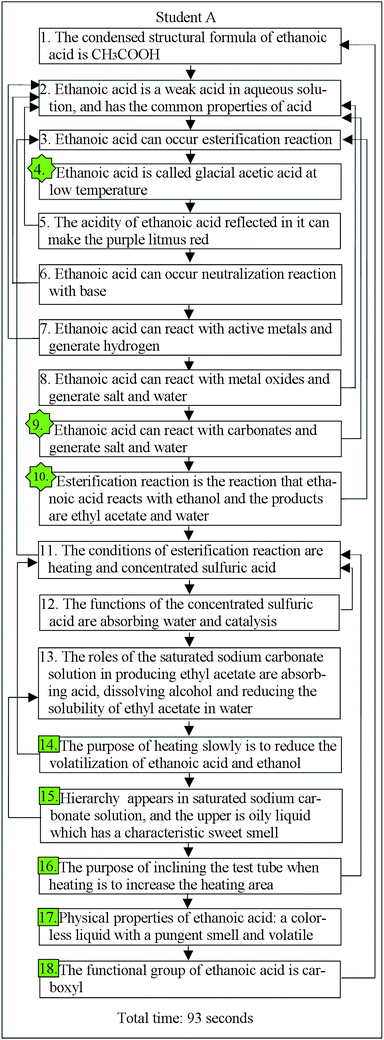
There are two types of arrows in the flow map. The liner or serial arrows show the sequential flow of the students' ideas, and the recurrent arrows indicate the relationships between the statements displayed in the flow map. Taking student A's flow map as an example, as shown in Fig. 1, the recall record of student A begins with the condensed structural formula of ethanoic acid, then the acidity of ethanoic acid, the esterification reaction, the common name of ethanoic acid and so on. For recurrent arrows, the statement 2, “Ethanoic acid is a weak acid in aqueous solution, and has the common properties of acid” states that ethanoic acid has the common properties of an acid, while statements 4, 5, 6, 7, 8 describe the specific characteristics of acidity, so there was a recurrent arrow drawn back to statement 2 from statements 4, 5, r, 7, 8 respectively. The numbers of linear and recurrent connections in the flow maps were calculated for the students' flow map scores. These scores were regarded as the indicators of conceptual achievement.
Fig. 1 and 2 are examples of the students' flow maps derived from the interview narratives of student A, student B and student C. Among them, student A was the one who had a high academic achievement, while student B and student C had medium and low academic achievement,respectively.
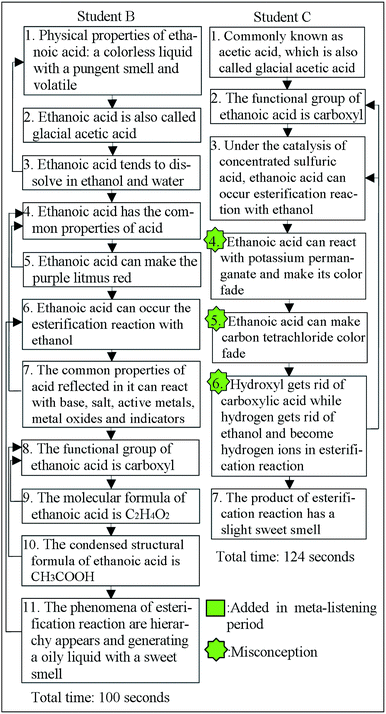 | ||
| Fig. 2 Two examples of students' flow maps based on students' (student B and student C) narratives on ethanoic acid. | ||
As a result, the following six different variables could be obtained from the flow maps to represent the students' cognitive structure outcomes in quantitative terms:
1. Extent: the total number of ideas shown in the flow map.
2. Richness: the total number of recurrent linkages which represent the connection between the two ideas.
3. Flexibility: flexibility indicates students' idea change as a result of the meta-listening period, which is equal to the total number of ideas minus the number of ideas elicited before the meta-listening period.
4. Integration: the connection of cognitive structures. This is equal to the number of recurrent linkages/(number of ideas + number of recurrent linkages).
5. Misconceptions: number of misconceptions shown in the flow map; a smaller number of misconceptions indicating greater correctness.
6. Information retrieval rate: information retrieval rate is represented by the statement per second. As described previously, the time measured was only applied to the first part of the interview; this variable is defined as the number of ideas elicited/total time used in that interview period.
Through a series of content analyses of the students' flow maps, the students' information processing modes could also be captured. In order to gain a deep understanding of the students' usage of these different modes of processing knowledge about ethanoic acid, the way each of the students' statements was presented in the flow maps was categorized into one of the following five levels (from low to high): defining, describing, comparing and contrasting, conditional inferring, and explaining, according to a system developed by Smith and Meux (1970). Taking ethanoic acid as the example, these categories were defined as follows (Tsai, 1999):
1. Defining: providing a definition of a concept or a scientific term, and the basic properties of a substance. E.g., an esterification reaction involves the reaction of a carboxylic acid with an alcohol to generate an ester and water.
2. Describing: depicting a phenomenon, a process or a fact. In this study, the descriptions relate to experimental phenomena, material properties and scientific terms. E.g., the chemical formula of ethanoic acid is C2H4O2, ethanoic acid can turn purple litmus (acid–base indicator) red.
3. Comparing and contrasting: describing the relationships between (or among) subjects, things, or methods. E.g., the acidity of ethanoic acid is weaker than that of sulfuric acid, hydrochloric acid and nitric acid, but is stronger than that of carbonic acid.
