Gold-catalyzed tandem cycloisomerization/Petasis–Ferrier rearrangement: a direct route to 3-alkoxyindanones from enynals and alcohols†
Ziping Cao*a,
Huaqing Zhanga,
Xiaoxiang Zhangb,
Ludan Zhanga,
Xin Menga,
Guang Chena,
Xian-En Zhaoa,
Xuejun Suna and
Jinmao You*a
aThe Key Laboratory of Life-Organic Analysis, Key Laboratory of Pharmaceutical Intermediates and Analysis of Natural Medicine, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, Shandong, P. R. China. E-mail: caozp_qfnu@163.com; jmyou6304@163.com
bCollege of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, P. R. China
First published on 27th November 2015
Abstract
A method to prepare 3-alkoxyindanones efficiently by gold-catalyzed tandem cycloisomerization/Petasis–Ferrier rearrangement from ortho-ethynylarylaldehydes with alcohols is described. The reaction was accomplished in moderate to excellent yields under mild reaction conditions and also offers a convenient synthetic route to 3-alkoxycyclopentenones.
Homogeneous gold catalysis has emerged in the past few years as one of the most powerful methods for the formation of cyclic compounds through the cycloisomerization reactions and skeleton reassembly.1,2 Among them, the cyclization processes involving carbonyl-yne motifs have been thoroughly studied to afford versatile products.3 Particularly, ortho-alkynylarylaldehydes as substrates could be converted to a variety of organic molecules.4 For example, Yamamoto et al.5 reported the synthesis of the naphthyl ketone derivatives from ortho-alkynylarylaldehydes and alkynes by gold catalysis (Scheme 1, eqn (1)). Dyker and other groups extended this methodology for the synthesis of other naphthalene derivatives.6 In addition, ortho-alkynylarylaldehydes could react with a series of nucleophiles to generate 1H-isochromene derivatives4,7 (Scheme 1, eqn (2)) or complex polycyclic molecules rapidly under mild reaction conditions.4,8 Following our ongoing interest in the development of gold-catalyzed tandem reactions,9 and encouraged by these pioneering works, we intended to explore new transformations of ortho-alkynylarylaldehydes.
We envisioned that o-ethynylbenzaldehyde 1a which proceeded through hemiacetalization/5-exo-dig cycloisomeriz-ation to give cyclic intermediate 2 under gold catalysis with alcohols would afford the skeleton rearrangement product 3 (Scheme 1, eqn (3)). In a related study, Toste and co-workers reported gold-catalyzed carboalkoxylation of pre-installed acetals with an alkyne motif for the synthesis of 3-alkoxyindanones.10 In addition, Chan group reported gold-catalyzed intramolecular tandem heterocyclization/Petasis–Ferrier rearrangement with the O- or N-linked aldehyde and alkyne as substrates.11 Furthermore, the intermolecular rearrangements of aldehydes or acetals with terminal alkynes were also reported.12
Based on these precedents, we anticipated that the designed tandem process would be possible (Scheme 1, eqn (3)). And the major challenges were to enable 5-exo-dig cycloisomerization with slow protodeauration and Petasis–Ferrier rearrangement to take place. Thus we utilized ortho-ethynylarylaldehydes with terminal alkynes as substrates to develop a gold catalyzed tandem reaction, which involves cycloisomerization/Petasis–Ferrier rearrangement sequence. Herein, we provide a method for the direct gold-catalyzed synthesis of 3-alkoxyindanones from ortho-ethynylarylaldehydes and alcohols. Notably, these new strategies also offer a practical and efficient way to synthesize indanone derivatives, which are very important building blocks in natural products and biological research.13
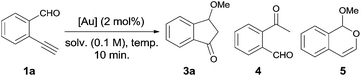
First, we tested the reaction of readily available o-ethynylbenzaldehyde 1a in different solvents by a variety of catalysts to establish the reaction conditions (Table 1). This revealed treating a solution of reaction containing 1a (0.1 mmol) and methanol (1 mL, 0.1 M) with 2 mol% of Ph3PAuNTf2 at 80 °C for 10 min gave 3a in 76% yield. In this reaction, the hydrolytic product 4 and 6-endo-dig cyclized product 5 as side products detected by 1H NMR spectroscopy were obtained in 5% and 6% yields, respectively (Table 1, entry 1). It is worth mentioning that the substrate 1a was consumed completely in 10 minutes without any protection. In an attempt to improve the yield of 3a, several gold catalysts were screened (Table 1, entries 2–7). When inorganic gold salt NaAuCl4·2H2O was used as the catalyst, the desired product 3a could be obtained in 88% yield after isolation, which was the best reaction conditions (Table 1, entry 7). Other catalysts such as PtCl2 can also afford the desired compound 3a in 35% yield (Table 1, entry 8). However, neither AgNTf2 nor HNTf2 as the catalyst could deliver the expected product 3a (Table 1, entries 9 and 10). In addition, both decreasing the reaction temperature and changing the solvents couldn't improve the yield of 3a (Table 1, entries 11–13). Finally, lower product yield would be obtained when the catalyst loading of 6 was reduced to 1 mol% (Table 1, entry 14).
