Diagnostic assessment of student misconceptions about the particulate nature of matter from ontological perspective
Dilek
Özalp
*a and
Ajda
Kahveci
 b
b
aUniversity of South Florida, USA. E-mail: dilekozalp@mail.usf.edu
bÇanakkale Onsekiz Mart University, Turkey. E-mail: ajdakahveci@comu.edu.tr
First published on 9th June 2015
Abstract
Student conceptions related with matter and the particulate nature of matter (PNM), are vital for advanced understanding in chemistry, and have been a research area of significant attention. Lacking in the literature are studies addressing chemical misconceptions from an ontological point of view. The purpose of the current study was to develop a diagnostic instrument by utilizing ontological categories as theoretical lens and to evaluate student understanding on the topic PNM through assessment based on the relationship between ontology and misconceptions. Aligned with content in the middle school curricula, an assessment instrument of 25 distractor-driven, multiple-choice items, 15 of which were two-tier, was constructed. Subsequent to content validity work, to utilize student feedback for the improvement of the items and the validity of the inferences, we pilot tested our instrument with 178 students. Cross-sectional survey methodology was employed for the larger assessment work across Grades 6–11. Data were collected from a randomly selected sample of 696 students attending primary and secondary schools. The overall trend emerged to be improved student knowledge with increasing grade level. The students most often tended to attribute the properties of macroscopic matter to its microscopic particles and had most difficulty in explaining dissolution at the particle level, confirming previous national and international findings. An understanding of misconceptions from an ontology perspective may further assist educators to enhance a radical conceptual change. Distractor-driven, two-tier multiple-choice items are envisioned to aid stakeholders in curriculum development and implementation by illuminating student conceptions and ways of thinking.
Introduction
Science education researchers and practitioners have widely recognized the importance of students' conceptions in the process of science learning. Because of this policy makers put an emphasis on materials that help teachers to assess students' conceptions of several topics in science (Keeley et al., 2005). If students do not understand the conceptions truly, their misunderstandings may result in misconceptions. The literature indicates that “misconceptions” is used for a wide range of terms, such as alternative frameworks (Driver, 1981), alternative conceptions (Hewson and Hewson, 1983; Arnaudin and Mintzes, 1985), preconceptions (Osborn and Freyberg, 1985; Gallegos et al., 1994), erroneous ideas (Sanders, 1993) and naïve beliefs (Caramazza et al., 1981). Smith et al. (1993) also define learner misconceptions as “faulty extensions of productive prior knowledge” (p. 152).Chemistry is the science of matter and its structure, composition and properties as well as the interactions between atoms and the changes that occur in matter during chemical reactions (Pinto and Silvestre, 2014). Evident from the definition and as a chemist would argue, matter is the fundamental topic constituting the foundation of chemistry. Thus, student conceptions related with matter and the PNM are crucial for advanced understanding in chemistry. Student conceptions related with the PNM has been a research area of interest for quite a while because it is strongly related to other primary and secondary school topics such as states of matter, chemical changes and chemical bonds.
Given its importance, misconceptions in understandings of the PNM have been researched by science educators for a long time (i.e., Griffths and Preston, 1992; Lee et al., 1993; Margel et al., 2004; Nakhleh et al., 2005; Boz, 2006; Othman et al., 2008). This body of research has exposed a variety of misconceptions about the PNM. For instance, some students think that water molecules are water droplets (Boz, 2006). Similarly, others think that molecules melt (Boz, 2006), water molecules expand when water is heated (Griffths and Preston, 1992; Lee et al., 1993; Kokkotas et al., 1998; Stepans, 2003; Kind, 2004), gold atoms are shiny and hard as gold itself (Stepans, 2003), and that there is air between particles (Kind, 2004). Also, some students think that particles (atoms and molecules) can be seen under a microscope (Griffths and Preston, 1992; Lee et al., 1993; Nakhleh et al., 2005; Tezcan and Salmaz, 2005); and some substances do not have atoms (Tezcan and Salmaz, 2005).
Øyehaug and Holt (2013) conducted a two-year longitudinal study to investigate four primary school students' understanding of the nature of matter and chemical reactions to find out their knowledge structures. Their findings indicated that although the students' understanding of matter and chemical reactions become somewhat more integrated and cohesive over time, at some points students expressed incomplete knowledge elements. For instance, the students' understanding about the difference between the smell particles and air particles were not clear, and their descriptions of how smell spreads were incomplete. Another finding was that the students' understandings advanced step by step. For instance, one of the students described particles in fluids as moving spheres, but over time he described them as molecule, atom and symbol.
Another study was conducted with students ranging from secondary to tertiary educational levels by Ayas et al. (2010) to identify their understanding of the PNM. They administered a questionnaire with five-item open-ended questions to 166 students. They identified some misconceptions such as the following: sunrise causes getting rid of the water molecules completely; air pressure is high in hot weather and low in cold weather, ice cubes in a jar melt and pass among the glass particles; ink particles affect the water particles by giving their color to the water particles and turning their color blue. Similar results were reported by Bridle and Yezierski (2012). They indicated that some students were not able to provide correct identification of the phases of matter, some students could not provide a description about the liquid behavior at the particulate level. They did not clearly distinguish between the particle spacing of a liquid and a gas. Students represented the change that occurs between the heated liquid in a beaker and the space above the liquid by breaking the substance apart into its elements. The students expressed that heating caused decomposition of a compound during boiling.
In their study, Nyachwaya et al. (2011) developed an open-ended drawing instrument to assess students' understandings of the PNM. The results indicated that students have problems with fundamental chemistry concepts related to PNM. For example, some students' drawings of ionic compounds did not indicate charged species. Most of the students used the same particulate model of bonding for both ionic and covalent compounds. In addition, they only used covalent models when they depicted the ionic compounds. For some of the cases, they tended to portray molecular compounds as having dissociated in solution. They reported that although the students received explicit instruction in representing the PNM, they still struggled to represent a chemical reaction correctly at the particulate level.
Conducted at college level, Kahveci (2009)’s research involving pre-service chemistry teachers revealed that the teacher candidates were challenged in reasoning at sub-microscopic level. Nearly half of the first year and 20–26% of the fourth and fifth (final) year students in the study could not differentiate between an element and a compound illustrated at sub-microscopic level. Based on the research findings, Kahveci (2009) drew attention to the need of chemistry and chemistry education curricula including a more explicit focus on particle level chemistry supported with inquiry-oriented teaching strategies and methods.
As many researchers agree, the PNM topic is critical in further understanding of sophisticated chemistry topics. Also conducted at college level, the purpose of Adadan (2014)’s research was to investigate the influence of pre-service chemistry teachers' understanding of the particle nature of matter on their conceptual understanding of solution chemistry in the context of multirepresentational (MR) instruction. The MR instruction, according to the researcher, allowed students to “build referential coherent links between the verbal and the corresponding visual mental representation, as well as integrating them with the relevant aspects of existing prior knowledge from long-term memory” (p. 221). The analysis of questionnaires about the PNM and interviews on solution chemistry indicated that although both groups of participants improved their understanding of solution chemistry, the participants with a high understanding of the PNM performed better than the participants with a low understanding of the PNM in terms of developing a more scientific conceptual understanding of the topic after the MR instruction.
In Ozmen's (2011) study on 4th, 5th, and 6th grade primary students' conceptions about the PNM in daily-life events, the interview analysis indicated that students in all grades have little knowledge of or misconceptions about the sub-microscopic particles such as atoms and molecules. For example, the students stated that the particle size and the number of the particles increase after heating; particles can freeze, get smaller when they are cooled; when a balloon is cooled the particles would disappear and the particles combine with each other and their numbers decrease; solids do not have particles; and there is no space between solid particles. Similar results were reported by Tsitsipis et al. (2011). The purpose of their study was to identify the effect of logical thinking, field dependence/independence, and convergent/divergent thinking on some specific students' misconceptions about the PNM. Three hundred twenty nine, ninth-grade junior high school pupils participated in their study. The results showed that the students have different misconceptions. Some of these were considering matter as being continuous; the same substance, molecules, other substance such as air exist between the particles of a substance; molecules especially in the solid do not move; molecules are always solid; and water molecules are composed of two or more solid spheres.
Gómez et al. (2006) examined the degree of coherence found in students' conceptions concerning the PNM. The analysis of the interviews that were conducted with 43 students indicated high degree of coherence in the replies given to different tasks. Students' replies to the confrontation questions indicated a greater degree of coherence than the predicting events. The analysis also indicated that for some students matter is continuous and static, for some of them it is continuous and stuffed with particles, or gaps, and there is a causal correlation with the movement of the particles and vacuum.
The studies identify several reasons for students' misconceptions. Some of these are everyday conversations, experience and communication at home and with media, science textbooks and creative thinking (Marques and Thompson, 1997; Goldston and Downey, 2012). Goldston and Downey (2012) state that the images and symbolic representations used in textbooks unintentionally foster misconceptions and construction of inaccurate understandings. In addition, storybooks and cartoons also cause students to misinterpret the world. They also argue that the partial or incorrect answers given to students by individuals such as parents, peers, grandparents and even teachers lead students to develop misunderstandings about the world.
Chi and Roscoe's (2002) comprehensive analysis reveals that the source of student misconceptions is the miscategorization of conceptions between ontological categories. Ontology is a science of entities, it searches entities' categories and it has been known since Aristoteles times (Chi and Hausmann, 2003). In the present research, students' misconceptions of the PNM were assessed based on ontological categories. The study aimed to identify how students miscategorize their conceptions in comparison with the ontological categories to which the concepts belong.
Purpose of the study
The main purpose of this study was to evaluate student understanding on the topic through assessment based on the relationship between ontology and misconceptions. Specifically, our study sought answers to the following research questions:(1) What are middle and high school students' misconceptions about the PNM?
(2) Do middle and high school students' understandings about the PNM differ based on grade levels or on extra science classes taken out of school?
(3) How do miscategorizations between the ontological categories explain students' misconceptions of the PNM?
Importance of the study
The definition of conceptual change and the factors that inhibit or enable conceptual change have been one of the concerns of researchers for a long time. Identification of student misconceptions based on ontological categories and figuring out their miscategorizations are important since these provide a base to understand the process and patterns of conceptual change. Using ontological categories helps to understand the organization of the concepts and how the categorization affects the subsequent understanding of the conceptions (Johnston and Southerland, 2000). According to Chi et al. (1994), how learners categorize new concepts into ontological categories that seem more appropriate to them. In this process, the concepts take their meaning based on the related concepts in the category to which they are assigned. This suggests that figuring out the expected category of the concept and comparing it to the category to which it is assigned is important because learners derive the meaning of the concept from the ontological characteristics of that category. Although in the literature there is plenty of research on student misconceptions, researchers argue for the importance of characterizing those conceptions instead of simply identifying them (Wandersee et al., 1994). Understanding student miscategorizations provides a way to figure out the underlying cause of common misconceptions. This helps not only finding ways to address those misconceptions but also finding the reasons of why some misconceptions are resistant to change which is an important problem in the conceptual change process. Knowing the possible reasons of the misconceptions may be useful for teachers and educators to structure their teaching to help students overcome erroneous thinking. Awareness of miscategorizations may shift the focus of teaching from misconceptions to relevant ontological debates which may enrich and strengthen the process of teaching for conceptual change.Contribution of the study
Research in ontology and science misconceptions has extensively been conducted in the disciplines physics and biology (Johnston and Southerland, 2000). On the other hand, research in chemistry education and particularly, in the topic PNM, has often focused on revealing student misconceptions through various methods of assessment, such as multiple choice testing or interviewing about the PNM. In this study, we explored students' misconceptions from an ontological point of view.The literature concerning misconceptions suggests that students' misconceptions about the PNM have been widely investigated. However, there is lack of research in the literature concerning student misconceptions about the PNM from an ontological point of view. This study addresses this gap by exploring the ontological categories of the concepts as well as those to which students assign the concepts, which may be different than the categories that they scientifically belong. Therefore, the study contributes to the literature by providing insight about the ontological nature of students' knowledge structures of the PNM.