4. Conditional inferring: a description of what will happen under certain conditions. E.g., ethanoic acid has the common properties of an acid, so it can undergo a neutralization reaction with a base.
5. Explaining: presenting an account to justify facts or events. E.g., ethanoic acid can be used to produce carbonic acid because its acidity is stronger than that of carbonic acid.
The reliability of the flow map method was determined by a second independent researcher (an expert in chemistry education) who was asked to draw diagrams of the students' interview narratives according to Anderson and Demetrius' procedure (Anderson and Demetrius, 1993). In this study, the inter-coder agreement for linear linkages was 0.90, and for recurrent linkages was 0.86. In general, it is considered sufficient for narrative analysis if the reliability is greater than 0.80 (Tsai and Huang, 2001; Wu and Tsai, 2005a, 2005b). Therefore, this method was deemed to be sufficiently reliable for the purposes of this study. Similarly, the inter-coder agreement for content analysis of information processing modes was 0.89, which indicated that the two researchers had the same way of categorization of 89% of the students' ideas of information processing modes. Therefore, the content analysis of the information processing modes in this study was viewed as being sufficiently reliable.
Results
Student's cognitive structure outcomes for ethanoic acid
Table 1 shows the quantitative outcomes of the three flow maps presented in Fig. 1 and 2. Clearly, among them student A seemed to construct the best cognitive structure of ethanoic acid and had the best cognitive structure outcomes in light of these cognitive structural variables. Although student B is not as good as student A in terms of extent, richness and flexibility and integration, there did not appear misconceptions in his narrative. Relatively, student C has the lowest information retrieval rate, he only recalled 7 ideas during 124 seconds, and 3 of them were misconceptions. We found that student C could not describe his ideas continuously and he needed a long time to recall each idea during the interview. The analysis indicates that higher academic achievers tend to construct a more enriched cognitive structure and have higher scores for these variables.| Variables | Student A | Student B | Student C |
|---|---|---|---|
| Extent | 18 | 11 | 7 |
| Richness | 12 | 6 | 2 |
| Integration | 12/(18 + 12) = 0.40 | 6/(11 + 6) = 0.35 | 2/(7 + 2) = 0.22 |
| Misconceptions | 3 | 0 | 3 |
| Flexibility | 5 | 0 | 0 |
| Information retrieval rate | 18/93 = 0.19 | 11/100 = 0.11 | 7/124 = 0.06 |
Table 2 Shows the correlation analyses of the students' academic achievement scores gathered from a paper-and-pencil test and the cognitive structure variables. The results indicate that the students' achievement score is closely related to the richness and flexibility (p < 0.01, p < 0.05). Higher achievers (according to the paper-and-pencil test) tend to have greater richness and stronger flexibility than lower achievers. The correlated matrix also suggests that the extent of students' cognitive structures is related to their richness and integration (p < 0.01, p < 0.05), and the richness of the cognitive structures is related to their integration and their flexibility (p < 0.01, p < 0.05). Even more, the misconception of students' cognitive structures is negative relative to their information retrieval rate, the number of misconceptions is greater, and the information retrieval rate tends to be lower.
| EXT | RICH | INTG | MISCON | FLEX | IRR | ACHV | |
|---|---|---|---|---|---|---|---|
| Key: EXT: extent; R1CH: richness; INTG: integration; MISCON: misconceptions; IRR: information retrieval rate, and FLEX: flexibility; ACHV: achievement score from paper-and-pencil test.a p < 0.01.b p < 0.05. | |||||||
| EXT | 796a | 0.462b | −0.074 | 0.106 | 0.189 | 0.359 | |
| RICH | 0.809a | −0.039 | 0.401b | 0.154 | 0.516a | ||
| INTE | −0.095 | 0.234 | 0.250 | 0.347 | |||
| MISCON | 0.22 | −0.409b | −0.091 | ||||
| FLEX | −0.098 | 0.381b | |||||
| IRR | 0.175 | ||||||
| ACHV | |||||||
Content analyses of students' information processing strategies
In this study, correlation analyses between the students' cognitive structure outcomes and information processing modes as well as correlation analyses between the students' academic achievement scores and information processing modes were also conducted. The results are shown in Table 3.| DFN | DSCB | CMPR | CNDTN | EXPLN | |
|---|---|---|---|---|---|
| Key: DFN: defining; DSCB: describing; CMPR: comparing; CNDTN: conditional inferring, and EXPLN: explaining; ACHV: achievement score from paper-and-pencil test.a p < 0.01.b p < 0.05. | |||||
| EXT | 0.173 | 0.663a | 0.283 | 0.470a | 0.739a |
| RICH | 0.219 | 0.579a | 0.235 | 0.557a | 0.749a |
| INTG | 0.106 | 0.276 | 0.208 | 0.361 | 0.567a |
| MISCON | 0.232 | 0.036 | −0.174 | −0.026 | 0.069 |
| IRR | −0.173 | 0.036 | 0.111 | −0.101 | 0.259 |
| FLEX | 0.093 | 0.284 | −0.12 | 0.183 | 0.277 |
| ACHV | −0.025 | 0.169 | 0.243 | 0.384b | 0.532a |
Table 3 shows the results of the content analyses of students' information processing strategies. The result of the correlation analysis of the students' cognitive structure outcomes and information processing strategies reveals that the cognitive structural variables “extent”, “richness” and “integration” are positively related to the highest information processing strategy, that is, “explaining” (p < 0.01), which is the one correlated with most of the cognitive structure variables. In addition, the “extent” and “richness” of the cognitive structure are also positively related to “describing” and “conditional inferring” information processing modes (p < 0.01).