| Entry | Cat. | Solv. | Temp. (°C) | Yieldb (3a, %) |
|---|---|---|---|---|
| a Reaction conditions: substrate 1a (0.1 mmol), catalyst (2 mol%), and solvent (0.1 M) in air unless otherwise specified.b Yields determined by 1H NMR spectroscopy with 1,3,5-trimethoxybenzene as an internal standard. Yields of isolated product given within parentheses.c 4 and 5 were detected as minor side products.d See Table 2, footnote a.e 1 mol% catalyst was used. | ||||
| 1 | Ph3PAuNTf2 | MeOH | 80 | 76c |
| 2 | Cy3PAuNTf2 | MeOH | 80 | 15 |
| 3 | (2,4-tBu2-C6H3O)3PAuNTf2 | MeOH | 80 | 10 |
| 4 | AuCl·Me2S | MeOH | 80 | 50 |
| 5 | AuCl3 | MeOH | 80 | 83 |
| 6 | NaAuCl4·2H2O (6) | MeOH | 80 | 89 |
| 7d | 6 | MeOH | 80 | 95 (88) |
| 8 | PtCl2 | MeOH | 80 | 35 |
| 9 | AgNTf2 | MeOH | 80 | 0 |
| 10 | HNTf2 | MeOH | 80 | 0 |
| 11d | 6 | MeOH | 60 | 73 |
| 12 | 6 | Toluene/MeOH(10/1) | 100 | 85 |
| 13 | 6 | DCE/MeOH(10/1) | 80 | 50 |
| 14e | 6 | MeOH | 80 | 82 |
Having established the optimized reaction conditions, the substrate scope was investigated with a variety of ortho-ethynylarylaldehydes. As shown in Table 2, substrates containing substituted groups such as alkyl, ethers, silyl ethers, and hydroxyl group on the aromatic ring were well-tolerated under the reaction conditions (3a–g). Furthermore, the reaction of the substrates with allyl ether proceeded smoothly in good yields (3h and i). Unfortunately, 2-ethynyl-5-methoxybenzaldehyde and 2-ethynyl-5-fluorobenzaldehyde were not good substrates for the formation of desired products, which resulted in complex mixture. Under the standard conditions, the other alcohols such as ethanol, allyl alcohol and cyclohexanol were not suitable for this transformation.
| a Typical reaction conditions: a solution of 1 (0.1 mmol) in the solvent (0.6 mL) was added to a solution of the catalyst (2 mol%) in the solvent (0.4 mL) via a syringe pump in 5 min at 80 °C and the mixture was stirred for another 5 min.b Isolated yields are shown.c At 50 °C.d Using Ph3PAuNTf2 as a catalyst at 50 °C. |
|---|
 |
Surprisingly, reactions of nonaromatic enynals (1j and k) catalyzed by NaAuCl4·2H2O gave a mixture of side products that could not be identified by 1H NMR analysis. When Ph3PAuNTf2 was used as a catalyst, the desired bicyclic compounds 3j–n were afforded in 64–86% yields (Table 2). Other gold catalysts were also examined, which could not improve the result.14 Notably, ethanol was also a suitable nucleophile for this transformation, which gave the corresponding cyclic products efficiently (3l–n).
