An investigation of the textural properties of mesostructured silica-based adsorbents for predicting CO2 adsorption capacity†
E. S. Sanz-Pérez,
A. Arencibia,
R. Sanz* and
G. Calleja
Department of Chemical and Energy Technology, ESCET, Universidad Rey Juan Carlos, C/ Tulipán s/n, 28933 Móstoles, Madrid, Spain. E-mail: raul.sanz@urjc.es
First published on 25th November 2015
Abstract
Among the technologies proposed to reduce greenhouse gas emissions, adsorption with porous solids has been widely studied in the past few years. Herein, up to 30 inorganic porous adsorbents have been studied, obtaining their CO2 uptake at 45 °C and ambient pressure, typical conditions of industrial post-combustion facilities after the desulphurization step. A clear relationship between CO2 adsorption capacity and the combination of surface area (SBET) and sorbent affinity towards gas molecules through C parameter was found. This study provides novel findings that allow the prediction of CO2 uptake in mesostructured silica physisorbents from their textural properties.
1. Introduction
Increasing CO2 atmospheric concentrations have motivated intense research efforts in order to reduce greenhouse gas emissions. Carbon capture and storage (CCS) with solid sorbents has been abundantly proposed in the recent literature. A great variety of materials, such as mesostructured and amorphous silica, alumina, zeolites, metal–organic frameworks, polymers or carbonaceous sorbents have been considered.1–5 When non-functionalized materials like the ones cited above are used the adsorption mechanism is restricted to physisorption, although some selectivity towards CO2 has been found in mixtures with N2, O2, H2, CH4 or Ar.6–8 This fact is explained by the CO2 adsorption mechanism, which is caused by two different kinds of forces.9 On the one hand, non-specific van der Waals forces (namely dispersion and repulsion) are present whenever a molecule is adsorbed. On the other, there are specific adsorbent–adsorbate electrostatic interactions that take place only for certain compounds such as CO2. This is due to its high quadrupole moment and its subsequent high polarizability.10 Physical adsorbents are generally characterized by their textural properties (mainly surface area, pore diameter and pore volume). For the assessment of surface area and related parameters, the model described by Brunauer–Emmett–Teller (BET)11 is widely accepted.12 Although there are several possibilities for the linearization of the original equation,13 the BET plot is commonly used following the initial linearization proposed:
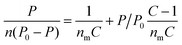 | (1) |
Unlike Langmuir adsorption isotherm, the BET model assumes that adsorption is not ideal and multilayer formation can occur for low coverage.11 This is also why adsorption in micropores is not accurately described by the BET equation, as multilayer adsorption in these narrow cavities is not possible.14 The BET model is still extensively used due to its two main advantages: it is easy to apply and provides two of the most relevant textural magnitudes, i.e., the monolayer capacity, and the C parameter. The monolayer capacity refers to the moles of gas adsorbed in a monomolecular layer that covers the whole surface of the adsorbent, which allows the estimation of specific surface area, a parameter commonly used to compare adsorbents. In addition, the C parameter is a thermodynamical magnitude related to the enthalpy of adsorption, which is defined as:
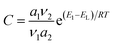 | (2) |
Since physical adsorption is a surface-dependent process, the amount of gas adsorbed depends both on the available surface and on the adsorption potential.17 Thus, surface area and C parameter obtained by means of nitrogen adsorption have been widely used to explain catalytic and adsorption behaviour of materials. Actually, textural properties determined by N2 adsorption are commonly correlated with the gas uptake of other adsorptives like Ar, H2, CH4 or CO2. Surface area,18–21 pore and micropore volume,22–24 free volume,25 pore shape, tortuosity and pore-structure irregularities,26 or combinations of several N2-obtained properties27,28 have been successfully associated to the mass uptake of gasses other than N2.
Regarding CO2 adsorption, some authors have reported correlations of the CO2 uptake by mesostructured materials with their textural properties, finding higher CO2 adsorption capacity for sorbents with larger surface area.29,30 A linear relationship between these variables was found for hybrid monolith aerogels of chitosan with increasing amounts of graphene oxide in their structure.30
In this work, more than 30 different samples of adsorbents prepared from mesostructured solids have been studied in order to find a valid correlation between CO2 adsorption capacity and textural properties such as available surface area, pore diameter, pore volume and adsorbate–adsorbent interactions (C parameter). Industrial post-combustion CO2 capture equipment is usually located after the desulphurization unit. After this step, flue gas is released at 45–55 °C and 1 bar.31 Since this study has a very practical interest for carbon capture and storage purposes, 45 °C and 1 bar have been the conditions considered for CO2 adsorption.