The results of the correlation analysis between students' academic achievement scores and information processing modes indicates that students' academic achievement scores are positively related to the “conditional inferring” and “explaining” information processing modes (p < 0.05, p < 0.01). This result suggests that higher academic achievers tend to express their ideas in relatively higher-order information processing modes (e.g., conditional inferring and explaining), which concurs with Tsai's (2001) conclusions about the atomic model.
The misconceptions and learning difficulties about ethanoic acid
In this study, the concepts students recalled about ethanoic acid were also analyzed by examining all of the flow maps derived from the 30 participants' interview narratives. All of the ideas on ethanoic acid displayed in the flow maps derived from the students' interview narratives are summarized in Table 4. The primary knowledge on ethanoic acid comprises the molecular constitution and structure of ethanoic acid, the physical properties of ethanoic acid, the chemical properties of ethanoic acid and its applications, and the chemical properties of ethanoic acid can be divided into its acidity and the esterification reaction (Xinqi Song et al., 2011; The Ministry of Education of China, 2006). By analyzing the concepts recalled in the interviews, both the correct and incorrect concepts recalled, educators gained information about what the students had learned about ethanoic acid in terms of the five aspects mentioned above, and also identified the students' misunderstandings about ethanoic acid during the learning. As a result, the teachers could determine whether the students had acquired the major ideas of the instruction, and gained some insight into the students' learning difficulties within the subject, so that they could arrange the teaching strategies effectively to enhance their instruction and the students' learning outcomes.| Major concepts students recalled | % | ||
|---|---|---|---|
| a Key: %: the percentage of students recalled the concept in the flow-map interviews, the total number of students participated in the interviews is 30(N = 30); MCS: molecular constitution and structure of ethanoic acid; PHY: physical properties of ethanoic acid; ACDT: acidity of ethanoic acid; ESTR: esterification reaction; APP: applications; CORCON: correct conceptions; MISCON: misconceptions. | |||
| MCS | CORCON | The functional group of ethanoic acid is carboxyl | 76.67 |
| The condensed structural formula of ethanoic acid is CH3COOH | 63.33 | ||
| The molecular formula of ethanoic acid is C2H4O2 | 56.67 | ||
| The properties of ethanoic acid are determined by its functional group, namely carboxyl | 20.00 | ||
| Ethanoic acid is an important derivative of hydrocarbon | 3.33 | ||
| MISCON | The chemical formula or molecular formula is CH3COOH 16.67 | ||
| The functional group of ethanoic acid is –COOH, which contains hydroxyl and hydrogen ion | 16.67 | ||
| The structural formula of ethanoic acid is C2H4O2 | 3.33 | ||
| PHY | CORCON | Ethanoic acid is a colorless liquid with a pungent smell | 83.33 |
| Commonly known as acetic acid and glacial acetic acid | 53.33 | ||
| Ethanoic acid tends to dissolve in ethanol and water | 36.67 | ||
| Glacial ethanoic acid is a pure substance | 26.67 | ||
| MISCON | Ethanoic acid is called glacial acetic acid at low temperature | 33.33 | |
| ACDT | CORCON | Ethanoic acid can make purple litmus red | 70.00 |
| Ethanoic acid has the common properties of an acid | 50.00 | ||
| Ethanoic acid reacts with active metals to produce hydrogen | 50.00 | ||
| Ethanoic acid reacts with base (sodium hydroxide), this is called a neutralization reaction | 46.67 | ||
| Ethanoic acid reacts with metal oxides to generate a salt and water | 43.33 | ||
| Ethanoic acid reacts with carbonates to generate a salt and water | 43.33 | ||
| The acidity of ethanoic acid is weaker than sulfuric acid, hydrochloric acid and nitric acid, but is stronger than that of carbonic acid | 16.67 | ||
| The acidity of ethanoic acid is relatively strong and it can be used to produce carbonic acid | 6.67 | ||
| ESTR | CORCON | Ethanoic acid undergoes an esterification reaction with ethyl alcohol to generate ethyl acetate and water | 96.67 |
| The conditions of esterification reaction are heating and the presence of concentrated sulfuric acid as a catalyst | 43.33 | ||
| The roles of saturated sodium carbonate solution in producing ethyl acetate are absorbing acid, dissolving alcohol and reducing the solubility of ethyl acetate in water | 40.00 | ||
| There is a colorless oily liquid with a sweet smell above the saturated sodium carbonate solution | 40.00 | ||
| The nature of the esterification reaction is the hydroxyl group getting rid of carboxylic acid while the hydrogen gets rid of alcohol | 36.67 | ||
| The functions of concentrated sulfuric acid are absorbing water and catalysis | 30.00 | ||
| In order to prevent suck-back, the glass tube must not be inserted below the liquid level in the experiment | 20.00 | ||
| The esterification reaction is a substitution reaction as well as a reversible reaction | 20.00 | ||
| The purpose of heating slowly is to reduce the volatilization of ethanoic acid and ethanol | 16.67 | ||
| The purpose of inclining the test tube when heating is to increase the heating area | 16.67 | ||
| The order of adding experiment reagents is ethyl alcohol, concentrated sulfuric acid and ethanoic acid | 13.33 | ||
| MISCON | The esterification reaction involves the reaction of ethanoic acid with ethanol and the products are ethyl acetate and water | 13.33 | |
| The hydroxyl group gets rid of alcohol while the hydrogen gets rid of carboxylic acid in the esterification reaction | 6.67 | ||
| Concentrated sulfuric acid is used to collect ethyl acetate | 3.33 | ||
| Ethanoic acid can occur in the addition reaction | 3.33 | ||
| Ethanoic acid can make the color of potassium permanganate and carbon tetrachloride fade | 3.33 | ||
| APP | CORCON | Commonly known as ethanoic acid, which is the major ingredient of vinegar | 53.33 |
| Ethanoic acid can be used as a condiment | 6.67 | ||
Conclusions and implications
Science educators encourage the use of multiple ways of assessing learners' learning outcomes in order to gain a better understanding of students' concepts in science (Wu and Tsai, 2005a, 2005b). Probing students' cognitive structures using the flow map method, enables educators to gain insight into the students' natural stream of thoughts and the interconnection of concepts with minimal intervention. In addition, by analyzing students' flow maps, educators can also analyze students' cognitive structures in quantitative terms using the cognitive structure variables as described previously.The findings of analysis about students' cognitive structures of ethanoic acid show that the individuals constructed different cognitive structures even in the same learning environment. As a result, their scores of cognitive structural variables are different, higher academic achievers tended to have higher scores for the extent, richness, integration, information retrieval rate and flexibility as well as lower scores for misconceptions than lower academic achievers. In addition, the correlation analysis revealed that students' academic scores are positively related to richness and flexibility of cognitive structures of ethanoic acid, which is different from Tsai's (2001) conclusion for the atomic model for which the academic scores were positively related to extent, richness, information retrieval rate and integration. The difference may due to the different subjects have different learning models and different students may have different cognitive characteristics. Moreover, different cultural context and teachers' different instruction styles may also be factors that affect students' cognitive characteristics.