Theoretical framework: ontology and ontological categories
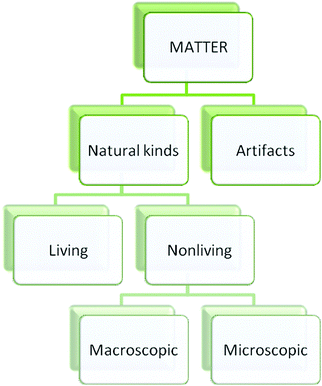
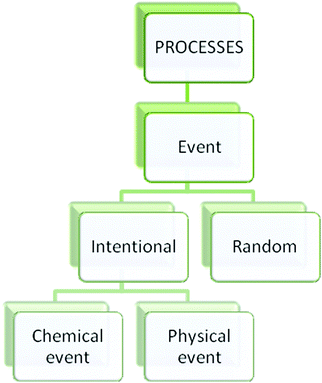
A number of researchers have focused on ontology with an attempt to understand conceptual change and sources of misconceptions. Ontology refers to the categorical structure of reality (Chi and Hausmann, 2003). It assumes that entities in the world essentially belong to different ontological categories (Chi and Hausmann, 2003). Chi and her colleagues propose three primary ontological categories; Matter, Processes and Mental States (Chi et al., 1994; Chi, 1997; Chi and Hausmann, 2003) (Fig. 1). Each category has ontological attributes and subcategories. According to Chi and Slotta (1993), “an ontological attribute is a property that an entity may potentially possess as a consequence of belonging to that category (or any of its subordinates)” (p. 252). Conceptions that belong to the Matter category have ontological attributes such as mass and volume, being storable and cumulative. Students can conceptualize these concepts more easily because they can interact with these entities (Johnston and Southerland, 2000). Table, pen, tree, etc. are the entities in the Matter category. Matter category has two subcategories: natural kinds and artifacts (Chi, 1997). Concepts in the Process category do not have physical attributes. For instance, reading, writing, talking, etc. belong to this category (Johnston and Southerland, 2000). Also, these concepts do not have color, mass, and volume. Therefore, Process and Matter categories are fundamentally distinct ontological categories. Process category has three subcategories. These are procedure, event and constraint-based interactions (Chi et al., 1994). One of the most important subcategories is constraint-based interactions because many physics and chemical concepts belong to constraint-based interactions category. For instance, heat, light, current, chemical bond belong to this category. Concepts which are about emotions, thoughts, wishes such as dream, wish, and fear belong to Mental States category (Chi et al., 1994).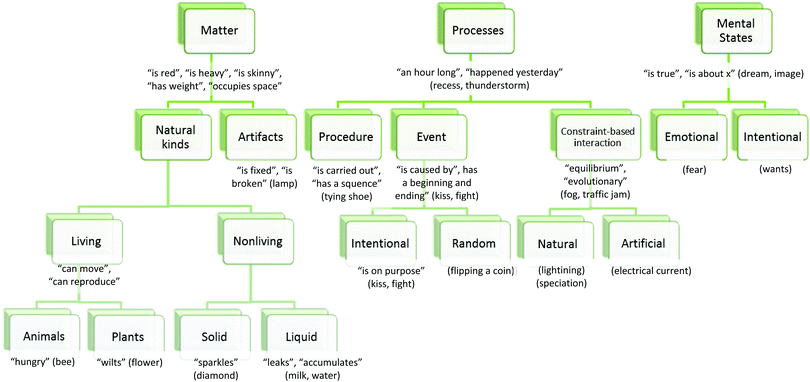 | ||
| Fig. 1 A plausible structure of ontological categories (Chi et al., 1994, p. 29). Reprinted from Chi M. T. H., Slotta J. D., and Leeuw N., (1994) From things to processes: a theory of conceptual change for learning science concepts, Learning and Instruction, 4(1), 27–43, Copyright (1994), with permission from Elsevier. | ||
Primary ontological categories are distinct from each other. Also, each subcategory of one ontological category is distinct from the other ontological category. The Processes category is ontologically different from any category of Matter since they have nonoverlapping sets of ontological attributes. For instance, an event in the Process category has an ontological attribute such as “being an hour long”, on the other hand an animal in the Matter category cannot be “an hour long”. This suggests they do not share “time” as an ontological attribute. As Chi and Slotta (1993) suggest, “…two categories are ontologically distinct if the attributes of one category cannot be applied to members of another category” (p. 252). On the other hand, the subcategories of the same primary ontological category have the potential to share ontological attributes. For instance, humans and animals are the subcategories of Matter and they are not ontologically distinct. Being hungry is one of the ontological attribute of humans and also of animals. Both humans and animals have the potential to be hungry.
Ontology and misconceptions
Chi (2008) defines categorizing as the process of identifying or assigning a concept to a category to which it belongs. As stated above, the concepts that are named as the members of an ontological category possess features of that category (Chi and Slotta, 1993; Chi, 1997; Chi and Hausmann, 2003). Once a concept is categorized it can take the attributes from its category membership. Therefore, categorization is very important in the learning process. When students confront a new concept, they assign it to a category which is more reasonable for them. A concept is assigned to a category through relating it with previous concepts.Although there are many other explanations, in ontology theory framework misconceptions are considered to be closely related with ontological categorizations, i.e., misconceptions involve the assignment of a concept to an inappropriate ontological category. In other words, from ontological perspective, students might assign the concepts to a wrong category which may result in misconceptions. Chi and Roscoe (2002) state that concepts are related to the notion of categories. Concepts are represented, understood and interpreted in the context of category membership. That means when a concept is assigned to a category then the concept inherits all the features of that category. This suggests misconceptions are the instances of miscategorization, not hierarchically but laterally (Chi and Roscoe, 2002). In other words, misconceptions involve the assignment of a concept to an inappropriate ontological category.
According to the researchers, if a concept is assigned to a different ontological category or subcategory than it scientifically belongs, a misconception is likely to emerge. For example, the concept of chemical bonding belongs to the Processes category; however, many students think that bonds have mass and volume, thus assign the concept to the Matter category. Eventually, this mismatch is likely to grow into a chemical misconception. Another example is electricity. If the concept of electricity is assigned to Matter category this is likely to lead to a misconception because electricity belongs to the Process category and does not have ontological attributes of matter (Chi and Roscoe, 2002).
Similarly, misconceptions may occur with relation to ontological subcategories which have lateral relationship. Chi (2008) refers to categories on different branches as “lateral” categories. For example, artifacts can be considered a lateral category to living beings. Artifacts include a set of subcategories, such as furniture and toys, and furniture includes subcategories such as tables and chairs but it does not include the subcategories of living beings, such as animals, bees, reptiles, birds, or robins (Fig. 1). Therefore, artifacts and living beings categories have distinct properties. For example, while living beings ‘can move’ artifacts cannot; and living beings ‘can reproduce’ but artifacts cannot. Being able to move is an ontological attribute of living beings. Chi et al. (1994) define ontological attribute as “an ontological attribute is a property that an entity may potentially possess as a consequence of belonging to that ontological category” (p. 29). That means, the entities in the living beings category potentially possess the ability of moving. For example, a cat in the living beings category can potentially move (an ontological attribute).
Chi et al. (1994) also provide the definitions of the defining attributes and a characteristic feature, and they explain how those definitions are different than the ontological attributes. They state that “defining attributes are those an entity must have, and a characteristic feature is one that an entity most frequently has”. They explain those definitions through the example of a coffee carafe. They state that “a coffee carafe which is an artifact in the Matter category must have a spout (a defining attribute). It is often made of glass, though not necessarily (a characteristic feature), but it can potentially be broken (an ontological attribute). “Thus, an ontological attribute is qualitatively different (and probably orthogonal) to either characteristic features or defining attributes” (p. 29).
As an entity, a car belongs to the non-living category. Being able to move is not an ontological attribute of that category because not all the entities in the non-living category have potential ability of moving. For instance, a table, a chair, a pen belong to non-living category and they cannot move; they potentially are not able to move. Therefore, being able to move for a car can be a defining attribute, not an ontological attribute. This suggests that “moving” of living beings is ontologically different than the moving of “a car”.
If a concept is assigned to a lateral category then a misconception is likely to emerge. For instance, if the concept of bee is assigned to the artifacts category (or its subcategory) instead of living beings category then the ontological attributes of the artifacts category is assigned to the concept of bee which is a misconception.
On the other hand, assigning a concept to a hierarchal category does not mean a misconception. Hierarchal categories are the ones that have a hierarchal relationship in the same ontological tree (Chi and Roscoe, 2002). For instance, Matter, living beings, animals have a hierarchical relationship. The concepts that have hierarchal relationships share the ontological attributes of the topmost category. For instance, animals share the ontological attributes of the living beings, such as ‘can move,’ living beings share the ontological attributes of the matters such as ‘has shape’. Since they share the ontological attributes, assigning a concept to a hierarchal category does not lead to a misconception. For instance, if the concept of ‘robin’ is assigned to living beings category instead of animals this does not result in misconception because robin has potential to share the ontological attributes of living beings or animals (Chi and Roscoe, 2002).
One of the most important reasons of why students miscategorize is the assignment of the new concept to a more familiar category. This happens because of lack of alternative ontological categories. When students encounter with a new concept, if they do not have the correct category they assign it to one of the existing categories that is more familiar. Once a concept is assigned to an existing category then the concept inherits all the features of that category (Chi and Roscoe, 2002). For example, students think that the earth is flat rather than spherical. Johnston and Southerland (2000) suggest that students categorize it in a way that does not allow it to be a planetary object. Instead, from an ontological categories perspective, we are more familiar with the notion of earth that acts as a container of entities rather than as an entity contained within some greater system. Therefore, students may assign the concept of earth to a flat object rather than a spherical object category.
Similarly, students think that atoms are alive. The reason from an ontological point of view is that they probably try to make a connection with atoms and another entity that they already know and that is very small like atoms. In their daily life, they know that microbes are very small. They think that atoms and small organisms such as microbes are of a similar kind. When students encounter the concept of atoms they try to find a category that they are more familiar with and have similar characteristics with atoms. Since they are familiar with the small organisms like microbes that “share” some characteristics with atoms such as being very small, cannot be seen and can move, they assign the concept of atoms to the living beings category. Hence, they think atoms are alive like microbes.