2. Experimental
2.1. Synthesis of siliceous materials
Materials of the families of conventional SBA-15,32 Al-SBA-15,33 pore-expanded SBA-15 (SBA-PE),34 conventional35 and pore-expanded36 MCM-41 and HMS37 were considered. Several synthesis temperatures and surfactant removal techniques were used in order to obtain the different supports. All the adsorbents used in this work were synthesized following the procedures described in the ESI,† except Silica Gel (SG), purchased from Merck (Silica Gel 60 F254).2.2. Synthesis of amine-modified materials
Some functionalized adsorbents were also used in this study. SBA-15 synthesis route was modified to load aminopropyl-trimethoxysilane (AP), ethylenediamine-trimethoxysilane (ED), and diethylenetriamine-trimethoxysilane (DT) organosilanes by co-condensation method. These organic molecules with one, two and three amino groups respectively yielded adsorbents named SBA-C-AP, SBA-C-ED and SBA-C-DT, where C indicates that the co-condensation method was followed. The structures of these organosilanes are shown in Fig. 1. | ||
| Fig. 1 Molecular structures of organosilane precursors containing (a) no amino groups, (b) tertiary amino groups and (c) primary and secondary amino groups. | ||
Calcined SBA-15 was functionalized by grafting with the following silanes: butyl-trimethoxysilane (BT), isobutyl-trimethoxysilane (IB) and octyl-trimethoxysilane (OC), without amino groups in their structures; and (N,N-dimethylaminopropyl)-trimethoxysilane (DM), (N,N-diethylaminopropyl)-trimethoxysilane (DE) and N-hydroxyethyl-N-methylaminopropyl-trimethoxysilane (HE), with tertiary amino groups (see Fig. 1 for structure details). Grafted materials were named SBA-Org, where Org refers to the type of organosilane used.
Detailed syntheses procedures and characterization techniques used can be found in the ESI.†
2.3. Physico-chemical characterization of supports
Prepared materials were characterized by low angle X-ray diffraction, using the CuKα monochromatic radiation in a powder diffractometer PHILIPS X-PERT MPD. Also, nitrogen adsorption–desorption isotherms at 77 K were obtained in a Micromeritics Tristar-3000 sorptometer. Siliceous materials were outgassed in N2 flow for 8 h at a temperature of 200 °C, while the temperature was only of 150 °C for functionalized samples in order to preserve their organic moieties. Elemental analyses of carbon, nitrogen and hydrogen were performed in a Vario EL III Elementar Analizer System GMHB. CO2 adsorption–desorption isotherms were obtained at 45 °C at pressures ranging from 0 to 6 bar by means of a VTI Scientific Instruments HVPA-100 volumetric equipment. All samples were outgassed at 110 °C for 2 h at a vacuum pressure of 5 × 10−3 mbar before each analysis. Two combined equilibration criteria were used to obtain isotherm points: a pressure drop below 0.2 mbar for 3 min or a maximum equilibration time of 50 min.3. Results and discussion
3.1. Physico-chemical characterization
A complete characterization of the synthesized solids is presented in Table 1, including d100 and a0 cell parameters measured by XRD, textural properties obtained by N2 adsorption–desorption at 77 K as well as nitrogen and total organic content measured by elemental analysis. Besides, a series of representative nitrogen adsorption–desorption isotherms is presented in Fig. 2, the rest of them being shown in the ESI (Fig. SI1 to SI3†).| Adsorbent | SBETa (m2 g−1) | DPb (nm) | VPc (cm3 g−1) | Ca | d100d (nm) | a0e (nm) | twf (nm) | Orgg (wt%) | Ng (wt%) | qh (mg CO2/g ads) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Specific surface and C parameter obtained by applying the BET equation in the P/P0 range from 0.05 to 0.20.b Pore diameter obtained from BJH pore size distribution.c Single pore volume measured at a relative pressure of 0.96.d Interplanar spacing obtained from the X-ray diffractogram by means of Bragg's Law.e Unit cell calculated from d100 values considering hexagonal close-packed mesopores.f Wall thickness calculated by subtracting DP from a0.g Total organic content (hydrogen, carbon and nitrogen) and nitrogen content measured by elemental analysis.h CO2 adsorption capacity obtained at 45 °C and 1 bar in a volumetric analyzer. | ||||||||||