The results of the analysis about students' information processing modes in this study indicate that students who have cognitive structures which are larger, richer and with a more integrated texture tended to express their ideas in relatively high information processing modes (e.g., conditional inferring and explaining). At the same time, higher academic achievers also tended to employ conditional inferring and explaining to organize their knowledge. According to the results, we can infer that the use of high-level information processing modes (e.g., conditional inferring and explaining) requires well developed cognitive structures and high academic scores, which concurs with Tsai's (1999, 2001) conclusions. In addition, we propose that the richer connections between concepts and the use of high-order information processing strategies may mutually reinforce one another, so chemistry teachers are encouraged to use effective teaching strategies to help students employ higher-order information processing operations, the use of POE (Prediction–Observation–Explanation) instructional activities (White and Gunstone, 1992; Liew and Treagust, 1995; Palmer, 1995) and cooperative learning strategies (Soyibo and Evans, 2002; Marinopoulos and Stavridou, 2002) are advised.
In this study, students' major ideas and learning difficulties were identified by the analysis of the content of 30 students' flow maps. It seems that students' cognitive structures about ethanoic acid put more emphasis on the molecular constitution and structure of ethanoic acid, the acidity of ethanoic acid and the esterification reaction. The esterification reaction, in particular, is important as well as difficult when teaching about ethanoic acid (Jiane, 2009; Jianjun et al., 2012), teachers always emphasis it in the classroom, so ideas about the esterification reaction appeared in most of the students' flow maps. While the concepts about physical properties and applications of ethanoic acid received relatively less attention from these students.
Furthermore, the results also suggest that the difficulties experienced by students when they learned about ethanoic acid mainly concerned its molecular constitution and structure and the esterification reaction. As for the molecular constitution and structure of ethanoic acid, students' misconceptions were mainly reflected in two aspects. First, the students were confused about the concepts and meanings of chemical/molecular formula, condensed structural formula and structural formula; second, students did not understand the structural feature of carboxyl correctly. This is because there are more chemical symbols used to represent organic matters (Yuqun, 2000; Xialin, 2014), the molecular formula, condensed structural formula and structural formula, which is completely different from inorganic chemistry learning in which the molecular formula is the only chemical symbol used to represent matter. On the other hand, there are too many functional groups in organic chemistry, making students confused and generating misconceptions. While, the structural features of matter are the foundation of further learning, high school chemistry teachers should emphasize the characteristics of these chemical symbols to help students remember and distinguish them correctly (Yaowu Guan, 2005; Min Xu, 2012). In addition, teachers should also pay attention to concept teaching because there are so many concepts in the learning of organic chemistry that students have difficulties in remembering and understanding.
Moreover, students' learning difficulties regarding the esterification reaction were mainly reflected in the understanding of the concept and nature of the esterification reaction, which mainly resulted from the students' poor knowledge transfer ability and their poor understanding of the nature of organic reactions (Yuqun, 2000; Xiaojie, 2011). The esterification reaction is first taught in China when students are taught about ethanoic acid, and the reaction of ethanol and ethanoic acid is used as an example of this type of reaction. As a result, most students cannot transfer the knowledge effectively and tend to acquire the knowledge that the esterification reaction is only the reaction involving ethanol and ethanoic acid to generate ethyl acetate and water. In addition, most high school chemistry teachers tend to tell students the conclusion about the reaction directly, instead of teaching it from the perspective of functional group and chemical bond breaking, so most students just remember simply, but do not understand it. As we know, the nature of organic reactions is the reaction of functional groups; teachers are not encouraged to instruct using specific matters, but expand to functional groups (Qiang, 2009; Min Xu, 2012). So high school chemistry teachers are encouraged to pay more attention to the cultivation of students' transfer ability in the classroom and employ the reaction mechanism in the instruction, which will promote better understanding by the students and will enhance students' meaningful learning (Qiang, 2009).