Another example is drawn from Moss and Abrams' (1999) study in which the researchers describe students' conceptions of models. Moss and Abrams found that students' understandings of models were related to the physical models rather than conceptual models which reflect a process or interaction. According to Johnston and Southerland (2000), from an ontological point of view the reason is students are more familiar with the physical models that represent the objects in the matters category rather than the conceptual models that represent the processes. Therefore, when they were asked about models, they referred the models that represent an entity which physically exists.
If misconceptions are not detected and altered in the learning process, the students are likely to have difficulties when faced with desired ideas (Duit and Treagust, 1995; Chu et al., 2009). Therefore, identification of student misconceptions is very important to support their learning. Student misconceptions need to be challenged and replaced with correct ones in order to achieve a deep understanding. This process is called as conceptual change. Conceptual change theories that are related to how learners restructure their conceptions are widely used by science educators. They have been discussed by many researchers for several decades yet there is a blurry picture of why change is difficult (Chi, 2008).
Chi (2008) claims that misconceptions are resistant to change because they are laterally or ontologically miscategorized. If a concept is assigned to a category and the desired conception belongs to another category then their characteristics and definitions conflict. In such cases, Chi (2008) suggests identifying the nature of misconceptions in order to figure out whether conceptions belong to different ontological categories. Researchers argue that correcting those misconceptions requires an ontological shift (Chi and Roscoe, 2002; Chi and Hausmann, 2003). From ontological perspective conceptual change is a shift of a concept between lateral categories. They claim that this process is not inherently difficult but very challenging when students lack awareness of their miscategorizations, of when a shift is necessary and of alternative categories that are needed to reassign the new concepts (Chi and Roscoe, 2002; Chi, 2008).
Lack of alternative categories is one of the main reasons of the difficulty of conceptual change (Chi and Hausmann, 2003). If the students do not have the correct category to assign the new concepts they will be challenged in doing the correct categorization. Therefore, conceptual change will not easily occur. On the other hand, even if students have alternative categories they still may have difficulties because they may not be aware of the need to shift the meaning of the concept from one ontological category to another. Therefore, Chi and her colleagues argue that “shifting on our own, without being told, may be more cumbersome, largely because we are not aware of the necessity to shift” (Chi and Hausmann, 2003, p. 13). So, they suggest that students should be made aware of the need to make a shift and attribute the features of the appropriate category to the new concept. In the learning experience, as explained next, students need to be involved in the radical conceptual change process. In this process, students learn about the new ontological category, reflect on the meaning of the concept within this category and finally the concept is reassigned to this new ontological category.
Understanding of student misconceptions from an ontology perspective may further assist educators to enhance a conceptual change for scientific competency. In this approach, conceptual change process involves reconsideration of ontology. Chi (1992) suggests radical conceptual change addressing misconceptions that requires a change across ontological categories. She defines radical conceptual change as a process that requires crossing the ontological boundaries.
Chi gives examples from physics in order to explain the process of radical conceptual change. The concepts such as heat and current belong to the constraint-based category, but students generally think that those concepts belong to the matter category. She states that in order for students to understand those concepts they need to understand that those concepts belong to the constraint-based interactions category rather than the matter category. That means their understandings require an ontological shift. For such misconceptions she suggests radical conceptual change that requires a three-step procedure summarized as following (Chi, 1992):
1. In this step, the properties of the new ontological category should be learned,
2. Then, the meaning of the concept within the new ontological category should be learned,
3. Finally, the concept should be reassigned to this new ontological category. This final step might be done in three different ways:
a. In the first way, the concept's original meaning can be replaced with the new meaning while the original meaning is abandoned.
b. In the second way, both meanings coexist in order to access them depending on context,
c. In the third way, the concept is replaced automatically depending on the coherence and strength of new meaning.
Chi suggests that in step 1, students should be directly told that the physics entities belong to a different ontological category (constraint-based category). She states that this explanation should be made directly since configuration of this explanation requires the process of discovery, which would otherwise be very difficult for students to find out themselves. In this step, students should learn the properties of constraint-based category. In step 2, she suggests that the learning process of the concepts (heat and current) should proceed as traditional instruction by using common acquisition processes. She also suggests that accretion and assimilation steps should be achieved in the context of the constraint-based categories. This will help students to start making connections between the properties of this category and those concepts. In step 3, students need to reassign those concepts to the constraint-based category. There are three different ways for the reassignment. In the first way (3a), the person has dual meanings for the concept and has to consciously think the new meaning of the concept. Consciously thinking of whales as a kind of mammals rather than a fish is an example of the first way. In the second way (3b), the concept takes two different meanings or assigned to two different categories and they are accessed under different circumstances. For example, in order to make predictions about the daily events physicists revert back and use the naïve conceptions. In the third way (3c), the reassignment occurs automatically and that may be because the new meaning of the concept is more reasonable and robust, while the original meaning is learned from the everyday environment. Chi states that radical conceptual change takes place after these three steps have occurred.
Research design and procedures
Within the frame of the 2004 curriculum reform initiatives of the Turkish Ministry of National Education, primary science curriculum had been revised in light of contemporary international science education literature and science curricula of developed countries such as US. The science curriculum in Turkey at the time of the study included the teaching of states of matter and changes in states of matter as well as phenomena such as expansion and contraction in Grades 4 and 5. In Grade 6, the PNM is introduced as a separate unit within which students learn about matter as being composed of tiny, invisible, and moving particles with space in between. Within the unit, students are gradually introduced to the concepts of atoms, molecules, elements, compounds, physical and chemical change, and states of matter as explained with the particle model (Ministry of National Education, [MEB], 2006). Included in reform-based curriculum, in essence, these topics thematically overlap with the related middle school Benchmarks for Science Literacy (American Association for the Advancement of Science [AAAS], 1993) and the National Science Education Standards (National Research Council [NRC], 1996).There is a large variety of research utilizing various instruments to assess PNM concepts. Kahveci (2013) provides an overview of diagnostic assessment research with a focus on the type and content of the instruments used for PNM assessment. The researcher particularly emphasizes that two-tiered assessment has more practical power in everyday classroom use. In our study, we constructed an assessment instrument of 25 distractor-driven, multiple-choice items (Sadler, 1998) aligned with content in middle school curricula (Appendix A). Fifteen of these were two-tier and 10 were one-tier diagnostic items. Ten of the two-tier items were developed based on misconceptions reported in the national and international literature regarding the PNM (Yezierski and Birk, 2006; Othman et al., 2008). In developing the instrument, Chi et al.'s (1994) theory of ontological categories was utilized as a theoretical lens. The misconceptions were framed within ontological categories. In other words, concepts addressed by those items were identified as entities in certain ontological categories. Subcategories related with chemistry were developed on the basis of Chi and Roscoe (2002)'s work on biological categories.
Distractor choices were statements constructed based on ontological categories, to which students might possibly assign the targeted concepts in a scientifically unacceptable way. The second tier of each of the items included possible reasons for the statements in the first tier. If students selected one of the distractors and the related explanation, then a misconception resulting from an ill-assigned concept to an ontological category would be evident. For instance, the first question of the test was concerned with student understanding of atoms in terms of their being living or nonliving entities. The ontological category Matter has two subcategories, living and nonliving (Chi and Slotta, 1993; Chi, 1997). If students assigned atoms to the ‘living’ category they would attribute properties of living beings to atoms, thus giving rise to further misconceptions.
The 25-item instrument was revised by experts in chemistry education for content validity. To validate the items which were adopted from nonnative language instruments (Yezierski and Birk, 2006; Othman et al., 2008) back translation method was utilized (permissions from the authors were obtained prior to use of the PNM items in their instruments). The items were first translated to Turkish by a group of bilingual chemistry teachers and then these translations were translated back to English by a second group of teachers. Both versions in the English language were compared, contrasted, and inconsistencies discussed and eliminated by the translating teachers. As a final step, the instrument was examined by a Turkish language expert for the editing and refinement of the items.
Pilot test
Following the scholarly recommendation of DeBoer et al. (2008), to utilize student feedback for the improvement of the items and the validity of the inferences we pilot tested our instrument with students, the majority of whom were middle graders. Pilot testing of the items was conducted with students attending a primary school and a private tutoring center in a metropolitan area in Turkey. A total of 178 students responded to the items, 128 of whom were middle school (Grades 6–8), and 50 were high school (Grades 9–11) students.In the pilot test, we asked the students a series of 15 questions about each test item, derived from DeBoer et al.'s (2008) sample feedback items (Appendix B). We basically asked the students if there was anything confusing about the particular test item, if there were words hard to understand, if there should have been any other answer choice, or any associated picture or graph. We asked if each choice in an item was correct or incorrect, and also solicited explanations about why a choice was correct or incorrect. For the two-tier items, we asked the feedback questions for the second tier, as well. Including the feedback items, in total, since we ended up with more items than students would be able to efficiently complete in a typical class period, we divided the instrument in three sections, each of which was administered to approximately 60 students in the six grade levels.
Pilot test findings
In this section, we illustrate the way we used validity work and student feedback to improve the test items by exemplifying our work on two of the items. We also exemplify ways of student thinking as revealed by student responses to the feedback questions for the test items.One of the reason options of each of the ten two-tier questions developed by the researchers was the statement “None of the above. For me, the reason is:…“. In this way, although we did not conduct interviews, we provided the students an opportunity to write their own reasons if they were different than the presented choices. Though, this open ended choice was selected only 34 times in the pilot test. It is likely that the provided choices reflected students' ways of reasoning to an adequate degree as these were constructed based on misconceptions research. Although this option was selected 34 times, 14 of them did not include any reason. Of the 20 written reasons, eight did not include a scientific explanation; instead the students wrote statements such as “I think all of them are same”, “I did not understand this question”, “it is not”, “the correct answer is not included in the choices”, and “it is not, choice E is correct”. Most of the other reasons written by the students were already included in the presented choices. In other words, the students did not provide sufficient feedback for this open ended option in order for us to make significant changes in the questions. However, we made changes based on student feedback to the 15 items derived from DeBoer et al.'s (2008) feedback items, which we explain next. The rest five two-tier questions adapted from Othman et al.'s (2008) test did not originally include an open ended option. We used DeBoer et al.'s (2008) feedback items to improve those questions, as well.