| SBA-15 | 692 | 9.0 | 1.03 | 145 | 10.5 | 12.1 | 3.1 | — | — | 21.2 |
| SBA-15e | 599 | 9.2 | 1.06 | 74 | 10.4 | 12.0 | 2.8 | 8.7 | — | 12.2 |
| Al-SBA (60) | 813 | 13.0 | 1.26 | 97 | 12.2 | 14.1 | 1.1 | — | — | 20.4 |
| Al-SBA (30) | 803 | 12.6 | 1.39 | 87 | 11.9 | 13.7 | 1.1 | — | — | 20.4 |
| Al-SBA (10) | 727 | 11.2 | 1.13 | 97 | — | — | 22.3 | |||
| SBA-PE-17c | 452 | 11.7 | 1.02 | 101 | 12.0 | 13.9 | 2.2 | — | — | 15.7 |
| SBA-PE-12c | 437 | 11.4 | 0.80 | 92 | 12.7 | 14.7 | 3.3 | — | — | 11.5 |
| SBA-PE-17e | 428 | 15.2 | 1.18 | 76 | 14.1 | 16.3 | 1.1 | 6.6 | — | 11.7 |
| SBA-PE-12e | 399 | 12.1 | 0.75 | 68 | 13.3 | 15.3 | 3.2 | 6.5 | — | 11.6 |
| HMS-18c | 643 | 1.7 | 0.59 | 62 | — | — | — | — | — | 12.0 |
| HMS-16c | 623 | 2.8 | 0.58 | 96 | — | — | — | — | — | 13.0 |
| HMS-12c | 1181 | 2.1 | 0.96 | 38 | — | — | — | — | — | 13.7 |
| HMS-12e | 1045 | 2.6 | 1.10 | 64 | — | — | — | 4.5 | 0.1 | 16.2 |
| HMS-10c | 918 | 2.1 | 0.72 | 48 | — | — | — | — | — | 14.8 |
| HMS-8c | 1056 | 1.5 | 0.72 | 39 | — | — | — | — | — | 15.9 |
| MCM-41 | 1088 | 2.6 | 0.83 | 61 | 3.8 | 4.4 | 1.9 | — | — | 16.1 |
| MCM-41e | 684 | 2.4 | 0.54 | 44 | 4.3 | 5.0 | 2.6 | 22.1 | 1.0 | 9.3 |
| MCM-PEc | 894 | 5.1 | 1.28 | 99 | 4.9 | 5.6 | 0.5 | — | — | 15.1 |
| SG | 263 | 10.2 | 0.70 | 119 | — | — | — | — | — | 10.4 |
 | ||
| Fig. 2 Textural characterization of SBA-15, SBA-PE-17e, HMS-12, MCM-PEc, and SG supports: (a) N2 adsorption–desorption isotherms at 77 K and (b) BJH pore size distributions. | ||
There is a wide diversity among the materials in terms of textural properties. Pore diameters are between 1.5 and 15.2 nm, in the range of large micropores or mesopores. Pore expanded SBA-PE-17e showed the highest pore diameter, up to 15.2 nm. Silica Gel (SG) has also large pores (10.2 nm) but with a less defined pore size distribution. Conventional SBA-15 and pore-expanded MCM-41 exhibited well-defined pore sizes centered in 9.0 and 5.1 nm respectively. Finally, HMS-12c presents a so-called wormhole structure, with homogeneously sized pores but lacking any bi or tridimensional structure.
C parameter values for calcined and ethanol-extracted SBA-15 are of 145 and 74 respectively (see Table 1). As detailed in the introduction section, C is related to the heat of adsorption (see eqn (2)). Hydrophilic materials present a high silanol (Si–OH) surface concentration and thus a strong interaction with N2, resulting in high C values. The amount of silanol groups in silica has shown to be higher for extracted materials compared to calcined ones.38,39 Accordingly, a higher C value could be expected for extracted SBA-15e compared to calcined SBA-15.
However, most of silanol groups in SBA-15e are hindered by the surfactant remaining after extraction (the organic content of SBA-15e is of 8.7%). For calcined SBA-15, which is pure silica, all silanol groups are available to N2 molecules, resulting in stronger interactions with N2 and hence, in higher C parameter than extracted SBA-15e. SBA-PE and MCM-41 materials also show C values after calcination and extraction which are in agreement with this remark (see Table 1).
When the structure of SBA-15 is modified by enlarging pores (SBA-PE) or including Al atoms in the structure, C values decreased from 145 to 70–100. Thus, it can be deduced that the modified structures present a lower affinity towards N2 molecules. This change can be ascribed to a loss of silanol groups or to a surface restructuration. The latter involves an increased surface roughness and reorientation of silanol groups leading to a lower surface polarity.40
Textural parameters of adsorbents prepared by co-condensation with AP, ED and DT, and by grafting with organosilanes containing just tertiary amino groups (DE, DM and HE) or no amino groups at all (IB, BT, OC) are summarised in Table 2. As seen, specific surface, pore diameter and pore volume are considerably lower than those of SBA-15 as a consequence of the pore-filling after organic functionalization.