In summary, the flow map method is a useful method that can be used to describe three major aspects of cognitive structures: the concepts or ideas acquired, the connections between concepts and the information processing strategies in quantitative and through visual formats (Tsai, 2001; Tsai and Huang, 2001, 2002; Dhindsa and Anderson, 2004; Bischoff et al., 2010). The analysis of students' flow maps can provide a rich variety of dimensions for teachers and professional evaluators to assess students' cognitive structures, and quantitative scores of cognitive structure variables (extent, richness, integration, misconceptions, information retrieval rate and flexibility) can be used as part of students assessment in addition to scores gathered from traditional paper-and-pencil tests. Otherwise, the information obtained through analyzing students' flow maps can also be used to identify students' cognitive development, misconceptions, the strengths and weakness of each student's knowledge within a specific scientific topic, which can not only help teachers to find the defects in their teaching, and to provide more information for teachers to optimize their teaching design, but can also help students engage in metacognitive learning and thus enhance students' learning outcomes. The findings derived from this study suggest that chemistry teachers could try to use the flow map method to probe students' cognitive structures and identify the learning difficulties of specific topics in addition to paper-and-pencil tests. In addition, in order to gain a better understanding of students' knowledge, researchers and teachers are encouraged to employ multiple methods to assess and evaluate students in science education together.
The limitations of the study and the further study
Although the flow map method is an effective method that can be used to obtain more and detailed information regarding the cognitive structure with minimum interference, there are some limitations in this study. Firstly, the students' response speed, how skilled the students were in expressing themselves and whether they were nervous in the interview, all influenced the outcomes of the students' cognitive structure.Secondly, in this study, we elicited the students' cognitive structures about ethanoic acid through the interviews. While, there is some implicit knowledge in students' memories, which is significant for learning and teaching but cannot be elicited directly (Taber, 2014). So the flow maps derived from students' narrative in the study may only be a part of students' cognitive structure but not the whole. On the other hand, the students' cognitive structures about the specific domain develop along with the learning, so the results of our study are only snapshots of students' cognitive structures (Taber, 2013). Therefore, as students' cognitive structures are so complex, multidimensional, as well as somewhat in flux (Mortimer, 1995; Petri and Niedderer, 1998; Taber, 2000, 2001, 2013, 2014), we cannot represent them with a simple fixed and monolithic underlying structure.
Thirdly, a lot of important knowledge about ethanoic acid cannot be described orally, such as its structural formula and the chemical equations of organic reactions, both of which were not mentioned in the narratives of the students. Therefore, a combination of verbal expression and writing should be used to provide more detailed information of students' cognitive structure, and to gain a more complete cognitive structure from the flow maps. This is the research direction our further studies will take.
In addition, the sample of this study was only 30 students' from the same class of a school, so the conclusions of this study may not be very representative.
However, this study was only a preliminary study. In this study, we explored 30 students' cognitive structures and identified the difficulties encountered when learning about ethanoic acid, so that we could determine the flaws in the instruction given and could provide some teaching strategies for instruction on ethanoic acid. From the results obtained in this study we conclude that most of the difficulties encountered when learning about ethanoic acid concerned the molecular constitution and structure of ethanoic acid and the esterification reaction. We intend to optimize the teaching design by emphasizing the conceptual change and reaction mechanism, and will then apply the optimized teaching design in teaching practice, and we will explore the students' cognitive structures using the flow map method to study the effects of the improved teaching strategies.
In addition, in our further study, we are planning to select more students from different classes and different schools, and choose more subjects, such as ethyl alcohol and acetaldehyde and so on to explore students' cognitive structures using the flow map method. So that we can understand the students' cognitive characteristics and learning model of organic chemistry better, as a result, teachers will be provided with effective teaching strategies for organic chemistry.
Appendix
1. Ethanoic acid is the major ingredient of vinegar, which of the following statements about acetic acid is correct (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. Ethanoic acid is a liquid with a pungent smell
b. There are four hydrogen atoms contained in the ethanoic, so ethanoic acid is not a monoacid
c. Ethanoic acid can undergo the esterification reaction at normal temperature
d. Ethanoic acid can't make purple litmus red because its acidity is relatively weak
2. Which of the following substances is carboxylic acid (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. CH3CH2OH b. CH3CHO
c. CH3COOH d. CH3COOCH2CH3
3. The esterification reaction is an important reaction type of organic reactions, which of the following statements about the esterification reaction is correct (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. Concentrated sulfuric acid is an essential reactant in the esterification reaction
b. The esterification reaction can be regarded as a substitution reaction
c. Ethanol is a necessary reactant in the esterification reaction
d. The reactants will completely convert into products in the esterification reaction
4. Vinegar is a common condiment, and it contains 3–5% ethanoic acid. Which of following statements about acetic acid is correct (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. Ethanoic acid tends to dissolve in ethanol and water
b. Vinegar can be used to dissolve the limescale in a kettle
c. Glacial acetic acid is the solid of acetic acid, and it is a mixture
d. Ethanoic acid is an ionic compound, because it will ionize and produce hydrogen ions when dissolved in water
5. Which of the following chemical symbols is correct (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. The structural formula of ethylene is CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2