Item 1 in the test was asking if the atoms should be considered as living or nonliving entities. The initial form of the item is shown in Fig. 2. After the pilot testing one of the reason choices was omitted since a number of both middle and high school students indicated that the reason choices were confusing. According to their comments, the confusion was caused by the similarity in the wording. To resolve the confusion we decided to leave the fifth reason alternative out since it was repetitive of the first and second alternatives.
 | ||
Fig. 2 Initial form of item 1 in the instrument and answer percentages from the pilot testing (N = 60). *![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Correct answer Correct answer | ||
Item 3 was related with dissolution. Its initial form is presented in Fig. 3. In accordance with subject matter expert comments, the phrase “When sugar is dissolved in water” was revised to exclude “dissolving” because one of the purposes of asking the question was to assess student understanding of what was happening to the sugar in the water. Also, according to student feedback from the pilot testing, water temperature should have been specified since dissolving was dependent on temperature. Another student pointed out that the amount of water should have been given. Considering these suggestions the item was revised as following: “When a teaspoon of sugar is added to a glass of water at room temperature a chemical reaction occurs.” Also, an image was added. An additional comment made by a Grade 11th student suggested that the first reason could have been perceived as correct since the mixture of sugar and water may be thought as new matter by some students, indicating a false negative response (DeBoer et al., 2008). To avoid this situation, the first reason was rewritten as following: “When sugar is dissolved in water a new compound forms.”
 | ||
Fig. 3 Initial form of Item 3 in the instrument and answer percentages from the pilot testing (N = 60). *![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Correct answer Correct answer | ||
An analysis of the percentage of correct responses for all items revealed a pattern of significantly lower correct percentages for the two-tier items (range: 1.6–18.3%) than those for the one-tier items (range: 18.0–57.9%). Similarly, the students were more successful in the first tier of the two-tier items, in agreement with previous research findings (Othman et al., 2008). Since the second tiers include justifications of responses in the first tiers, clearly, two-tier questions probe for a meaningful learning of the concepts as opposed to a factual, rote learning. Failing in these questions is an indication of unsatisfactory student understanding of the topic and existence of misconceptions.
Larger study
Cross-sectional survey methodology (Fraenkel and Wallen, 2003) was employed to evaluate student understanding of the PNM across Grades 6–11. According to Fraenkel and Wallen (2003);a cross-sectional survey collects information from a sample that has been drawn from a predetermined population. Furthermore, the information is collected at just one point in time, although the time it takes to collect all of the data may take anywhere from a day to a few weeks or more (p. 398).
In this research, the population was middle and high school students, and the randomly selected sample was drawn from this population. The data were collected at one point in time in a period of about ten days.
Sampling of the participants
Following the pilot study and subsequent revisions of the instrument, data were collected from a randomly selected sample of 696 students attending primary and secondary schools in a metropolitan area in Turkey. 382 of these students were attending middle school, and 314 were attending high school. The selection process involved cluster and stratified random selection techniques. The accessible population was defined as all middle and high school students in the metropolitan area.First, four municipalities out of 33 were randomly selected by coding each and entering the codes into the SPSS software. Then, information on all primary and secondary schools in these four municipalities was accessed and listed. The schools were stratified as primary and secondary schools and one school from each stratum was randomly selected. This process was repeated for each of the four municipalities until a total of eight schools were determined. Three classes from each high school (Grades 9–10–11) and three from each primary school (Grade 6–7–8), were selected. The test was administered in a period of about ten days. With active cooperation of the teachers, the students were motivated to carefully respond to the items in one lecture hour.
For this research, ethical considerations were taken into account. In Turkey, the researchers have to obtain the consent firstly from the Provincial Directorate for National Education. After reviewing our application materials including the research proposal in which we explained the purpose, method, sample and population, and research instrument of the study, the Provincial Directorate for National Education approved the research and provided us a consent report. After reading the report, each school principal and the teacher of each class in the study informed us they approved the administration of our instrument. Before the administration, in each class the researchers explained to the students in detail why they were requested to participate, how the research was important, what kind of questions they were asked to answer, how they needed to answer the questions, and how long it would take. The purpose of the research was explained and the students were informed that the data would be anonymously used in research reports. In addition, this information was available on the first page of the research instrument. The instrument was administered after the students voluntarily consented to participate.
Analyses and findings
Data were coded and entered into SPSS spreadsheets and subsequent analyses performed. First, cumulative scores for the one-tier, two-tier and all items were computed by scoring each correct answer as 1 and false answer as 0. For the two-tier items, only students who answered both tiers correctly received a score of 1 with the rest receiving 0. The total scores were converted to percent values. Next, these variables were checked for normal distribution on both individual item and cumulative score levels. As small significance values (<0.05) were obtained from one-sample Kolmogorov–Smirnov tests, the distributions were found not to be corresponding to normal. Therefore, nonparametric techniques were employed to further analyze the variables. In addition, reliability statistics yielded a Cronbach's alpha value of 0.78, indicating an acceptable reliability level for the test.In Table 1, overall percent correct mean scores by grade levels for the ten one-tier items, 15 two-tier items and all 25 items as three separate variables are reported. In addition, conducted for grade-to-grade comparisons, a Kruskal–Wallis test indicated that the difference among the grades was significant (X2(5) = 225.751, p < 0.05) for all items combined. Subsequent Mann–Whitney U tests revealed the significant differences to be between Grades 6 and 7, 6 and 8, 6 and 9, 6 and 10, 6 and 11, 7 and 9, 7 and 10, 7 and 11, 8 and 9, 8 and 10, and 8 and 11. The overall trend emerged to be improved student knowledge with increasing grade level. The greatest difference between two consecutive grade levels was observed between Grades 8 and 9, the final year in primary school and the first year in high school.
| Question type | Grade 6 (N = 138) | Grade 7 (N = 134) | Grade 8 (N = 110) | Grade 9 (N = 110) | Grade 10 (N = 109) | Grade 11 (N = 95) |
|---|---|---|---|---|---|---|
| One-tier items | 21.6 | 25.7 | 27.2 | 44.4 | 45.1 | 46.8 |
| Two-tier items | 11.6 | 14.5 | 17.8 | 30.6 | 29.8 | 33.4 |
| All items | 15.6 | 19.0 | 21.6 | 36.1 | 36.0 | 39.0 |
Another pattern revealed higher overall percent correct scores for the one-tier items than the two-tier items for all grade levels. Since the second tiers include justifications of responses in the first tiers, clearly, two-tier questions probe for a meaningful learning of the concepts as opposed to a factual, rote learning. Failing in these questions is an indication of unsatisfactory student understanding of the topic, factual knowledge and misconceptions.
Students' scores on 100 point scale were also analyzed. Since there were 25 questions in the test every correct answer counted for 4 points. For the two-tier items, only students who answered both tiers correctly received a score of 4 with the rest receiving 0. Conducted for grade-to-grade comparisons, a Kruskal–Wallis test indicated that the difference among the grades was significant for two-tier items (X2(5) = 187.653 p < 0.01) (Table 2). Subsequent Mann–Whitney U tests revealed the significant differences to be between Grades 6 and 9, 6 and 10, 6 and 11, 7 and 9, 7 and 10, 7 and 11, 8 and 9, 8 and 10, 8 and 11.
| Question Type | Grade | N | Mean Rank | X 2 | df | p |
|---|---|---|---|---|---|---|
| One-tier | 6 | 138 | 234.31 | 160.635 | 5 | <0.01 |
| 7 | 134 | 273.08 | ||||
| 8 | 110 | 287.73 | ||||
| 9 | 110 | 447.45 | ||||
| 10 | 109 | 452.20 | ||||
| 11 | 95 | 457.57 | ||||
| Two-tier | 6 | 138 | 219.86 | 187.653 | 5 | <0.01 |
| 7 | 134 | 259.52 | ||||
| 8 | 110 | 304.00 | ||||
| 9 | 110 | 448.65 | ||||
| 10 | 109 | 453.45 | ||||
| 11 | 95 | 476.02 | ||||
| Total | 6 | 138 | 207.88 | 225.751 | 5 | <0.01 |
| 7 | 134 | 254.69 | ||||
| 8 | 110 | 288.90 | ||||
| 9 | 110 | 461.44 | ||||
| 10 | 109 | 470.00 | ||||
| 11 | 95 | 483.92 | ||||
Similar results were observed for the one-tier items. Generally, secondary school students' scores were higher than the primary school students'. Conducted for grade-to-grade comparisons, a Kruskal–Wallis test indicated that the difference among the grades was significant for one-tier items (X2(5) = 160.635, p < 0.01) (Table 2). Mann–Whitney U tests revealed the significant differences to be between Grades 6 and 8, 6 and 9, 6 and 10, 6 and 11, 7 and 9, 7 and 10, 7 and 11, 8 and 9, 8 and 10, 8 and 11.
In Turkey, students have to take the annual High School Entrance Examination conducted nationwide in order to attend a high school and they have to take the annual University Entrance Examination also conducted nationwide to attend an undergraduate program. Those exams consist of only multiple choice questions from topics learnt during middle and high school, respectively. In Turkey, there are tutoring centers which prepare students for those exams. Students who want to receive support from outside of school attend to a tutoring center. When students attend to a tutoring center, they have the opportunity to learn the topic PNM in a different context which means they learn it from a different teacher and using different materials in a different class environment. This might help them to better learn the concepts of the PNM. Also, since the primary aim of these centers is to prepare the students for the nationwide exam, they focus on multiple choice questions in all science topics including the PNM. In general, the distractors of multiple choice questions are constructed based on student misconceptions. Therefore, the educational practices of those centers include activities that lead students to learn about the desired concepts and also experience misconceptions in the distractors. For these reasons in this research we wanted to explore whether the tutoring centers helped students to better understand the PNM.
In order to find out whether the tutoring centers are successful in helping students to better understand the topic matter; we also compared student scores based on whether they attended or did not attend a tutoring center. The Mann–Whitney U analysis indicated that there was a statistically significant difference between the scores of attending and non-attending students (Table 3). The analysis indicated that the scores of students who attended science tutoring sessions were higher than those of the students who did not attend. Score differences on one- and two-tier questions revealed similar results.
| Question type | Attendance to tutoring center | N | Mean rank | Sum of ranks | U | p |
|---|---|---|---|---|---|---|
| One-tier | Attending | 236 | 405.11 | 95605.00 | 40213.000 | <0.01 |
| Non-attending | 457 | 316.99 | 144866.00 | |||
| Two-tier | Attend | 236 | 403.69 | 95257.00 | 40561.000 | <0.01 |
| Non-attending | 457 | 317.75 | 145214.00 | |||
| Total | Attend | 236 | 412.51 | 97352.00 | 38466.000 | <0.01 |
| Non-attending | 457 | 313.17 | 143119.00 | |||
According to a percent correct analysis for each item, nine of the items received a percent correct score of 10% or lower in at least one of the grade levels (Table 4). The items that more than two grade levels scored 10% or lower correct were dealing with: (a) dissolving of sugar in water as physical change with explanation at the particle level, (b) molecules not being altered during freezing, (c) atoms not being altered during melting, and (d) sulphur having characteristics such as brittleness or a certain melting point but not sulphur atoms. Most students scoring false in these items were the middle school students. On the other hand, seven of the items in total received a percent correct score of 40% or higher in at least three of the grade levels.