| Adsorbent | SBET (m2 g−1) | DP (nm) | VP (cm3 g−1) | C | Org. (wt%) | N (wt%) | q (mg CO2/g ads) |
|---|---|---|---|---|---|---|---|
| SBA-15 | 692 | 9.0 | 1.03 | 145 | — | — | 21.2 |
| SBA-OC | 621 | 8.2 | 0.92 | 88 | 5.3 | — | 17.0 |
| SBA-BT | 612 | 8.3 | 0.90 | 99 | 5.6 | — | 15.0 |
| SBA-IB | 650 | 8.5 | 0.97 | 101 | 4.1 | — | 16.8 |
| SBA-DE | 307 | 7.2 | 0.51 | 30 | 18.7 | 2.7 | 6.9 |
| SBA-DM | 266 | 7.4 | 0.47 | 28 | 16.2 | 3.1 | 4.6 |
| SBA-HE | 207 | 7.1 | 0.35 | 34 | 19.5 | 4.0 | 5.5 |
| SBA-C-AP | 572 | 8.2 | 0.87 | 68 | 4.7 | 1.3 | 10.1 |
| SBA-C-ED | 508 | 8.3 | 0.78 | 63 | 7.8 | 2.7 | 11.2 |
| SBA-C-DT | 477 | 8.1 | 0.41 | 57 | 7.3 | 2.6 | 10.2 |
Samples functionalized with amine-containing organosilanes also present a certain amount of nitrogen loaded (1.3–4.0%). This variation is consistent with the decrease observed in the textural properties.
C parameter took values between 28 and 101, much smaller than the one corresponding to siliceous SBA-15, which was 145. This difference is in agreement with the previous results obtained for extracted materials, where the presence of organic content led to a weaker affinity between N2 and the organically-covered sorbent surface and thus to lower C values. Even more, in this case there is not only an increase of organic content, but also a decrease in the silanol surface concentration due to the grafting of organosilanes.
3.2. CO2 adsorption
Pure CO2 adsorption–desorption isotherms obtained at 45 °C for a selection of siliceous and functionalized materials are displayed in Fig. 3a and b respectively. The complete set of isotherms for all the adsorbents is shown in the ESI (Fig. SI4 to SI7†). | ||
| Fig. 3 Pure CO2 adsorption–desorption isotherms at 45 °C of (a) bare supports SBA-15, SBA-PE-17e, HMS-12c and SG and (b) functionalized SBA-15 adsorbents. | ||
The isotherms of non-functionalized supports are characteristic of physical adsorption, as previously described.41 The CO2 uptake determined at 1 bar does not seem to correlate with textural properties (Table 1). For example, samples as different as SBA-PE-17e and HMS-18c, with no common features in their textural properties, presented analogous CO2 uptakes, around 12 mg CO2/g ads. Likewise, samples as similar as SBA-15 and SBA-15e showed very different CO2 adsorption capacities, 21.2 and 12.2 mg CO2/g ads respectively.
The functionalization of SBA-15 led to interesting findings (Fig. 3b and Table 2). The grafting with organosilanes containing tertiary amino groups (DE, DM and HE) or no amino groups at all (IB, BT and OC) as well as the co-condensation of molecules with primary and secondary groups (AP, ED and DT) resulted in adsorbents with a lesser CO2 uptake than siliceous SBA-15. This result was originated by the surface reduction occurred during the functionalization process and the fact that no active groups for CO2 adsorption were present in these samples. Silanes and functionalization methods were specifically selected in order to yield amine-modified adsorbents that only present physical adsorption of CO2. Amines loaded by co-condensation (AP, ED and DT) using an acidic media are protonated42 and end up embedded in the silica walls, so they provide little interaction with CO2.41 Besides, tertiary amines (DM, DE and HE) have been extensively described as not active for CO2 adsorption in dry conditions.43,44 Fig. 3b (and SI7†) confirm that the shape of the CO2 adsorption desorption isotherms is clearly due to just physisorption. This is in agreement with many theoretical and experimental works that discard the occurrence of chemical adsorption on samples with analogous behaviour,45–47 thus ruling out the presence of chemical adsorption on the samples above described.
In order to present definite proof regarding the absence of chemical adsorption in the samples presented upon here, isosteric heat of adsorption was estimated for SBA-15 pure silica and SBA-C-ED. Experimental adsorption isotherms were acquired at 35, 45, 55 and 65 °C and modeled according to Sips equation (eqn (3)):48
 | (3) |
Fig. 4 displays experimental adsorption points as well as the Sips isotherm model fits for SBA-15 and SBA-C-ED adsorbents. Suitable fittings with R2 values around 0.999 were observed in all cases. Parameters included in Sips equation as well as R2 fitting values for each isotherm are listed in Table S1 (ESI†).
 | ||
| Fig. 4 CO2 adsorption equilibrium points at temperatures between 35 °C and 65 °C for (a) SBA-15 silica and (b) co-condensed SBA-C-ED with 2.7%N. The lines correspond to the Sips isotherm model fit. | ||
Clausius–Clapeyron equation (eqn (4)) was used to obtain isosteric heat of adsorption, as shown in Fig. SI9.†
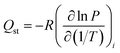 | (4) |
Finally, the isosteric heat of adsorption was plotted against CO2 uptake, obtaining the curves presented in Fig. 5. According to these results, isosteric heat of adsorption at the minimum coverage considered was of 33.6 and 36.5 kJ mol−1 for SBA-15 and SBA-C-ED respectively.
