Green coating by coordination of tannic acid and iron ions for antioxidant nanofiltration membranes†
Lin Fanab,
Yanyan Maab,
Yanlei Su*ab,
Runnan Zhangab,
Yanan Liuab,
Qi Zhangab and
Zhongyi Jiangab
aKey Laboratory for Green Chemical Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: suyanlei@tju.edu.cn; Fax: +86-22-27890882
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin University, Tianjin 300072, China
First published on 14th December 2015
Abstract
A novel green coating method was proposed to prepare composite nanofiltration (NF) membranes without using organic solutions or toxic reagents in the formation of the active layer compared with traditional interfacial polymerization. Tannic acid (TA) and iron(III) chloride (FeCl3) were chosen as the two reactive monomers dissolved in the aqueous phase. The stable metal–polyphenol complex coating was formed via the coordination reaction between TA and iron ions (FeIII) upon porous support. Fourier transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and water contact angle were used to characterize the chemical features of the prepared TA–FeIII/polyethersulfone (PES) composite NF membranes. Scanning electron microscope (SEM) and atomic force microscopy (AFM) were utilized to observe the surface morphologies. The effects of reactive monomer concentration and reaction time on the permeability of water and rejection of dyes and inorganic salts were investigated, respectively. The TA–FeIII/PES composite NF membranes possessed good structural stability and oxidation resistance ability.
1. Introduction
Nanofiltration (NF), as a new pressure-driven membrane separation process, has suitable permeability with pore size of about 0.5–2.0 nm and nominal molecular weight cut-off ranging from 200 to 1000 Da between ultrafiltration and reverse osmosis systems.1 With the advantages of energy saving, low operation pressure and high rejection ratio of divalent ion, the NF technique is widely applied in separation, concentration and purification in food, pharmaceutical, and textile industries, and wastewater treatment.2–6Interfacial polymerization, the most commonly used method for the preparation of NF membranes, is based on a polycondensation reaction between two reactive monomers from aqueous phase and organic phase, respectively. In general, reactive monomers are dissolved in two incompatible solutions, typically water and organic solutions. The polycondensation reaction takes place at the interface of two phases, forming an ultrathin active layer directly attached to the porous support.7 However, the organic solutions utilized in polymerization reaction are often harmful and toxic to natural environment and human body, such as harm respiratory system, nervous system, liver and kidney, even induce chronic skin diseases.8 Trimesoyl chloride (TMC), toxic reagent for human and animal, is widespread employed as the reactive monomer in organic phase, due to its acyl chloride groups providing bonding sites for polycondensation reaction.9 Therefore, it is significant to search the reactive monomer dissolved in harmless solution to replace TMC organic solution for polycondensation reaction.
Tannic acid (TA), as a typical kind of natural polyphenols from multiple plants, was composed of a central glucose core and surrounding covalently attached digalloyl ester groups containing luxuriant phenolic hydroxyl groups.10 TA could provide polydentate ligands for metal ions to coordination, forming metal–polyphenol complex and implementing bioadhesion, typically achieved by TA–FeIII complex.11,12 Actuality, some researchers have focused on TA–FeIII complex to form surface coating, utilizing hydroxyl groups from catechol and/or galloyl adhering on the surfaces via covalent and/or non-covalent bonding,13–15 which achieved the multifunctional modification of surfaces in biomedical and environmental applications.
In our previous study, TA has been used to prepare composite NF membranes by interfacial polymerization method.16 TA was dissolved in water as water phase monomer, which supplied phenol groups performing cross-linking reaction with acyl chloride groups of TMC dissolved in organic phase. Water flux of the prepared TA–TMC composite NF membranes was reached 46 ± 2.0 L m−2 h−1 and orange GII rejection ratios could reach 99.8% at operation pressure of 0.20 MPa. Besides having excellent antifouling property, the TA–TMC composite NF membranes were much stable than polyamide thin films from piperazine (PIP)–TMC composite NF membranes in sodium hypochlorite solution, which was because of the better chemical stability of ester groups from the polyester active layer performed by the cross-linking of TA and TMC than the amide groups of PIP–TMC polycondensation. However, the TA–TMC composite NF membranes still couldn't avoid using the harmful organic solution and toxic reagent.