| Number | Item description | Percentage by Grade | |||||
|---|---|---|---|---|---|---|---|
| 6th | 7th | 8th | 9th | 10th | 11th | ||
| 1 | Atoms are not living beings | 10.9 | 16.4 | 20.0 | 32.7 | 37.6 | 42.1 |
| 2 | Gold atoms not having the same physical properties of gold | 8.0 | 11.2 | 7.3 | 18.2 | 16.5 | 14.7 |
| 3 | Dissolving of sugar in water as physical change with explanation at the particle level | 3.6 | 3.7 | 0.9 | 10.9 | 7.3 | 14.7 |
| 4 | The size of water molecules not altered during phase change | 13.8 | 28.4 | 29.1 | 45.5 | 27.5 | 49.5 |
| 5 | Iron atoms in solid phase move | 31.3 | 24.6 | 29.1 | 39.1 | 53.2 | 34.7 |
| 6 | Shape of water molecules not altered depending on the shape of container | 15.9 | 22.4 | 27.3 | 54.5 | 46.8 | 55.8 |
| 7 | Molecules cannot be in solid or liquid phase | 2.9 | 8.2 | 15.5 | 26.4 | 33.0 | 36.8 |
| 8 | Alcohol consists of alcohol molecules; sugar consists of sugar molecules | 11.6 | 17.9 | 27.3 | 32.7 | 37.6 | 32.6 |
| 9 | Molecules not being altered during freezing | 7.2 | 6.0 | 9.1 | 28.2 | 17.4 | 30.5 |
| 10 | Atoms not being altered during melting | 7.2 | 9.7 | 3.6 | 31.8 | 25.7 | 37.9 |
| 11 | Bubbles in water during boiling consist of water vapor | 22.5 | 30.6 | 40.9 | 38.2 | 47.7 | 36.8 |
| 12 | Total weight of iodine in a closed container not altered during sublimation | 17.4 | 20.9 | 27.3 | 57.3 | 59.6 | 66.3 |
| 13 | Space between water molecules increases during evaporation | 5.1 | 6.0 | 13.6 | 26.4 | 21.1 | 23.2 |
| 14 | Dissolving of sugar in water as physical change | 11.6 | 9.0 | 10.9 | 8.2 | 10.1 | 18.9 |
| 15 | Sulphur having characteristics such as brittleness or a melting point but not sulphur atoms | 2.2 | 2.2 | 5.5 | 9.1 | 5.5 | 13.7 |
| 16 | Water molecules move faster in gas phase than in liquid and solid phases | 21.7 | 37.3 | 37.3 | 68.2 | 72.5 | 66.3 |
| 17 | Size of molecules not being altered during phase change | 5.8 | 14.2 | 14.5 | 34.5 | 19.3 | 25.3 |
| 18 | Liquid water turning into water vapor (gas) during evaporation | 48.6 | 51.5 | 48.2 | 45.5 | 54.1 | 65.3 |
| 19 | Space between molecules of water decreasing during condensation | 14.5 | 18.7 | 31.8 | 39.1 | 47.7 | 47.4 |
| 20 | Liquid water turning into water vapor (gas) during evaporation | 24.6 | 29.1 | 24.5 | 36.4 | 54.1 | 61.1 |
| 21 | Molecules moving faster if water is heated | 19.6 | 20.9 | 30.0 | 53.6 | 52.3 | 63.2 |
| 22 | Size of molecules not being altered in phase change | 4.3 | 11.2 | 9.1 | 32.7 | 25.7 | 36.8 |
| 23 | Space between molecules of dry ice not being altered in melting | 25.4 | 20.1 | 14.5 | 27.3 | 15.6 | 14.7 |
| 24 | Molecules vibrating in solid phase | 20.3 | 27.6 | 26.4 | 52.7 | 57.8 | 42.1 |
| 25 | Space between molecules of ammonia increases in evaporation | 31.2 | 26.9 | 35.5 | 53.6 | 51.4 | 46.3 |
A distractor-level analysis was also conducted to investigate how common the listed misconceptions were for the students at each grade level, shown in Table 5. The most common misconceptions in the sample were the ideas that: (a) when dry ice melts (turns liquid), the space between its molecules increases (51.9%), (b) ice molecules are solid because ice is solid and water molecules are liquid because water is liquid (38.6%), (c) when a few sugar cubes are put into water they would get heat from the surroundings, melt and then the liquid formed would mix with the water (35.2%), (d) when iron is in the solid phase its atoms do not move because there is not any space between atoms of solids (32%), (e) when water at 24 °C is cooled to 0 °C and freezes, water molecules stop moving (31.6%), and (f) freezing makes water molecules larger (30.5%). One of the most interesting and surprising findings was that in some cases a higher percentage of high school (especially Grade 11) than middle school (especially Grade 6) students appeared to hold certain misconceptions. This finding is contrary to an expectation that misconceptions would be lesser or weaker in higher grade levels as students become more proficient in chemistry. For instance, 25.3% of the Grade 11 students believed that “during melting iron heats up, thus the iron atoms are also heating up, melting and expanding” in contrast to 8.7% of the Grade 6 students. Further, statistically significant difference was found between the percent correct responses of the students to the item in favor of the 6th graders. Although the grade gap is much wider in this study, similar findings were obtained in Herrmann-Abell and DeBoer's (2008) field test research where overall, 7th graders were found to be more successful than 8th graders on the topic.
| Misconception |
Grade 6 (N = 138)
% (n) |
Grade 7 (N = 134)
% (n) |
Grade 8 (N = 110)
% (n) |
Grade 9 (N = 110)
% (n) |
Grade 10 (N = 109)
% (n) |
Grade 11 (N = 95)
% (n) |
Total (N = 696)
% (n) |
|---|---|---|---|---|---|---|---|
| When dry ice melts (turns liquid), the space between its molecules increases. | 31.2 (43) | 35.8 (48) | 50 (55) | 59.1 (65) | 74.3 (81) | 72.6 (69) | 51.9 (361) |
| Ice molecules are solid because ice is solid and water molecules are liquid because water is liquid. | 46.4 (64) | 41 (55) | 42.7 (47) | 36.4 (40) | 39.4 (43) | 21 (20) | 38.6 (269) |
| When a few sugar cubes are put into water they would get heat from the surroundings, melt and then the liquid formed would mix with the water. | 35.5 (49) | 38.1 (51) | 34.5 (38) | 37.3 (41) | 35.8 (39) | 28.4 (27) | 35.2 (245) |
| When iron is in the solid phase its atoms do not move because there is not any space between atoms of solids. | 31.2 (43) | 44.8 (60) | 31.8 (35) | 27.3 (30) | 21.1 (23) | 33.7 (32) | 32 (223) |
| When water at 24 °C is cooled to 0 °C and freezes, the water molecules stop moving. | 28.3 (39) | 36.6 (49) | 30.9 (34) | 31.8 (35) | 24.8 (27) | 37.9 (36) | 31.6 (220) |
| Freezing makes water molecules larger. | 29 (40) | 29.9 (40) | 23.6 (26) | 26.4 (29) | 45 (49) | 29.5 (28) | 30.5 (212) |
| Atoms in a leave are alive since leave is alive. | 28.3 (39) | 27.6 (37) | 29.1 (32) | 28.2 (31) | 26.6 (29) | 28.4 (27) | 28 (195) |
| Water molecules break up to oxygen and hydrogen atoms when water evaporates. | 14.5 (20) | 20.1 (27) | 20 (22) | 41.8 (46) | 37.6 (41) | 20 (19) | 25.1 (175) |
| The shape of water molecules changes depending on the shape of the container because water molecules are flexible. | 23.2 (32) | 32.8 (44) | 30 (33) | 15.5 (17) | 21.1 (23) | 24.7 (23) | 24.7 (172) |
| When sugar is put into water a chemical reaction happens with the water because sugar melts in water. | 31.9 (44) | 23.1 (31) | 33.6 (37) | 20.9 (23) | 13.8 (15) | 12.6 (12) | 23.3 (162) |
| During melting iron heats up, thus the iron atoms are also heating up, melting and expanding. | 8.7 (12) | 17.2 (23) | 14.5 (16) | 18.2 (20) | 27.5 (30) | 25.3 (24) | 18 (125) |
| When iodine in the solid phase is heated and changes to gas phase, its weight decreases because weight of gases is less than weight of solids. | 20.3 (28) | 19.4 (26) | 19.1 (21) | 15.5 (17) | 12.8 (14) | 10.5 (10) | 16.7 (116) |
| Ammonia breaks up to nitrogen and hydrogen gas when turning from liquid to gas phase. | 15.2 (21) | 14.9 (20) | 16.4 (18) | 10.9 (12) | 9.2 (10) | 8.4 (8) | 12.8 (89) |
As we mentioned before, ten of the two-tier questions in the test included an open ended reason option “None of the above. For me, the reason is:…”. Table 6 shows the percentage of the students who selected this option. Although this option was selected by a number of students, the feedback provided was not sufficient enough to make substantial changes.
| Item # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 690 | 684 | 695 | 680 | 691 | 693 | 693 | 689 | 688 | 670 |
| Percentage | 2% | 5% | 7.3% | 5.7% | 2% | 12.1% | 9.2% | 8.9% | 4.5% | 2.2% |
In this research, we also analyzed students' misconceptions based on ontological categories. First, we identified their misconceptions by using the diagnostic test as explained above. Then we aimed to compare the categories that the students' concepts belonged with the anticipated ontological categories of the concepts. As a result of this analysis, we demonstrated how students' misconceptions regarding PNM could be explained in terms of ontological miscategorization of the concepts. That means a miscategorization does not lead to a misconception as if in a cause–effect relationship. The misconceptions are an indication of miscategorization between the ontological categories. The students already have the misconceptions. We did not ask the students explicitly to categorize their understandings based on ontology but we used ontology as a theoretical lens to understand how students reasoned when explaining phenomena.
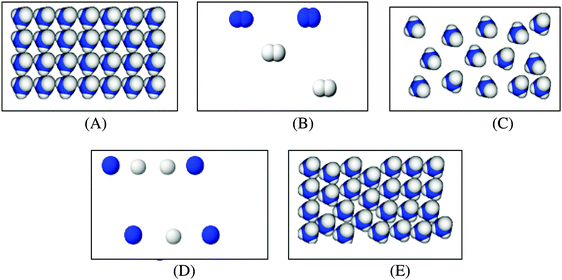
It became evident that students' often fell into miscategorization between macroscopic matter and microscopic particle categories (Fig. 4). Chi et al. (1994) state that Fig. 1 shows the possible/plausible structure of the ontological categories, therefore there might be other major ontological categories and subcategories as well. From our research, it emerged that macroscopic matter and microscopic particle categories are two possible subcategories of nonliving. Students might assume a microscopic particle to be as macroscopic matter; this is an indication of miscategorization between the two categories. In this case, students attribute properties of macroscopic matter to microscopic particles.
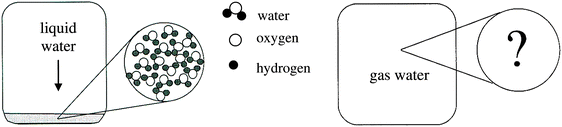
For instance, one of the identified misconceptions was ‘since ice is solid its molecules are solid and since water is liquid its molecules are liquid’. Water and ice belong to macroscopic matter category because being in a physical phase is an attribute of macroscopic matter. In contrast, water and ice molecules belong to the microscopic particle category and so, they cannot be in solid or liquid phase. An ontological perspective would suggest that students were miscategorizing between these two categories by attributing properties of the macroscopic matter to its microscopic particles.