 | ||
| Fig. 5 Isosteric heat of adsorption for CO2 on (a) siliceous SBA-15 and (b) amine-containing SBA-C-ED. | ||
Values measured by calorimetry for mesoporous silicas have been reported to range between 20 and 35 kJ mol−1,51–53 with these low values being associated to physical adsorption. On the contrary, samples with available amino groups from AP, ED and DT organosilanes showed much higher values, from 50 to 70 kJ mol−1,53–55 being ascribed to chemical adsorption. Thus, it can be inferred that both SBA-15 silica and ED-containing SBA-C-ED are interacting with CO2 just by physical adsorption.
Contrasting with physical adsorbents presented in this work, many researchers use amines that actually react with CO2, resulting in chemisorption processes. These materials achieve much higher CO2 uptake53,56–61 and their CO2 adsorption isotherms are very different to those described here. Namely, the CO2 uptake at low pressures is considerable, its dependence with pressure is much lower than in the case of physisorption and the reversibility of the process is not complete (i.e., adsorption and desorption branches do not overlap).41,45 When chemisorption strongly contributes to the overall CO2 adsorption capacity, this variable is mainly governed by the kind, amount and distribution of amino groups loaded, as extensively reasoned in the literature.9,62–65 Since functionalized samples prepared in this work adsorb CO2 only by physisorption, there is no dependence on any magnitude related to the amino groups loaded when such moieties are present.
3.3. Correlation between CO2 uptake and textural properties
In an attempt to find a relationship between physical CO2 adsorption and textural properties, the CO2 uptake of all the adsorbents was plotted against the main magnitudes obtained from N2 adsorption–desorption isotherms, i.e., specific surface area, pore diameter, pore volume and C parameter (Fig. 6).As seen, these results reveal that none of these parameters individually considered correlates with the CO2 uptake. Detailed fitting parameters are listed in Table 3, where very low values of the regression parameter R2 are listed in the four first cases. This confirms that specific surface area, pore diameter, pore volume and C parameter are poor predictors of CO2 uptake if considered individually.
| Individual fitting q(CO2) = a + kx | |||||||
|---|---|---|---|---|---|---|---|
| x | R2 | Slope (k) | Std. error | Perc. error (%) | Intcp. (a) | Std. error | Perc. error (%) |
| SBET | 0.392 | 0.011 | 0.003 | 24 | 6.6 | 1.8 | 26 |
| DP | 0.021 | 0.16 | 0.22 | 132 | 12 | 2 | 15 |
| VP | 0.572 | 12 | 2 | 17 | 3.0 | 1.9 | 61 |
| C | 0.337 | 0.090 | 0.024 | 27 | 6.7 | 1.9 | 29 |
| CSBET | 0.780 | 1.7 × 10−4 | 0.2 × 10−4 | 10 | 5.4 | 0.9 | 17 |
| CVP | 0.578 | 0.089 | 0.015 | 17 | 7.6 | 1.1 | 15 |
In contrast with these results, many papers claim that pore diameter plays an important role in CO2 adsorption.66–68 Though this statement is correct, it only applies to microporous adsorbents. On the contrary, mesoporous materials are formed by much wider pores and confination effects are not significant. Consequently, there is no clear effect of this variable in the CO2 uptake, as seen in Fig. 6b.
For mesoporous adsorbents, Alhwaige et al. have recently reported a robust linear correlation between CO2 uptake and BET surface using a chitosan aerogel with different amounts of graphene oxide (GO), with GO being responsible of the increment detected in porosity.30 However, the authors stated that chemisorption on GO active sites was likely the underlying adsorption mechanism, so there may be a chemisorption contribution to the overall CO2 uptake in this case.
Regarding CO2 physisorption, it is well known that the available surface area is very likely playing a role in CO2 capture, although probably not as an individual variable. A similar conclusion was drawn by Xu and Hedin by using more than 30 microporous adsorbents.29 These authors observed the influence of surface area in CO2 adsorption but did not get a robust linear correlation, concluding that surface area was not predicting CO2 uptake by itself.
Additionally, it is reasonable to assume that the surface chemistry is also a determining factor in the amount of gas adsorbed. In this study, C parameter is considered in order to quantify the affinity of a given surface towards an adsorptive. Despite C parameter is calculated from the N2 adsorption isotherm, it constitutes a fair approximation to the affinity between the adsorbent surface and the gas adsorbed by means of weak interactions. This assumption is coherent with the number of references correlating C parameter or BET surface area (both of them deriving from the BET equation) with the adsorption capacity of gases other than N2.19–21 Thus, CO2 uptake was fitted against the product of the available surface area (SBET) and its affinity towards an adsorptive (C parameter). As shown in Fig. 7, the points are now much better fitted to a straight line, with no significant scattering. Also, the value of R2 parameter is much higher than those resulting from fitting the CO2 uptake against individual textural parameters. A similar correlation was tested considering the product of C and VP, but the fitting was not so good (see Table 3).