b. The empirical formula of acetic acid is CH2O
c. The condensed structural formula of ethanol is C2H6O
d. The condensed structural formula of ethanoic acid is
HCOOCH3
6. Which of the following mixtures can be separated using a separating funnel (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. Ethanol and acetic acid
b. Ethanol and water
c. Acetic acid and water
d. Benzene and water
7. Which of the statements about the esterification reaction between acetic acid and ethanol is correct (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. The hydroxyls of acetic acid combine with the hydrogen atoms of ethanol to form water
b. The hydroxyls of acetic acid combine with the hydrogen atoms of the hydroxyls of ethanol to form water
c. The hydrogen atoms of the hydroxyls of acetic acid combine with the hydroxyls of ethanol to form water
d. The hydrogen atoms of acetic acid combine with the hydroxyls of ethanol to form water
8. Which of the following statements is correct (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. The reactions between acid and alcohol must be esterification reactions
b. In the esterification reaction, carboxylic acid removes the hydroxyl of carboxyl and alcohol removes the hydrogen atom of hydroxyl
c. Concentrated sulfuric acid is only playing a catalytic role in the esterification reaction
d. In order to separate and purify the ester produced by the esterification reaction, the guide tube should be inserted into the level of saturated sodium carbonate solution below, and then separated by liquid-separation
9. Malic acid is a common organic acid, and its condensed structural formula is
Which of the following reactions does malic acid undergo (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
– Reaction with sodium hydroxide solution
– Making purple litmus red
– It can react with sodium to produce hydrogen
– It will react with acetic acid under certain conditions, this is called the esterification reaction
– It will react with ethanol under certain conditions, this is called the esterification reaction
a. ①②③ b. ①②③④ c. ①②③⑤ d. ①②③④⑤
10. Which of the following statements is incorrect (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. The functional group of ethanoic acid is carboxyl, while the functional group of ethanol is hydroxyl
b. Ethanoic acid can react with sodium carbonate to produce carbon dioxide, which indicates that the acidity of ethanoic acid is stronger than the acidity of carbonic acid
c. The reaction that ethanoic acid reacts with ethanol and produces ethyl acetate belongs to the acid–base neutralization reaction
d. Ethyl acetate is a colorless oily liquid with a sweet smell, which is less dense than water and insoluble in water
11. Which of the following statements can indicate that ethanoic acid is a weak acid (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. Ethanoic acid can't make phenolphthalein solution red
b. Ethanoic acid can make purple litmus test solution red
c. Ethanoic acid can react with sodium carbonate to produce carbon dioxide
d. The hydrogen ion concentration of the 0.10 M ethanoic acid solution is about 0.01 M
12. What is the relative molecular mass of water that is produced by the esterification reaction between acetic acid in which all of the oxygen atoms are 18O and ethanol in which the oxygen atom is 16O (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. 16 b. 18 c. 20 d. 22
13. The ethyl acetate produced from the reaction of ethanoic acid and ethanol often contains a small amount of ethanoic acid and ethanol, which of the following reagents is best chosen to remove the ethanoic acid and ethanol (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. Sodium hydroxide solution
b. Saturated sodium carbonate solution
c. Saturated sodium bicarbonate solution
d. Dilute sodium carbonate solution
14. Which of the following statements about the properties of ethanoic acid is incorrect (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. Ethanoic acid can react with carbonate solution to produce carbon dioxide because the acidity of acetic acid is stronger than the acidity of carbonic acid
b. Ethanoic acid can react with sodium to produce hydrogen
c. There is a carbon–oxygen double bond in the ethanoic acid molecule, so it can make the color of bromine water fade
d. Ethanoic acid will condense into glacial crystals when the temperature is lower than 16.6 degrees Celsius
15. Butyl acetate can be produced by the esterification reaction between 1-butanol and ethanoic acid with concentrated sulfuric acid as catalyst and the reaction temperature is 115–125 °C. The reaction device figure is shown below, which of the following statements about the experiment is incorrect (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. The experiment cannot be carried out with water-bath heating
b. The function of the glass tube is reflux condensation
c. Water and sodium hydroxide solution are needed for washing when purifying butyl acetate
d. Adding excess ethanoic acid will improve the conversion ratio of 1-butanol
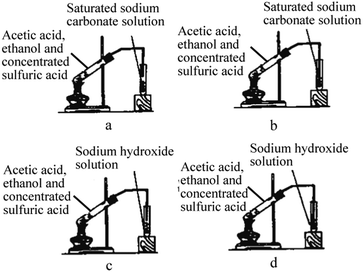
16. Which of the following reaction equipments for producing ethyl acetate is correct (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
17. A small amount of ethyl acetate can be produced using the reaction equipment below
Answer the following questions:
(1) 2 mL concentrated sulfuric acid, 2 mL ethanoic acid and 2 mL ethanol need to be added to tube a respectively, what is the correct order of the reagents addition and operation?
(2) Write the chemical equation and the reaction type of the reaction in tube a.
(3) What are the purposes of heating the test tube?
(4) What are the functions of saturated sodium carbonate solution in test tube b? (![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )
)
a. Neutralizing the ethanoic acid and ethanol
b. Reacting with ethanoic acid and partially absorbing ethanol
c. Facilitating the separation of ethyl acetate because the solubility of ethyl acetate in saturated sodium carbonate solution is less than in water
d. Promoting the generation and productivity of ethyl acetate
e. Why is the guide tube not inserted below the liquid level in test tube b? What experimental operations should apply to separate the ethyl acetate in test tube b?
18. Milk will go sour after a long time, which results from a lot of lactose in the milk decomposing to produce lactic acid because of the effect of microorganisms. Lactic acid is also called 2-hydroxyl propionic acid, its condensed structural formula is
Answer the following questions:
(1) Write the names of the functional groups of lactic acid.
(2) Write the chemical equation of the reaction between lactic acid and enough sodium metal.
(3) Write the chemical equation of the reaction between lactic acid and sodium carbonate solution.