To obtain a more detailed insight into the reaction mechanism, the deuterium-labelled methanol was utilized under the standard reaction condition (Table 1, entry 7). The 1H NMR analysis of the crude product obtained from the reaction of 1e in CH3OD under gold catalysis revealed that incorporation of deuterium has occurred at the α position of the carbonyl group (Scheme 2, eqn (1) and (2)). Speculatively, it would be quite likely to have deuterium exchange with the hydrogen of the terminal alkyne via the intermediate gold(I) acetylide.15,16
Based on the above studies, we tentatively propose the following mechanism for gold-catalyzed tandem cycloisomerization/Petasis–Ferrier rearrangement reaction (Scheme 3).11,17 The hemiacetalization of the substrate 1e in MeOD under gold catalyst forms intermediate A, accompanying with the H/D exchange of the terminal alkyne. Subsequently, coordination of the intermediate A to gold catalyst leads to the gold–alkyne complex B, followed by 5-exo-dig cycloisomerization reaction to give the vinyl gold species C. The intermediate D would be formed after C–O bond cleavage and isomerization of C, which is further transformed to the aurated complex E via Petasis–Ferrier rearrangement.18 Finally, the protodeauration of E provides the desired product 7, along with the regeneration of catalyst for the next cycle.
It is important to note that gold-catalyzed rearrangement of ortho-ethynylarylaldehydes and alcohols undergoes a 5-exo-dig cyclization, which is an interesting regioselective nucleophilic addition, and only a few cases were reported using other metal catalysts.19
In summary, we have developed a gold-catalyzed tandem cycloisomerization/Petasis–Ferrier rearrangement process for the synthesis of 3-alkoxyindanones and 4-alkoxycyclopentenones from easily accessible ortho-ethynylarylaldehydes or nonaromatic enynals substrates. This efficient transformation represents a novel reaction cascade to the previously reported strategies. A further study on the detailed reaction mechanism is currently underway in our laboratories.
Acknowledgements
The authors are grateful to the NNSFC (21402106, 21302096), the Project-sponsored by SRF for ROCS, SEM, and the Natural Science Foundation of Shandong Province (ZR2014BQ024), and Research Start-up Foundation of Qufu Normal University (bsqd20130115).Notes and references
- For selected reviews for gold catalysis, see: (a) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028 CrossRef CAS PubMed; (b) A. Fürstner, Chem. Soc. Rev., 2009, 38, 3208 RSC; (c) A. Corma, A. Leyva-Pérez and M. J. Sabater, Chem. Rev., 2011, 111, 1657 CrossRef CAS PubMed; (d) S. M. A. Sohel and R.-S. Liu, Chem. Soc. Rev., 2009, 38, 2269 RSC; (e) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed; (f) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS PubMed; (g) A. Arcadi, Chem. Rev., 2008, 108, 3266 CrossRef CAS PubMed; (h) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS PubMed; (i) L. Zhang, J. Sun and S. A. Kozmin, Adv. Synth. Catal., 2006, 348, 2271 CrossRef CAS; (j) P. Belmont and E. Parker, Eur. J. Org. Chem., 2009, 6075 CrossRef CAS; (k) V. Michelet, P. Y. Toullec and J.-P. Genêt, Angew. Chem., Int. Ed., 2008, 47, 4268 CrossRef CAS PubMed; (l) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (m) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS PubMed; (n) B. J. Ayers and P. W. H. Chan, Synlett, 2015, 26, 1305 CrossRef CAS.