The correlation between CO2 uptake and the product of C and surface area in the present study is still valid at pressures higher than the atmospheric. When data at 4.5 bar were considered, the fitting showed an R2 value of 0.818, much higher than any of the other correlations previously considered.
Finally, to investigate the possibility of mixed interactions between textural parameters, multivariable fittings (y = αx1 + βx2) were studied, with y being the CO2 uptake in all cases. In the first two experiments, C parameter was selected as the first independent variable (x1), with SBET and VP being considered as the second variable (x2). Two additional multivariable fitting were carried out, considering the product between surface area and C parameter as one independent variable and either VP or SBET as the second one. However, none of these correlations yielded substantial increases in the goodness of the fit compared to the previous one shown in Fig. 7. Moreover, they entailed higher relative errors in the parameters obtained, so it can be inferred that adding the influence of these variables does not significantly improve the fitting.
All things considered, it can be concluded that in an extensive number of materials, physisorbed CO2 can be directly related to the combined influence of the available surface area (SBET) and the affinity of this surface towards physisorption (C parameter), measured in a simple way by means of the BET equation (CSBET).
When mesoporous silica adsorbents are used for CO2 capture presenting just physisorption mechanism, with no chemical contribution, a linear relationship between CO2 uptake and the product of C parameter and BET surface was established. As a uniform rule inferred from the fitting in Fig. 7, it can be concluded that at 45 °C and 1 bar the linear dependence was found to be:
| qCO2 = 1.714 × 10−4(SBETC) + 5.38 | (5) |
It is noteworthy to remark that this finding is in agreement with the well-known surface-dependent nature of physical adsorption, which explains the dependence of the gas uptake on both the available surface and the adsorption potential.17 Moreover, this is an important outcome, since the CO2 uptake of a mesostructured material can be predicted from its textural properties using eqn (5).
This correlation explains for example, that SBA-BT and HMS-10c exhibited almost the same CO2 adsorption capacity (15.0 and 14.8 mg g−1) but having significantly different values of BET surface and C parameter (612 m2 g−1 and 99 for the former; and 918 m2 g−1 and 48 for the latter). That means that the same CO2 uptake can be obtained with materials having few physical sorption sites (accounted by the BET surface area) with high affinity, or else with adsorbents displaying many physisorption sites, although less active.
4. Conclusions
In this work, more than 30 physisorbents were subjected to N2 adsorption at −193 °C and CO2 adsorption at 45 °C. The CO2 uptake of these samples was found to correlate with their textural parameters, namely the product of the available surface area (SBET) and the affinity of the surface toward adsorptives (C parameter). Although R2 values obtained for this linear fitting are not too high (0.78 and 0.82 at 1 bar and 4.5 bar respectively), the correlation found is significant taking into account that more than 30 materials prepared by a variety of procedures were considered. This is a novel finding that allows the estimation of CO2 uptake values from physical adsorbents based on mesostructured silicas directly from their textural properties. The significance of this new correlation lies in the industrial importance of post-combustion CO2 capture, an application for which all these materials have been extensively proposed.Notes and references
- J. Wang, L. Huang, R. Yang, Z. Zhang, J. Wu, Y. Gao, Q. Wang, D. O'Hare and Z. Zhong, Energy Environ. Sci., 2014, 7, 3478 CAS.
- A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 2012, 51, 1438 CrossRef CAS.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796 CrossRef CAS PubMed.
- N. Chalal, H. Bouhali, H. Hamaizi, B. Lebeau and A. Bengueddach, Microporous Mesoporous Mater., 2015, 210, 32 CrossRef CAS.
- A. Erto, A. Silvestre-Albero, J. Silvestre-Albero, F. Rodríguez-Reinoso, M. Balsamo, A. Lancia and F. Montagnaro, J. Colloid Interface Sci., 2015, 448, 41–50 CrossRef CAS PubMed.
- R. V. Siriwardane, M. S. Shen, E. P. Fisher and J. A. Poston, Energy Fuels, 2001, 15, 279 CrossRef CAS.
- Y. Belmabkhout, G. de Weireld and A. Sayari, Langmuir, 2009, 25, 13275 CrossRef CAS PubMed.
- S. Yang, J. Sun, A. J. Ramírez-Cuesta, S. K. Callear, W. I. F. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schröder, Nat. Chem., 2012, 4, 887 CrossRef CAS PubMed.
- E. S. Sanz-Pérez, M. Olivares-Marín, A. Arencibia, R. Sanz, G. Calleja and M. M. Maroto-Valer, Int. J. Greenhouse Gas Control, 2013, 17, 366 CrossRef.