(4) With concentrated sulfuric acid as catalyst, two molecules of lactic acid can react and the product is an annular structure. Write the condensed structural formula of the product.
(5) With concentrated sulfuric acid as catalyst, three molecules of lactic acid can react and the product is a chain. Write the condensed structural formula of the product
Acknowledgements
We would like to acknowledge the support of the Chinese National 985 Innovation Platform for Development Outstanding Universities of China. We gratefully acknowledge the support and collaboration of the teachers and students in Class 3 and Grade 1 of Dong Yuan Road Senior High School in Xi'an, the capital city of Shaanxi province in China who participated in the study.Notes and references
- Anderson O. R., (1991), Neurocognitive models of information processing and knowledge acquisition, Prog. Sens. Physiol., 12, 115–192.
- Anderson O. R., (1997), A neurocognitive perspective on current learning theory and science instructional strategies, Sci. Educ., 81, 67–89.
- Anderson O. R. and Demetrius O. J., (1993), A flow-map method of representing cognitive structure based on respondents' narrative using science content, J. Res. Sci. Teach., 30, 953–969.
- Ausubel D. P., (1963), Cognitive structure and the facilitation of meaningful verbal learning, J. Teach. Educ., 14, 217–221.
- Bischoff P. J., (1999), The development of ideational networks, systemic knowledge, and application abilities among preservice elementary teachers who studied a laboratory unit on ecology, J. Geo. Acad. Sci., 3, 210–223.
- Bischoff P. J., (2002), The role of knowledge structures in the ability of preservice elementary teachers to diagnose a child's understanding of molecular kinetics, Sci. Educ., 9, 936–951.
- Bischoff P. J., et al., (2010), An Analysis of Knowledge Structure, Diversity and Diagnostic Abilities Among Pre-Service Science Teachers Within the Domain of Oxidation and Reduction Chemistry, J. Sci. Teach. Educ., 21, 411–429.
- Bonder G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63(10), 873–878.
- Champagne A. B., Klopfer L. E., Desena A. T. and Squires D. A., (1981), Structural representations of students' knowledge before and after science instruction, J. Res. Sci. Teach., 18, 97–111.
- Dhindsa H. S. and Anderson O. R., (2004), Using a conceptual-change approach to help preservice science teachers reorganize their knowledge structures for constructivist teaching, J. Sci. Teach. Educ., 15, 63–85.
- Fosnot C. T., (1996), Constructivism: a psychological theory of learning, in Fosnot C. T. (ed.), Constructivism: theory, perspectives and practice, New York: Teachers College Press, pp. 3–7.
- Grove N. P. and Bretz S. L., (2010), Perry's Scheme of Intellectual and Epistemological Development as a framework for describing student difficulties in learning organic chemistry, Chem. Educ. Res. Pract., 11, 207–211.
- Gunstone R. F., (1980), Word association and the description of cognitive structure, Res. Sci. Educ., 10, 45–53.
- Ifenthaler D., Masduki I. and Seel N. M., (2009), The mystery of cognitive structure and how we can detect it: tracking the development of cognitive structures over time, Instr. Sci., 4, 162–179.
- Jiane B., (2009), The course and video and teaching reflection of ethanoic acid under the new curriculum reform, Chin. J. Chem. Educ., 11, 17–19.
- Jianjun W., Jiliang Y. and Xing W., (2012), Teaching design, teaching implementation and teaching reflection of ethanoic acid, Educ. Chem., 5, 39–41.
- Jonassen D. H., (1987), Assessing cognitive structure: verifying a method using pattern notes, J. Res. Dev. Educ., 20(3), 1–14.
- Jonassen D. H., Beissner K. and Yacci M., (1993), Structural knowledge: Techniques for representing, conveying, and acquiring structural knowledge, Hilsdale, NJ: Lawrence Erlbaum.
- Leilei M., (2014), Analysis of the Chemistry Learning Barriers with the Diagnostic Assessment, J. Shanghai Univ., 28(4), 71–74.
- Liew C. W. and Treagust D. F., (1995), A predict-observe-explain teaching sequence for learning about students' understanding of heat and expansion of liquids, Aus. Sci. Teach. J., 41(1), 68–71.
- Ling Z. and Houxiong W., (2011), The metacognitive factors and teaching strategies of high school students' chemistry learning difficulties, Theor. Pract. Educ., 7, 56–58.
- Marinopoulos D. and Stavridou H., (2002), The influence of a collaborative learning environment on primary students' conceptions about acid rain, J. Biol. Educ., 37(1), 18–24.
- Min Xu, (2012), The exploration about the cause of teaching difficult points and solving strategies of the basic organic chemistry, Master's thesis, Capital Normal University.
- Mortimer E. F. (1995). Conceptual change or Conceptual Profile change? Sci. Educ., 4(3), 267–285. DOI: http://10.1007/BF00486624.
- Nakiboglu C., (2008), Using word associations for non major science students' knowledge structure before and after general chemistry instruction: the case of atomic structure, Chem. Educ. Res. Pract., 9, 309–322.
- Novak J. D., (1990), Concept mapping: a useful tool for science education, J. Res. Sci. Teach., 14, 5–13.
- Novak J. D. and Gowin D. B., (1984), Learning how to learn, Cambridge University Press: New York.
- Oskay Ö. Ö. and Dinçol S., (2011), The Effects of Internet-Assisted Chemistry Applications on Prospective Chemistry Teachers' Cognitive Structure, Proc. Soc. Behav. Sci., 15, 927–931.