Another misconception related with these two categories was reflected in the response ‘since iron heats during it melts, its atoms also heat and so atoms melt and their volume increases'. Because iron belongs to the macroscopic matter category it is an appropriate expression to tell that when iron melts its volume increases. However, iron atoms belong to the microscopic particle category and thinking that they melt would be an erroneous idea. In this case, students again engage in miscategorization by attributing the properties of macroscopic iron (melting) to the iron atoms. These and similar misconceptions are an indication of miscategorizing between the macroscopic matter and microscopic particle categories.
Another example is the misconception that ‘freezing makes water molecules larger’ (expressed by 30.5% of students in total). Freezing is a property of macroscopic matter. When water freezes, its volume increases but the volume of water molecules does not change. By thinking that water molecules get larger, students attribute the property of water to its molecules. This misconception suggests that students miscategorize between the macroscopic matter and microscopic particle categories.
About a quarter of the student sample thought that ‘water molecules break up to oxygen and hydrogen atoms when water evaporates’ (25.1%). The concept of evaporation was considered as a chemical event instead of a physical event. From ontological perspective, the students engaged in miscategorization between two Processes sub-categories, physical event and chemical event (Fig. 5).
There were two other misconceptions relevant to such miscategorization. One was the idea that ‘ammonia breaks up to nitrogen and hydrogen gas when it turns from liquid to gas phase’ (12.8%). Evaporation of ammonia is a physical event but the break up of ammonia into nitrogen and hydrogen gases is a chemical event. A similar miscategorization occurred related with sugar dissolution in water. Students thought that ‘when sugar is put into water a chemical reaction happens with the water because sugar melts in water’ (23.3%). Although the dissolution of sugar is a physical event since it dissolves in water but does not melt, the students assumed it a chemical reaction. This again suggests that students miscategorize between the physical event (dissolving) category and chemical event category of Processes.
Discussion
In this study, firstly we developed a diagnostic instrument by utilizing ontological categories as theoretical lens. Secondly, we evaluated student understanding on the topic PNM through assessment based on the relationship between ontology and misconceptions. The instrument development involved rigorous validity work. Some of the items were subject to revisions pertaining to validation and pilot testing. With these revisions we anticipate that our test items as well as the validity of the inferences drawn from the test results improved. For instance, our expectation is that in a further study with the instrument as revised, the percentage of students selecting reason 1 in item 3, would decrease, reducing the false negative responses. Similarly, given the clarification made in item 1, we anticipate that more students would concentrate on the meaning and respond to the item correctly. Nevertheless, we strongly recommend further revision of the items via pilot testing with diverse samples of national and international students.The relatively low percent correct levels (highest: 46.8% for 11th graders in one-tier items) for the Turkish students as compared to others [i.e., Herrmann-Abell and DeBoer's (2008) field test sample] may be explained in terms of the recently reformed middle school science curriculum at the time of the study to include the topic of the PNM, not given specific attention before. This may also explain the unexpected higher percentage of Grade 11 students than Grade 6 students in some of the misconceptions as the high school students primarily experienced learning about matter at the macro level until this time. Grade 6 students in the sample took science classes which were reformed based on the new curricula (MEB, 2006).
The new curriculum specifically focuses on the PNM. For instance, in Grade 6, the third unit is titled “The Particulate Nature of Matter”. In this unit, students learn about matter as being composed of tiny, invisible, and moving particles with space in between. They also learn about the concepts atom, molecule, element and compound. In addition, they classify the changes in matter as physical and chemical by making connection to the particles of matter. Grade 7 curriculum focuses on the symbols of elements and formulas of compounds, the meaning of electron, proton and neutron. In addition, one aim is that students know the relationship between dissolving and the PNM. In Grade 8, students learn the periodic table, chemical bonds and reactions by making connections to the PNM. This suggests that middle school students in the sample were explicitly taught the PNM but the high school students were less exposed to this topic when they were in middle school. This might affect their understandings of PNM concepts resulting in higher percentages of certain misconceptions. For instance, in general more of the high than middle school students thought that “when dry ice melts (turns liquid), the space between its molecules increases”, “when water at 24 °C is cooled to 0 °C and freezes, the water molecules stop moving”, “freezing makes water molecules larger” “water molecules break up to oxygen and hydrogen atoms when water evaporates”, and “during melting iron heats up, thus the iron atoms are also heating up, melting and expanding.” Those misconceptions are related with phase changes and their connection to the particles of matter which is the focus of the new curriculum but not of the old curriculum. This might explain why certain misconceptions were prevalent in high school students.
In this research, students' achievement on the test increased with increasing level of grades. Both one- and two-tier items were answered more successfully by the upper level students than the lower level students. Upper grade students' achievement on the two-tier items revealed that these students were better in understanding the underlying reasons of phenomena. Since the PNM is a difficult topic to learn, it requires higher level cognitive abilities for most primary school students (Johnson and Papageorgiou, 2010), and maturation by age is an important factor in progression of students' understanding (Rahayu and Kita, 2010). Some studies suggest that the PNM unit should be taught in higher grade levels in the curriculum (Fensham, 1994; Harrison and Treagust, 2002). These suggestions are consistent with Piaget's cognitive development theory. The theory states that older students have more developed formal thinking ability. Therefore, the result that we identified also might be related with students' cognitive development. Based on Piaget's cognitive development levels children who are 7–11 years old are in concrete operational stage and children who are 11 years and older are in formal operational stage. In this view, upper grade students have developed abstract thinking ability but this ability is not fully developed by primary school students. Therefore, two-tier items are likely to be answered more properly by secondary school students.
On the other hand, Liu and Lesniak (2006) suggest that “children's matter concept development is attributable to not only maturation by age but also school context, such as curriculum and instruction” (p. 322). Therefore, this result might be also related with the science curriculum that is conducted in middle and high schools in Turkey. Students are taught the unit of matter and other related units several times from middle school to high school (MEB, 2006). That means, for instance, students in 11th grade learn the unit of matter both in middle and high school. In middle school they learn the PNM, the phases, changes and properties of matter; in high school these topics are covered again but in more detail. Therefore, their understandings might have deepened as they progress grade wise.
Conclusions
Our findings suggest that overall, both middle and high school students have misconceptions about the PNM. An unexpected finding of this study was that in some cases misconception percentages were higher for high school than middle school students. This study also shed light on the ontological categories of the concepts as well as the categories to which the students assigned the concepts. The results suggested that the categories that the students assigned the concepts were ontologically different than the categories they scientifically belonged.Considering the most prevalent misconceptions as well as the items with percent correct of 10% or lower, it is clear that the students in the sample most often tended to attribute the properties of macroscopic matter to its microscopic particles, and that they had most difficulty in explaining dissolving at the particle level. For instance, ideas such as “since leaves are alive, atoms in the leaves are also alive; if water takes the shape of the container then water molecules also change shape to fit” were amongst the misconception choices selected by at least 20% of the students. These findings relate to findings in other studies conducted both in Turkey and in other countries concluding that students often think of properties of bulk matter to be observed in its particles, as well. For instance, it is reported that some students think that when matter is heated, the atoms would heat up (Lee et al., 1993; Boz, 2006), melt (Boz, 2006), vaporize (Griffths and Preston, 1992; Kokkotas et al., 1998), expand (Griffths and Preston, 1992; Lee et al., 1993; Kokkotas et al., 1998; Stepans, 2003; Kind, 2004), or condense (Lee et al., 1993). In these cases, students are attributing the characteristics of one ontological subcategory, macroscopic matter, to cases of another, microscopic matter. From an ontological point of view, mismatching reasoning as evident in the findings of the current study and previous research, results in misconceptions.
The misconceptions revealed in this research were related with two ontological categories (Matter, Processes) and most of them occurred resulting from a mismatch between macroscopic matter and microscopic particle categories. This is a similar result with Othman et al.'s (2008). The researchers observed that 34.3% of the 9th grade and 25.8% of the 10th grade students thought that an atom is the smallest particle which has the same properties of elements. Students thought that atoms have the same physical properties of their elements. This misconception was held by 55.7% of the 9th grade and 54.2% of the 10th grade students. Students attributed the properties of the macroscopic matter to their atoms and molecules.
Overall, this study explored the ontological categories of the concepts as well as those to which the students assigned the concepts, which may be different than the categories that they scientifically belong. The results of this study add to the literature documenting student misconceptions about the PNM and provide insight about the ontological nature of students' knowledge structures of the PNM.
Implications
This research has important implications for chemistry education. The research results could have a directive role for teachers. Also, this study is an important step for understanding misconceptions regarding the particulate nature of matter on the basis of ontological categories.In this research, misconceptions were approached from ontology perspective because this perspective illuminates reasons of student misconceptions in more depth. In other words, ontology facilitates to explain the way students think and possess misconceptions. Being sensitive to misconceptions and their underlying reasons might benefit both teachers and students. Teachers who are aware of their students' misconceptions may plan their teaching based on student thinking and so, may provide better teaching context. Also, as in the process of radical conceptual change, they can teach the ontological categories in their classrooms and help their students to ontologically categorize their concepts.
According to Sadler (1998) distractor-driven multiple choice assessments based on alternative conceptions identified through qualitative studies are powerful and reliable tests measuring conceptual understanding. In this study, we also attempted to augment the power of distractor-driven multiple choice items with a second tier requiring reasoning. Furthermore, our construction of the distractors addressing chemical misconceptions of the PNM was informed by the theory of ontological categories. Studies such as the current one are important for science educators and teachers as they evaluate student conceptions and reveal misconceptions in specific topics significant to enhance achievement in science.
Distractor-driven, two-tier multiple-choice items such as those developed in the current work may serve to aid chemistry teachers, teacher educators and stakeholders in curriculum development and implementation by illuminating student conceptions and ways of thinking in depth. Furthermore, understanding student misconceptions from ontology perspective may assist educators to effectively enhance a conceptual change via experiences of radical conceptual change toward scientific competency. Such an analysis is promising in terms of evaluating curricula, finding weak areas in our teaching and optimizing teaching methods to meet the goals of science education reforms.
Appendix A: the particulate nature of matter diagnostic test
In this test, there are 25 questions about the particulate nature of matter. Please read the questions carefully. In the first 15 questions, select the correct answer choice of the question then select your reason statement that most appropriately explains why your answer is correct. Since there are no reason statements after the 16th question, you only need to mark one of the correct answer choices. Please circle the correct answers.(1) Green leaves (the ones on the tree) are comprised of living cells, and these cells involve atoms. Also, the element iron is comprised of atoms. Accordingly:
(A) The atoms in the leaf are alive.
(B) The atoms in the iron are alive.
(C) The atoms in the leaf and iron are nonliving.
(D) The atoms in the leaf and iron are alive.
Reason:
1. The atoms in the iron are alive because they are moving.
2. Atoms do not have living characteristics.
3. Since leaves are alive their atoms are also alive.
4. No matter the kind all atoms are alive.
5. None of the above. For me, the reason is:
(2) Which statements below is true regarding to the properties of gold atoms?