- D. M. Ruthven, Principles of Adsorption and Adsorption Processes, Wiley Interscience, New York, 1984 Search PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051 CrossRef CAS.
- F. Salvador, C. Sánchez-Jiménez, M. J. Sánchez-Montero and A. A. Salvador, Stud. Surf. Sci. Catal., 2002, 144, 379 CrossRef CAS.
- M. J. Remy and G. A. A. Poncelet, J. Phys. Chem., 1995, 99(2), 773 CrossRef CAS.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press Inc., London, 2nd edn, 1982 Search PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- A. Ansón, J. Jagiello, J. B. Parra, M. L. Sanjuán, A. M. Benito, W. K. Maser and M. T. Martínez, J. Phys. Chem. B, 2004, 108, 15820 CrossRef.
- R. Ströbel, L. Jörissen, T. Schliermann, V. Trapp, W. Schütz, K. Bohmhammel, G. Wolf and J. Garche, J. Power Sources, 1999, 84, 221 CrossRef.
- A. Züttel, P. Sudan, P. Mauron and P. Wenger, Appl. Phys. A: Mater. Sci. Process., 2004, 78, 941 CrossRef.
- M. Rzepka, P. Lamp and M. A. de la Casa-Lillo, J. Phys. Chem. B, 1998, 102(52), 10894 CrossRef CAS.
- T. C. Drage, A. Arenillas, K. M. Smith, C. Pevida, S. Piippo and C. E. Snape, Fuel, 2007, 86, 22 CrossRef CAS.
- M. Seredych, J. Lison, U. Jans and T. J. Bandosz, Carbon, 2009, 47, 2491 CrossRef CAS.
- J. B. Parra, C. O. Ania, A. Arenillas, F. Rubiera, J. M. Palacios and J. J. Pis, J. Alloys Compd., 2004, 379, 280 CrossRef CAS.
- R. Gadiou, S. E. Saadallah, T. Piquero, P. David, J. Parmentier and C. Vix-Guterl, Microporous Mesoporous Mater., 2005, 79, 121 CrossRef CAS.
- J. Kim, L.-C. Lin, J. A. Swisher, M. Haranczyk and B. Smit, J. Am. Chem. Soc., 2012, 134(46), 18940 CrossRef CAS PubMed.
- C. C. Ahn, Y. Ye, B. V. Ratnakumar, C. Witham, R. C. Bowman Jr and B. Fultz, Appl. Phys. Lett., 1998, 73(23), 3378 CrossRef CAS.
- M. Bastos-Neto, D. V. Canabrava, A. E. B. Torres, E. Rodriguez-Castello, A. Jiménez-López, D. C. S. Azevedo and C. L. Cavalcante Jr, Appl. Surf. Sci., 2007, 253, 5721 CrossRef CAS.
- M. Jordá-Beneyto, F. Suárez-García, D. Lozano-Castelló, D. Cazorla-Amorós and A. Linares-Solano, Carbon, 2007, 45, 293 CrossRef.
- C. Xu and N. Hedin, J. Mater. Chem. A, 2013, 1(10), 3406 CAS.
- A. A. Alhwaige, T. Agag, H. Ishida and S. Qutubuddin, RSC Adv., 2013, 3, 16011 RSC.
- R. Gaikwad, W. L. Boward and W. dePriest, Wet flue gas desulfurization technology evaluation, National Lime Association, Sargent & Lundy, Chicago, 2003 Search PubMed.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. Fredrickson, B. Chmelka and G. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- Y. Yue, A. Gédéon, J.-L. Bonardet, J.-B. D'Espinose, J. Fraissard and N. Melosh, Chem. Commun., 1999, 1967–1968 RSC.
- L. Cao, T. Man and M. Kruk, Chem. Mater., 2009, 21, 1144–1153 CrossRef CAS.
- W. Lin, Q. Cai, W. Pang, Y. Yue and B. Zou, Microporous Mesoporous Mater., 1999, 33, 187–196 CrossRef CAS.
- M. Kruk, M. Jaroniec and A. Sayari, Microporous Mesoporous Mater., 2000, 35–36, 545–553 CrossRef CAS.
- P. T. Tanev and T. J. Pinnavaia, Science, 1995, 267, 865–867 CrossRef CAS PubMed.
- V. Ya Davidov, A. V. Kiselev and L. T. Zhuravlev, Trans. Faraday Soc., 1964, 60, 2254 RSC.
- L. T. Zhuravlev, Langmuir, 1987, 3, 316 CrossRef CAS.
- J. Chaignon, Y. Bouizi, L. Davin, N. Calin, B. Albela and L. Bonneviot, Green Chem., 2015, 17, 3130 RSC.
- R. Sanz, G. Calleja, A. Arencibia and E. S. Sanz-Pérez, Microporous Mesoporous Mater., 2012, 158, 309 CrossRef CAS.