- For selected recent examples for gold catalysis, see: (a) P. Morán-Poladura, E. Rubio and J. M. González, Angew. Chem., Int. Ed., 2015, 54, 3052 CrossRef PubMed; (b) S. Guven, M. S. Ozer, S. Kaya, N. Menges and M. Balci, Org. Lett., 2015, 17, 2660 CrossRef CAS PubMed; (c) K. Speck, K. Karaghiosoff and T. Magauer, Org. Lett., 2015, 17, 1982 CrossRef CAS PubMed; (d) C. Yu, B. Chen, T. Zhou, Q. Tian and G. Zhang, Angew. Chem., Int. Ed., 2015, 54, 10903 CrossRef CAS PubMed; (e) H. Wu, W. Zi, G. Li, H. Lu and F. D. Toste, Angew. Chem., Int. Ed., 2015, 54, 8529 CrossRef CAS PubMed; (f) Y. Tokimizu, S. Oishi, N. Fujii and H. Ohno, Angew. Chem., Int. Ed., 2015, 54, 7862 CrossRef CAS PubMed; (g) H. Peng, N. G. Akhmedov, Y.-F. Liang, N. Jiao and X. Shi, J. Am. Chem. Soc., 2015, 137, 8912 CrossRef CAS PubMed; (h) X.-Q. Mou, Z.-L. Xu, S.-H. Wang, D.-Y. Zhu, J. Wang, W. Bao, S.-J. Zhou, C. Yang and D. Zhang, Chem. Commun., 2015, 51, 12064 RSC; (i) P. Aillard, P. Retailleau, A. Voituriez and A. Marinetti, Chem.–Eur. J., 2015, 21, 11989 CrossRef CAS PubMed; (j) R. K. Shiroodi, M. Sugawara, M. Ratushnyy, D. C. Yarbrough, D. J. Wink and V. Gevorgyan, Org. Lett., 2015, 17, 4062 CrossRef PubMed; (k) W. Rao, D. Susanti, B. J. Ayers and P. W. H. Chan, J. Am. Chem. Soc., 2015, 137, 6350 CrossRef CAS PubMed.
- (a) M. Gulias, J. R. Rodriguez, L. Castedo and J. L. Mascareñas, Org. Lett., 2003, 5, 1975 CrossRef CAS PubMed; (b) A. S. K. Hashmi, L. Schwarz, J.-H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285 CrossRef CAS; (c) S. F. Kirsch, J. T. Binder, C. Liébert and H. Menz, Angew. Chem., Int. Ed., 2006, 45, 5878 CrossRef CAS PubMed; (d) J. Zhang and H.-G. Schmalz, Angew. Chem., Int. Ed., 2006, 45, 6704 CrossRef CAS PubMed; (e) Y. Liu, M. Liu, S. Guo, H. Tu, Y. Zhou and H. Gao, Org. Lett., 2006, 8, 3445 CrossRef CAS PubMed; (f) T. Yao, X. Zhang and R. C. Larock, J. Am. Chem. Soc., 2004, 126, 11164 CrossRef CAS PubMed; (g) T. Yao, X. Zhang and R. C. Larock, J. Org. Chem., 2005, 70, 7679 CrossRef CAS PubMed; (h) E. Jiménez-Núñez, C. K. Claverie, C. Nieto-Oberhuber and A. M. Echavarren, Angew. Chem., Int. Ed., 2006, 45, 5452 CrossRef PubMed; (i) J.-M. Tang, T.-A. Liu and R.-S. Liu, J. Org. Chem., 2008, 73, 8479 CrossRef CAS PubMed; (j) A. Das, H.-K. Chang, C.-H. Yang and R.-S. Liu, Org. Lett., 2008, 10, 4061 CrossRef CAS PubMed; (k) L.-P. Liu, D. Malhotra, R. S. Paton, K. N. Houk and G. B. Hammond, Angew. Chem., Int. Ed., 2010, 49, 9132 CrossRef CAS PubMed; (l) G. Li, X. Huang and L. Zhang, J. Am. Chem. Soc., 2008, 130, 6944 CrossRef CAS PubMed; (m) J. J. Kennedy-Smith, S. T. Staben and F. D. Toste, J. Am. Chem. Soc., 2004, 126, 4526 CrossRef CAS PubMed.