I. Gold atoms are shiny and hard.
II. If gold is heated, its atoms heat up as well.
III. When gold is shaped its atoms take the same shape.
IV. Most of the volume of gold atoms is empty space.
(A) Only I (B) Only II (C) Only III (D) Only IV (E) I, II, and III
Reason:
1. Any outside change made to gold affects the gold atoms in the same way.
2. If we compare the volume of nucleus and atom, the volume of the nucleus is so much smaller than the volume of the atom (If atomic volume is considered as a football field, volume of the nucleus would be like a ball in this field). Therefore, the rest of the volume of the atom is empty space.
3. All properties of gold exist in its atoms as well.
4. None of the above. For me, the reason is:
(3) If a teaspoon of sugar is put into a glass of water at room temperature, a chemical reaction occurs between sugar and water.
(A) True (B) False
Reason:
1. When sugar is dissolved in water, a new compound forms.
2. Sugar melts in water.
3. When sugar is dissolved in water, it turns into water.
4. When sugar is dissolved in water, water molecules surround sugar molecules.
5. None of the above. For me, the reason is:
(4) Which statement below is true regarding to the properties of gold atoms?
(A) The size of the water molecules in the solid phase is the largest, in the liquid phase is the smallest.
(B) The size of the water molecules in the solid phase is the smallest, in the gas phase is the largest.
(C) The size of the water molecules is same in the solid, liquid and gas phase.
(D) The size of the water molecules in the liquid phase is the largest, in the solid phase is the smallest.
Reason:
1. The volume of a molecule increases from solid to liquid phase and from liquid to gas phase.
2. The volume of molecules does not alter during phase change.
3. The volume of a molecule decreases from solid to liquid phase and from liquid to gas phase.
4. None of the above. For me, the reason is:
(5) The atoms of iron do not move in solid phase.
(A) True (B) False
Reason:
1. Atoms vibrate in solid phase.
2. Atoms do not move in solid phase because there is no space in between them.
3. Atoms do not move in solid phase since it is the most regular phase of matter.
4. None of the above. For me, the reason is:
(6) Liquids take the shape of their container. Accordingly;
The shape of water molecules changes depending on the shape of the container.
(A) True (B) False
Reason:
1. Since water molecules are solid their shape does not change.
2. Water molecules are flexible.
3. Whatever the shape of the container is, the shape of the molecules does not change.
4. Water molecules have the shape of water droplets.
5. None of the above. For me, the reason is:
(7) Which statement is true regarding ice and water molecules?
(A) Ice molecules are solid, water molecules are liquid.
(B) Both ice and water molecules are solid.
(C) Both ice and water molecules are liquid.
(D) Molecules cannot be in solid or liquid phase
Reason:
1. Being in solid or liquid phase of matter is related with the interactions between its molecules
2. Molecules are always in liquid phase.
3. Ice molecules are solid because ice is solid and water molecules are liquid because water is liquid.
4. Molecules are always in solid phase.
5. None of the above. For me, the reason is:
(8) The smallest particle of alcohol is a drop of alcohol, the smallest particle of sugar is a sugar crystal.
(A) True (B) False
Reason:
1. The particles of sugar and alcohol are the same.
2. Alcohol consists of alcohol molecules; sugar consists of sugar molecules.
3. The smallest particles of sugar and alcohol are their smallest parts that are visible.
4. None of the above. For me, the reason is:
(9) When water is held in the refrigerator, it freezes and turns into ice. During this, water molecules................
I. Cool down II. Freeze III. Shrink IV. Expand V. Do not alter
(A) Only IV (B) Only V (C) I and II (D) I, II and III (E) I, II and IV
Reason:
1. Since the temperature decreases during freezing, the temperature of molecules decrease as well, therefore molecules freeze.
2. Since the temperature decreases during freezing, the temperature of molecules decrease therefore molecules freeze and their volume decrease.
3. Freezing do not make any change on molecules.
4. Since the temperature decreases during freezing, the temperature of molecules decrease therefore molecules freeze and their volume increase.
5. Since volume of water increases during freezing, its molecules expand.
6. None of the above. For me, the reason is:
(10) When a piece of iron melts through heating, iron atoms...............
I. Heat up II. Melt III. Expand IV. Do not alter V. Shrink
(A) Only IV (B) Only V (C) I and II (D) II and III (E) I, II and III
Reason:
1. Since volume decreases during melting, iron atoms shrink.
2. Since iron is heated during melting, its atoms heat up as well, therefore the atoms melt and their volume increases.
3. Melting does not cause a change in atoms.
4. The temperature of atoms does not change during melting but atoms melt therefore their volume increases.
5. Since the temperature increases during melting, atoms heat up and melt. No other changes happen.
6. None of the above. For me, the reason is:
(11) Assume a beaker (heat resistant glass container) of pure water has been boiling for 30 minutes. What is/are in the bubbles in the boiling water?
(A) Air
(B) Oxygen gas and hydrogen gas
(C) Oxygen
(D) Water vapor (water in the gaseous state)
(E) Heat
Reason:
1. The hydrogen and oxygen atoms in water molecules break away from each other to form gases.
2. Heating gives the particles more energy and they are able to break away from their attractions. As the particles break apart, the air between the particles is released in the form of bubbles.
3. Heat energy is absorbed by the water and released as bubbles.
4. The forces between the water molecules are overcome, and the water molecules break free from the liquid to form steam.
Oxygen dissolved in water is expelled as air bubbles.
(12) Information: Iodine has chemical symbol I and atomic number 53; it is an element in group VIIA of the Periodic Table. It is a dark grey-dark purple solid at room temperature.
A 1.0 g sample of solid iodine is placed in a tube and the tube is sealed after all of the air is removed. The total mass of the tube and the solid iodine is 27.0 g.
The tube is then heated until all of the iodine evaporates and the tube is filled with iodine gas. The mass after heating will be
(A) less than 27.0 g
(B) 27.0 g
(C) more than 27.0 g
Reason:
1. A gas weighs less than a solid.
2. Mass is conserved.
3. The particles become more spread out when the iodine becomes a gas.
4. Iodine gas is lighter than air.
(13) The circle on the left shows a magnified view of a very small portion of liquid water in a closed container.
What would the magnified view show after the water evaporates?
Reason:
1. Water molecules have decomposed into oxygen atoms and hydrogen atoms.
2. Water molecules have escaped into the air.
3. Water molecules have decomposed into oxygen and hydrogen gas.
4. Water molecules have spread further apart.
5. A mixture of water molecules, oxygen atoms and hydrogen atoms is produced.
(14) Crystals of sugar are placed in a beaker (heat-resistant glass container) of water (Case I). If the mixture is left to stand long enough, the sugar crystals eventually can no longer be seen, and the water will taste sweet (Case II).
(A) True (B) False
Reason:
1. The sugar molecules gain heat from the surrounding and melt, forming a liquid. This liquid mixes with the water.
2. The sugar fills the air spaces in the water and therefore ‘disappears’.
3. Water molecules surround sugar molecules on the surfaces of the crystals and pull them away from the crystal lattice.
4. The sugar crystals will only dissolve when stirred. Stirring causes the sugar crystals to break up into smaller particles that will then spread in the water and can no longer be seen.
(15) Information: Sulphur has chemical symbol S and atomic number 16; it is an element in group VIA of the Periodic Table. It is a lemon yellow solid at room temperature.
A sample of solid sulphur has the following properties:
I. brittle,
II. melting point 113 °C.
Which, if any, of the above properties would be the same for one single atom of sulphur obtained from the sample?
(A) I and II
(B) I only
(C) II only
(D) None of the properties
Reason:
1. An atom is the smallest particle of an element that has the same properties as the element.
2. A sulphur atom has smooth faces and sharp edges and so breaks easily when a force is applied.
3. Sulphur is a non-metal therefore the sulphur atom melts at a relatively low temperature.
4. The properties of an element are a result of the interactions of its individual particles.
(16) Consider three samples of water in three phases. The first is solid water (ice) at 0 °C, the second is liquid water at 24 °C, and the third is gaseous water at 100 °C. The water molecules in the liquid phase __________ the water molecules in the solid phase.
(A) Move faster than
(B) Move slower than
(C) Move at the same speed as
(D) Move less randomly than
(E) Travel in the same direction as
(17) Which of the following processes will make water molecules larger?
(A) Freezing
(B) Melting
(C) Evaporation
(D) Condensation
(E) None of the above
(18) When water is vaporized, it is changed to
(A) Hydrogen and oxygen
(B) Hydrogen only
(C) Gaseous water
(D) Air, hydrogen, and oxygen
(E) Oxygen only
(19) A pot of water on a hot stove begins to boil rapidly. A glass lid is placed on the pot and water droplets begin forming on the inside of the lid. What happened?
(A) The lid became sweaty.
(B) Steam cools and water molecules move closer together.
(C) Water from outside leaked into the pot.
(D) Hydrogen and oxygen combined to form water.
(E) Steam combined with the air to wet the inside of the lid.
(20) A wet dinner plate is left on the counter after it has been washed. After a while it is dry. What happens to the water that did not drip onto the counter?
(A) It changes to carbon dioxide.
(B) It just dries up and no longer exists as anything.
(C) It goes into the air as molecules of water.
(D) It goes into the plate.
(E) It changes to oxygen and hydrogen in the air.
(21) When water molecules in the gas phase are heated, the molecules themselves
(A) Expand.
(B) Move faster.
(C) Become less massive.
(D) Change to a liquid.
(E) Release air.
(22) Which of the following processes will make molecules smaller?
(A) Freezing
(B) Melting
(C) Evaporation
(D) Condensation
(E) None of the above
(23) A diagram representing carbon dioxide molecules in the solid phase, also known as dry ice, is shown below.
Which of these molecular diagrams best shows what dry ice would look like after it melts (changes to a liquid)?
(24) When water at 24 °C is cooled to 0 °C and freezes, the water molecules
(A) Become less organized.
(B) Move much faster.
(C) Stop moving.
(D) Break apart.
(E) Move much more slowly.
(25) A sample of liquid ammonia (NH3) is completely evaporated (changed to a gas) in a closed container as shown:
Which of the following diagrams best represents what you would “see” in the same area of the magnified view of the vapor?
Appendix B: questions asked during pilot testing
1. Is there anything about this test question that was confusing? Explain.2. Circle any words on the test question you don't understand or aren't familiar with.
3. Is answer choice A correct? Yes No Not Sure
Explain why or why not.
4. Is answer choice B correct? Yes No Not Sure
Explain why or why not.
5. Is answer choice C correct? Yes No Not Sure
Explain why or why not.
6. Is answer choice D correct? Yes No Not Sure
Explain why or why not.
7. Is answer choice E correct? Yes No Not Sure
Explain why or why not.
8. Did you guess? Yes No
9. Should there be any other answer choice? Yes No
If you think so, what are they?
10. Was the picture or graph helpful? Yes No
Why or why not? [Or, if there was no picture…]
Would a picture or graph be helpful? Why or why not? Yes No
11. Have you studied this topic in school? Yes No Not Sure
12. Have you learned about it somewhere else? Yes No Not Sure
Where? (TV, museum, etc.)?