- A. S. Maria Chong, X. S. Zhao, A. T. Kustedjo and S. Z. Qiao, Microporous Mesoporous Mater., 2004, 72, 33 CrossRef.
- S. A. Didas, A. R. Kulkarni, D. S. Sholl and C. W. Jones, ChemSusChem, 2012, 5, 2058 CrossRef CAS PubMed.
- M. Caplow, J. Am. Chem. Soc., 1968, 24, 6795 CrossRef.
- R. Serna-Guerrero, Y. Belmabkhout and A. Sayari, Chem. Eng. J., 2010, 161, 173 CrossRef CAS.
- R. Sanz, G. Calleja, A. Arencibia and E. S. Sanz-Pérez, Energy Fuels, 2013, 27, 7637 CrossRef CAS.
- B. Aziz, N. Hedin and Z. Bacsik, Microporous Mesoporous Mater., 2012, 150, 42–49 CrossRef.
- R. Sips, J. Chem. Phys., 1948, 16, 490 CrossRef CAS.
- S. K. Papageorgiou, F. K. Katsaros, E. P. Kouvelos, J. W. Nolan, H. le Deit and N. K. Kanellopoulos, J. Hazard. Mater., 2006, 137, 1765–1772 CrossRef CAS PubMed.
- W. Rudzinski and D. H. Everett, Adsorption of Gases on Heterogeneous Surfaces, Elsevier, 1992 Search PubMed.
- G. P. Knowles, J. V. Graham, S. W. Delaney and A. L. Chaffee, Fuel Process. Technol., 2005, 86, 1435–1448 CrossRef CAS.
- C. Knöfel, C. Martin, V. Hornebecq and P. L. Llewellyn, J. Phys. Chem. C, 2009, 113, 21726–21734 Search PubMed.
- G. P. Knowles, S. W. Delaney and A. L. Chaffee, Ind. Eng. Chem. Res., 2006, 2626–2633 CrossRef CAS.
- O. Leal, C. Bolívar, G. Sepúlveda, G. Molleja, G. Martínez and L. Esparragoza, EE.UU. Patent 2.087.597, 1992.
- A. C. C. Chang, S. S. C. Chuang, M. Gray and Y. Soong, Energy Fuels, 2003, 17, 468–473 CrossRef CAS.
- W. Klinthong, C.-H. Huang and C.-S. Tan, Microporous Mesoporous Mater., 2014, 197, 278 CrossRef CAS.
- J. Fujiki, H. Yamada and K. Yogo, Microporous Mesoporous Mater., 2015, 215, 76 CrossRef CAS.
- N. Minju, P. Abhilash, B. N. Nair, A. P. Mohamed and S. Ananthakumar, Chem. Eng. J., 2015, 269, 335 CrossRef CAS.
- E. Vilarrasa-Garcia, E. M. O. Moya, J. A. Cecilia, C. L. Cavalcante Jr, J. Jiménez-Jiménez, D. C. S. Azevedo and E. Rodríguez-Castellón, Microporous Mesoporous Mater., 2015, 209, 172 CrossRef CAS.
- J. A. A. Gibson, A. V. Gromov, S. Brandani and E. E. B. Campbell, Microporous Mesoporous Mater., 2015, 208, 129 CrossRef CAS.
- E. G. Moschetta, M. A. Sakwa-Novak, J. L. Greenfield and C. W. Jones, Langmuir, 2015, 31, 2218 CrossRef CAS PubMed.
- A. L. Chaffee, Fuel Process. Technol., 2005, 86, 1473 CrossRef CAS.
- G. P. Knowles, S. W. Delaney and A. L. Chaffee, Ind. Eng. Chem. Res., 2006, 45, 2626 CrossRef CAS.
- P. J. E. Harlick and A. Sayari, Ind. Eng. Chem. Res., 2007, 46, 446 CrossRef CAS.
- W. Lu, J. P. Sculley, D. Yuan, R. Krishna, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51(30), 7480 CrossRef CAS PubMed.
- M. Sevilla, J. B. Parra and A. B. Fuertes, ACS Appl. Mater. Interfaces, 2013, 5, 6360 CAS.
- V. Presser, J. McDonough, S.-H. Yeon and Y. Gogotsi, Energy Environ. Sci., 2011, 4, 3059 CAS.
- A. Silvestre-Albero, S. Rico-Francés, F. Rodríguez-Reinoso, A. M. Kern, M. Klumpp, B. J. M. Etzold and J. Silvestre-Albero, Carbon, 2013, 59, 221 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental methodology, N2 adsorption–desorption isotherms at 77 K, CO2 adsorption–desorption isotherms at 45 °C and isosteric heat of adsorption calculation details. See DOI: 10.1039/c5ra19105j |
| This journal is © The Royal Society of Chemistry 2015 |