- H. Wang, Y. Kuang and J. Wu, Asian J. Org. Chem., 2012, 1, 302 CrossRef CAS.
- (a) N. Asao, K. Takahashi, S. Lee, T. Kasahara and Y. Yamamoto, J. Am. Chem. Soc., 2002, 124, 12650 CrossRef CAS PubMed; (b) N. Asao, T. Nogami, S. Lee and Y. Yamamoto, J. Am. Chem. Soc., 2003, 125, 10921 CrossRef CAS PubMed; (c) N. Asao, H. Aikawa and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 7458 CrossRef CAS PubMed.
- (a) G. Dyker, D. Hildebrandt, J. Liu and K. Merz, Angew. Chem., Int. Ed., 2003, 42, 4399 CrossRef CAS PubMed; (b) N. Asao and H. Aikawa, J. Org. Chem., 2006, 71, 5249 CrossRef CAS PubMed; (c) N. Asao and K. Sato, Org. Lett., 2006, 8, 5361 CrossRef CAS PubMed; (d) A. K. Gupta, C. Y. Rhim, C. H. Oh, R. S. Mane and S.-H. Han, Green Chem., 2006, 8, 25 RSC; (e) J. Zhu, A. R. Germain and J. A. Porco Jr, Angew. Chem., Int. Ed., 2004, 43, 1239 CrossRef CAS PubMed.
- (a) X. Yao and C.-J. Li, Org. Lett., 2006, 8, 1953 CrossRef CAS PubMed; (b) A. B. Beeler, S. Su, C. A. Singleton and J. A. Porco Jr, J. Am. Chem. Soc., 2007, 129, 1413 CrossRef CAS PubMed; (c) L.-P. Liu and G. B. Hammond, Org. Lett., 2010, 12, 4640 CrossRef CAS PubMed; (d) S. Obika, H. Kono, Y. Yasui, R. Yanada and Y. Takemoto, J. Org. Chem., 2007, 72, 4462 CrossRef CAS PubMed; (e) S. Handa and L. M. Slaughter, Angew. Chem., Int. Ed., 2012, 51, 2912 CrossRef CAS PubMed; (f) A. Kotera, J. I. Uenishi and M. Uemura, Tetrahedron Lett., 2010, 51, 1166 CrossRef CAS.
- (a) S. Bhunia, K.-C. Wang and R.-S. Liu, Angew. Chem., Int. Ed., 2008, 47, 5063 CrossRef CAS PubMed; (b) S. Zhu, Z. Guo, Z. Huang and H. Jiang, Chem.–Eur. J., 2014, 20, 2425 CrossRef CAS PubMed; (c) S. Zhu, Z. Zhang, X. Huang, H. Jiang and Z. Guo, Chem.–Eur. J., 2013, 19, 4695 CrossRef CAS PubMed.
- Z. Cao and F. Gagosz, Angew. Chem., Int. Ed., 2013, 52, 9014 CrossRef CAS PubMed.
- (a) W. Zi and F. D. Toste, J. Am. Chem. Soc., 2013, 135, 12600 CrossRef CAS PubMed; For Pt catalysis, see: (b) I. Nakamura, G. B. Bajracharya, H. Wu, K. Oishi, Y. Mizushima, I. D. Gridnev and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 15423 CrossRef CAS PubMed; (c) I. Nakamura, Y. Mizushima and Y. Yamamoto, J. Am. Chem. Soc., 2005, 127, 15022 CrossRef CAS PubMed.
- (a) E. M. L. Sze, W. Rao, M. J. Koh and P. W. H. Chan, Chem.–Eur. J., 2011, 17, 1437 CrossRef CAS PubMed; (b) E. M. L. Sze, M. J. Koh, Y. M. Tjia, W. Rao and P. W. H. Chan, Tetrahedron, 2013, 69, 5558 CrossRef CAS.
- (a) D. M. Schultz, N. R. Babij and J. P. Wolfe, Adv. Synth. Catal., 2012, 354, 3451 CrossRef CAS; (b) M. Zhang, Y. Wang, Y. Yang and X. Hu, Adv. Synth. Catal., 2012, 354, 981 CrossRef CAS.