13. Is there anything about the reasons of this test question that was confusing? Explain.
14. Circle any words on the reasons of this test question you don't understand or aren't familiar with.
15. Should there be any other reason choice? Yes No
If you think so, what are they?
References
- Adadan E., (2014), Investigating the influence of pre-service chemistry teachers' understanding of the particle nature of matter on their conceptual understanding of solution chemistry, Chem. Educ. Res. Pract., 15, 219–238.
- American Association for the Advancement of Science, (1993), Benchmarks for science literacy, Project 2061, New York: Oxford University Press.
- Arnaudin M. W. and Mintzes J. J., (1985), Students' alternative conceptions of the human circulatory system: across age study, Sci. Educ., 69(5), 721–733.
- Ayas A., Özmen H. and Çalik M., (2010), Students' conceptions of the PNM at secondary and tertiary level, Int. J. Sci. Math. Educ., 8, 165–184.
- Boz Y., (2006), Turkish pupils' conceptions of the particulate nature of matter, J. Sci. Educ. Technol., 15(2), 203–213.
- Bridle C. A. and Yezierski E. J., (2012), Evidence for the effectiveness of inquiry-based, particulate-level instruction on conceptions of the PNM, J. Chem. Educ., 89, 192–198.
- Caramazza A., McCloskey M. and Green B., (1981), Naive beliefs in “sophisticated” subjects: Misconceptions about trajectories of objects, Cognition, 9, 117–123.
- Chi M. T. H., (1992), Conceptual change within and across ontological categories: Examples from learning and discovery in science, in Giere R. (ed.), Cognitive Models of Science: Minnesota Studies in the Philosophy of Science, Minneapolis, MN: University of Minnesota Press, pp. 129–186.
- Chi M. T. H., (1997), Creativity: Shifting across ontological categories flexibly, in Ward T. B., Smith S. M. and Vaid J. (ed.), Conceptual Structures and Processes: Emergence, Discovery and Change, Washington, D.C.: American Psychological Association, pp. 209–234.
- Chi M. T. H., (2008), Three types of conceptual change: belief revision, mental model transformation, and categorical shift, in Vosniadou S. (ed.), Handbook of research on conceptual change, Hillsdale, NJ: Erlbaum, pp. 61–82.
- Chi M. T. H. and Hausmann R. G. M., (2003), Do radical discoveries require ontological shifts? in Shavinina L. V. and Sternberg R. (ed.), International Handbook on Innovation, New York: Elsevier Science, pp. 430–444.
- Chi M. T. H. and Roscoe R. D., (2002), The processes and challenges of conceptual change, in Limon M. and Mason L. (ed.), Reconsidering Conceptual Change: Issues in Theory and Practice, The Netherlands: Kluwer Academic Publishers, pp. 3–27.
- Chi M. T. H. and Slotta J. D., (1993), The ontological coherence of intuitive physics, Cognition Instruct., 10, 249–260.
- Chi M. T. H., Slotta J. D. and Leeuw N., (1994), From things to processes: a theory of conceptual change for learning science concepts, Learn. Instr., 4(1), 27–43.
- Chu H., Treagust D. F. and Chandrasegaran A. L., (2009), A stratified study of students' understanding of basic optics concepts in different contexts using two-tier multiple-choice items, Res. Sci. Technol Educ., 27, 253–265.
- DeBoer G. E., Dubois N., Herrmann-Abell C. F. and Lennon K., (2008), Assessment linked to middle school science learning goals: using pilot testing in item development, paper presented at the Annual Meeting of the National Association for Research in Science Teaching, Baltimore, MD.
- Driver R., (1981), Pupils' alternative frameworks in science, Eur. J. Sci. Educ., 3(1), 93–101.
- Duit R. and Treagust D. F., (1995), Students' conceptions and constructivist teaching approaches, Chicago, IL: The National Society for the Study of Education.
- Fensham P., (1994), Beginning to teach chemistry, in Fensham P., Gunstone R., White R. (ed.), The content of science: a constructivist approach to its teaching and learning, London: Falmer, pp. 14–28.
- Fraenkel J. R. and Wallen N. E., (2003), How to design and evaluate research in education, 5th edn, New York: McGraw-Hill.
- Gallegos L., Jerezano M. E. and Flores F., (1994), Preconceptions and relations used by children in the construction of food chains, J. Res. Sci. Teach., 31(3), 259–272.
- Goldston M. J. and Downey L., (2012), Your Science Classroom: Becoming an Elementary/Middle School Science Teacher, Thousand Oaks, CA: Sage Publications.
- Gómez E. J., Benarroch A. and Marín N., (2006), Evaluation of the degree of coherence found in students' conceptions concerning the PNM, J. Res. Sci. Teach., 43(6), 577–598.
- Griffths A. K. and Preston K. R., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29(6), 611–628.
- Harrison A. G. and Treagust D. F., (2002), The particulate nature of matter: challenges in understanding the microscopic world, in Gilbert J. K. et al. (ed.), Chemical Education: Towards Research-Based Practice, Dordrecht: Kluwer Academic.
- Herrmann-Abell C. F. and DeBoer G. E., (2008), An analysis of field test results for assessment items aligned to the middle school topic of atoms, molecules, and states of matter, paper presented at the Annual Meeting of the National Association for Research in Science Teaching, Baltimore, MD.
- Hewson M. G. and Hewson P. W., (1983), Effect of instruction using students' prior knowledge and conceptual change strategies on science learning, J. Res. Sci. Teach., 20, 731–743.
- Johnson P. and Papageorgiou G., (2010), Rethinking the introduction of particle theory: a substance-based framework, J. Res. Sci. Teach., 47(2), 130–150.
- Johnston A. T. and Southerland S. A., (2000), A reconsideration of science misconceptions using ontological categories, paper presented at the Annual Meeting of the National Association for Research in Science Teaching, New Orleans, LA.
- Kahveci A., (2009), Exploring chemistry teacher candidates' profile characteristics, teaching attitudes and beliefs, and chemistry conceptions, Chem. Educ. Res. Pract., 10, 109–120.
- Kahveci A., (2013), Diagnostic assessment of student understanding of the particulate nature of matter: Decades of research, in Tsaparlis G. and Sevian H. (ed.), Concepts of Matter in Science Education, Dordrecht: Springer, pp. 249–278.
- Keeley P., Eberle F. and Farrin L., (2005), Uncovering student ideas in science, Arlington, VA: NSTA Press.
- Kind V., (2004), Beyond appearances: students' misconceptions about basic chemical ideas, 2nd edn, Durham: Royal Society of Chemistry.
- Kokkotas P., Vlachos I. and Koulaidis V., (1998), Teaching the topic of the particulate nature of matter in prospective teachers' training courses, Int. J. Sci. Educ., 20(3), 291–303.
- Lee O., Eichinger D. C. Anderson C. W., Berkheimer G. D. and Blakeslee T. D., (1993), Changing middle school students' conceptions of matter and molecules, J. Res. Sci. Teach., 30(3), 249–270.
- Liu X. and Lesniak K. M., (2006), Progression in children's understanding of the matter concept from elementary to high school, J. Res. Sci. Teach., 43(3), 320–347.
- Margel H., Eylon B. and Scherz Z., (2004), “We actually saw atoms with our own eyes”. Conceptions and convictions in using the scanning tunneling microscope in junior high school, J. Chem. Educ., 81(4), 558–566.
- Marques L. and Thompson D., (1997), Misconceptions and conceptual changes concerning continental drift and plate tectonics among Portuguese students aged 16–17, Res. Sci. Technol. Educ., 15, 195–222.
- MEB, (2006), Ilkogretim fen ve teknoloji dersi (6, 7 ve 8. siniflar) ogretim programi. Ankara: Milli Egitim Bakanligi Talim ve Terbiye Kurulu Başkanlığı.
- Moss D. M. and Abrams E. D., (1999), Describing student understandings of unifying concepts in science, paper presented at the Annual Meeting of the National Association for Research in Science Teaching, Boston, MA.
- Nakhleh M. B., Samarapungavan A. and Sağlam Y., (2005), Middle school students' beliefs about matter, J. Res. Sci. Teach., 42(5), 581–612.
- National Research Council., (1996), National Science Education Standards, Washington, D.C.: National Academy Press: National Research Council.
- Nyachwaya J. M., Mohamed A. R., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students' understanding of the PNM, Chem. Educ. Res. Pract., 12, 121–132.
- Osborn R. J. and Freyberg P., (1985), Learning in science: the implications of children's science, Auckland: Heinemann.
- Othman J., Treagust D. and Chandrasegaran A. L., (2008), An investigation into the relationship between students' conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 1–20.
- Øyehaug A. B. and Holt A., (2013), Students' understanding of the nature of matter and chemical reactions – a longitudinal study of conceptual restructuring, Chem. Educ. Res. Pract., 14, 450–467.
- Ozmen H., (2011), Turkish primary students' conceptions about the PNM, Int. J. Environ. Sci. Educ., 6(1), 99–121.
- Pinto R. M. A. and Silvestre S. M., (2014), Pharmaceutical green chemistry: is just green chemistry or is something else more? J. Chem. Eng. Chem. Res., 1(5), 290–301.
- Rahayu S. and Kita M., (2010), An analysis of Indonesian and Japanese students' understandings of macroscopic and submicroscopic levels of representing matter and its changes, Int. J. Sci. Math. Educ., 8(4), 667–688.
- Sadler P. M., (1998), Psychometric models of student conceptions in science: reconciling qualitative studies and distractor-driven assessment instruments, J. Res. Sci. Teach., 35(3), 265–296.
- Sanders M., (1993), Erroneous ideas about respiration: the teacher factor, J. Res. Sci. Teach., 30(8), 919–934.
- Smith J. P., diSessa A. A. and Roschelle J., (1993), Misconceptions reconceived: a constructivist analysis of knowledge in transition, J. Learn. Sci., 3(2), 115–163.
- Stepans J., (2003), Targeting students' science misconceptions: physical science concepts using the conceptual change model, Tampa, FL: Showboard.
- Tezcan H. and Salmaz, Ç., (2005), Atomun yapısının kavratılmasında ve yanlış kavramaların giderilmesinde bütünleştirici ve geleneksel ögretim yöntemlerinin etkileri, Gazi Eğitim Fakültesi Dergisi, 25(1), 41–54.
- Tsitsipis G., Stamovlasis D. and Papageorgiou G., (2011), A probabilistic model for students' errors and misconceptions on the structure of matter in relation to three cognitive variables, Int. J. Sci. Math. Educ., 10, 777–802.
- Wandersee J. H., Mintzees J. J. and Novak J. D., (1994), Research on Alternative Conceptions in Science, in Gabel D. (ed.), Handbook of Research on Science Teaching and Learning, New York: Macmillan Publishing Company, pp. 177–210.
- Yezierski E. J. and Birk J. P., (2006), Misconceptions about the particulate nature of matter, J. Chem. Educ., 83(6), 954–960.
| This journal is © The Royal Society of Chemistry 2015 |