- Oskay Ö. Ö., Temel S., Özgür S. D. and Erdem E., (2012), Determination of preservice chemistry teachers' cognitive structures via flow map method and their knowledge level on “greenhouse gases and their effects” topic, Eur. J. Phys. Chem. Educ., 4(1), 30–45.
- Palmer D., (1995), The POE in the primary school: an evaluation, Res. Sci. Educ., 25(3), 323–332.
- Petri J. and Niedderer H. (1998). A learning pathway in high-school level quantum atomic physics, Int. J. Sci. Educ., 20(9), 1075–1088.
- Qiang C., (2009), The study about senior high school students' learning difficulties and relevant solving strategies in organic chemistry learning, Master's thesis, Northeast Normal University.
- Shanshan L. and Hualin B., (2013), Knowledge types and cognitive analysis of the high students' chemistry learning difficulties, Chin. J. Chem. Educ., 6, 7–9.
- Shavelson R. J., (1972), Some aspects of the correspondence between content structure and cognitive structure in Physics education. J. Educ. Psychol., 63(3), 225–234.
- Shavelson R. J., (1974), Methods for examining representations of a subject-matter structure in student memory, J. Res. Sci. Teach., 11, 231–249.
- Smith B. O. and Meux M. O., (1970), Smith system, in Simon A. and Boyer E. G. (eds.), Mirrors for Behavior II: an anthology of observation instruments, Philadelphia, PA: Classroom Interaction Newslet-ter.
- Snow R. E., (1989), Toward assessment of cognitive and conative structures in learning, Educ. Res., 18(9), 8–14.
- Snow R. E. and Lohman D. F., (1990), Implications of cognitive psychology for educational measurement, J. Educ. Res., 14(5), 455–473.
- Soyibo K. and Evans H. G., (2002), Effects of co-operative learning strategy on ninth-graders' understanding of human nutrition, Aus. Sci. Teach. J., 48(2), 32–35.
- Taber K. S., (2000), Multiple frameworks? Evidence of manifold conceptions in individual cognitive structure, Int. J. Sci. Educ., 4, 399–417.
- Taber K. S., (2001), Shifting sands: a case study of conceptual development as competition between alternative conceptions, Int. J. Sci. Educ., 23(7), 731–753.
- Taber K. S., (2009), Progressing Science Education: Constructing the scientific research programme into the contingent nature of learning science, Dordrecht: Spring.
- Taber K. S., (2013), Modelling Learners and Learning in Science Education: Developing representations of concepts, conceptual structure and conceptual change to inform teaching and research, Dordrecht: Springer.
- Taber K. S., (2014), The significance of implicit knowledge in teaching and learning chemistry, Chem. Educ. Res. Pract., 15(4), 447–461.
- The Ministry of Education of China, (2006), The ordinary high school chemical curriculum standards (experiment), The People's Education Press, p. 13.
- Tsai C. C., (1998), An analysis of Taiwanese eighth graders' science achievement, scientific epistemological beliefs and cognitive structure outcomes after learning basic atomic theory, Inter. J. Sci. Educ., 20, 413–425.
- Tsai C. C., (1999), Content analysis of Taiwanese 14 year olds' information processing operations shown in cognitive structures following physics instruction, with relations to science attainment and scientific epistemological beliefs, Res. Sci. Technol. Educ., 17, 125–138.
- Tsai C. C., (2000), The effects of STS-oriented instruction on female tenth graders' cognitive structure outcomes and the role of student scientific epistemological beliefs, Inter. J. Sci. Educ., 22, 1099–1115.
- Tsai C. C., (2001), Probing students' cognitive structures in science: the use of a flow map method coupled with a metalistening technique, Stud. Educ. Eval., 27, 257–268.
- Tsai C. C. and Huang C. M., (2001), Development of cognitive structures and information processing strategies of elementary school students learning about biological reproduction, J. Biol. Educ., 36, 21–26.
- Tsai C. C. and Huang C. M., (2002), Exploring students' cognitive structure in learning science: a review of relevant methods, J. Biol. Educ., 36, 163–169.
- White R. and Gunstone R., (1992), Prediction-observation-explanation, in White R. and Gunstone R. (eds.), Probing understanding, London: The Falmer Press, pp. 44–64.
- Wu Y. T. and Tsai C. C., (2005a), Effects of constructivist-oriented instruction on elementary school students' cognitive structures, J. Biol. Educ., 39, 113–119.
- Wu Y. T. and Tsai C.-C., (2005b), Development of elementary school preservice teachers' cognitive structures and information processing strategies under long-term constructivist oriented instruction, Sci. Educ., 89, 822–846.
- Xialin W., (2014), The investigation about the learning difficulties and the causes of organic chemistry when senior grade 1 students first learned, Master's thesis, Shaanxi Normal University.
- Xiaojie W., (2011), The survey and analysis of senior high school students' cognitive structures about organic chemistry, Master's thesis, Capital Normal University.
- Xinqi Song, Jing Wang and Wending Li, (2011), Chemistry compulsory teachingmaterials2 for Senior High School. The People's Education Press, pp. 75–76.
- Yaowu Guan, (2005), The study about senior high school students' learning difficulties and the solving strategies in organic chemistry learning, Master's thesis, Northeast Normal University.
- Yujuan Li, (2006), The exploration about senior high school students' chemistry learning difficulties, Master's thesis, Shandong Normal University.
- Yuqun H., (2000), The investigation and revelation of senior high school students' learning difficulties when they were first taught organic chemistry, Chin. J. Chem. Educ., 4, 22–23.
| This journal is © The Royal Society of Chemistry 2015 |