- (a) R. Benkrief, A.-L. Skaltsounis, F. Tillequin, M. Koch and J. Pusset, Planta Med., 1991, 57, 79 CrossRef CAS PubMed; (b) S. J. Uddin, T. L. H. Jason, K. D. Beattie, D. Grice and E. Tiralongo, J. Nat. Prod., 2011, 74, 2010 CrossRef CAS PubMed; (c) S.-H. Kim, S. H. Kwon, S.-H. Park, J. K. Lee, H.-S. Bang, S.-J. Nam, H. C. Kwon, J. Shin and D.-C. Oh, Org. Lett., 2013, 15, 1834 CrossRef CAS PubMed.
- For the transformation of the enynal to 3j, the reaction conditions including catalyst and temperature were screened. The isolated yields using Cy3PAuNTf2, JohnPhosAuNTf2, CyJohnPhosAuNTf2 and (p-CF3C6H4)3PAuNTf2 as catalysts were 21%, 10%, 0% and 56%, respectively. Also, changing the temperature could not improve the yield of 3j.
- For reviews on gold acetylides, see: (a) C.-J. Li, Acc. Chem. Res., 2010, 43, 581 CrossRef CAS PubMed; (b) T. de Haro and C. Nevado, Synthesis, 2011, 2530 CAS. For selected examples, see: (c) T. J. Brown and R. A. Widenhoefer, Organometallics, 2011, 30, 6003 CrossRef CAS; (d) A. Gómez-Suárez and S. P. Nolan, Angew. Chem., Int. Ed., 2012, 51, 8156 CrossRef PubMed; (e) A. Gómez-Suárez, S. Dupuy, A. M. Z. Slawin and S. P. Nolan, Angew. Chem., Int. Ed., 2013, 52, 938 CrossRef PubMed; (f) A. S. K. Hashmi, T. Lauterbach, P. L. Nösel, M. H. Vilhelmsen, M. Rudolph and F. Rominger, Chem.–Eur. J., 2013, 19, 1058 CrossRef CAS PubMed; (g) P. H.-Y. Cheong, P. Morganelli, M. R. Luzung, K. N. Houk and F. D. Toste, J. Am. Chem. Soc., 2008, 130, 4517 CrossRef CAS PubMed; (h) A. Grirrane, H. Garcia, A. Corma and E. Alvare, ACS Catal., 2011, 1, 1647 CrossRef CAS; (i) L. Ye, Y. Wang, D. H. Aue and L. Zhang, J. Am. Chem. Soc., 2012, 134, 31 CrossRef CAS PubMed; (j) A. S. K. Hashmi, I. Braun, P. Nösel, J. Schädlich, M. Wieteck, M. Rudolph and F. Rominger, Angew. Chem., Int. Ed., 2012, 51, 4456 CrossRef CAS PubMed; (k) M. H. Larsen, K. N. Houk and A. S. K. Hashmi, J. Am. Chem. Soc., 2015, 137, 10668 CrossRef CAS PubMed.
- The unsuccessful transformation of 3e to 7 under reaction condition excludes the possibility of the H/D exchange at the active methylene group.
- A possible mechanism for gold(I) catalysis could be similar to gold(III).
- N. A. Petasis and S.-P. Lu, Tetrahedron Lett., 1996, 37, 141 CrossRef CAS.
- (a) N. Asao, T. Nogami, K. Takahashi and Y. Yamamoto, J. Am. Chem. Soc., 2002, 124, 764 CrossRef CAS PubMed; (b) N. T. Patil and Y. Yamamoto, J. Org. Chem., 2004, 69, 5139 CrossRef CAS PubMed; (c) T. Godet, C. Vaxelaire, C. Michel, A. Milet and P. Belmont, Chem.–Eur. J., 2007, 13, 563 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and analysis data for new compounds. See DOI: 10.1039/c5ra24541a |
| This journal is © The Royal Society of Chemistry 2015 |