In the present study, a new green method for NF membranes with TA was proposed to prepare by the coordination complex of TA and FeIII ions only in water environment. TA and iron(III) chloride (FeCl3) were chosen as the two reactive monomers both dissolved in aqueous phase. A stable metal–polyphenol complex coating was formed via the coordination reaction between them upon porous support. This green coating for NF membranes might completely avoid using toxic organic reagents. The influences of reaction time, concentration of TA and FeIII ions in aqueous phase on the permeation and separation properties of the TA–FeIII/PES composite NF membranes were evaluated. The stability of the TA–FeIII/PES composite NF membranes after immersion into different pH aqueous solutions and NaClO solution was investigated. It was worth mentioning that the TA–FeIII/PES composite NF membranes had excellent antioxidant property after a long term immersion into NaClO solution.
2. Experimental section
2.1 Materials
Tannic acid (TA) was obtained from Guangfu Chemical Reagent Co. (Tianjin, China). Iron chloride (FeCl3) was purchased from J&K Scientific Ltd. (Beijing, China). Polyethersulfone (PES, E6020P, Mw = 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), from BASF Co. (Germany), was dried at 80 °C over 24 h before used. Poly(ethylene glycol) (PEG, Mw = 2000 Da), N,N-dimethylformamide (DMF), Na2SO4, orange GII (C16H10N2Na2O7S2, Mw = 452), congo red (C32H22N6Na2O6S2, Mw = 696) and other reagents were purchased from Kewei Chemical Reagent Co. (Tianjin, China). The used water was the deionized water prepared by reverse osmosis system.
000 Da), from BASF Co. (Germany), was dried at 80 °C over 24 h before used. Poly(ethylene glycol) (PEG, Mw = 2000 Da), N,N-dimethylformamide (DMF), Na2SO4, orange GII (C16H10N2Na2O7S2, Mw = 452), congo red (C32H22N6Na2O6S2, Mw = 696) and other reagents were purchased from Kewei Chemical Reagent Co. (Tianjin, China). The used water was the deionized water prepared by reverse osmosis system.
2.2 Preparation of TA–FeIII/PES composite NF membranes
PES ultrafiltration membranes were used as the porous support of composite membranes according to our previous works.17,18 Pure water flux of PES ultrafiltration membranes was about 160 L m−2 h−1 at pressure of 0.10 MPa and molecular weight cut off was about 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da. The schematic illustration for the preparation of the TA–FeIII/PES composite NF membranes was presented in Fig. 1. The PES support membranes were firstly immersed into aqueous solution of TA (0.5–3.0 g L−1, 30 mL) about 20 min, the excess TA solution was removed via pressing gently between filter papers. The PES membranes containing TA adsorption layer were subsequently soaked in the FeCl3 aqueous solution (0.5–2.0 g L−1, 30 mL) to react with TA for a certain time to complete the coordination function. The metal–polyphenol coating layer was formed upon the PES support, followed by drying at room temperature for about 40 min, then the resultant membranes with TA–FeIII coating were rinsed by water and also stored in water prior to use.
000 Da. The schematic illustration for the preparation of the TA–FeIII/PES composite NF membranes was presented in Fig. 1. The PES support membranes were firstly immersed into aqueous solution of TA (0.5–3.0 g L−1, 30 mL) about 20 min, the excess TA solution was removed via pressing gently between filter papers. The PES membranes containing TA adsorption layer were subsequently soaked in the FeCl3 aqueous solution (0.5–2.0 g L−1, 30 mL) to react with TA for a certain time to complete the coordination function. The metal–polyphenol coating layer was formed upon the PES support, followed by drying at room temperature for about 40 min, then the resultant membranes with TA–FeIII coating were rinsed by water and also stored in water prior to use.
2.3 Characterization of TA–FeIII/PES composite NF membranes
Fourier transform infrared spectrometer (ATR-FTIR, Vectot 22 FTIR spectrometer, Bruker Optics) and DXR Smart Raman spectrometer (Thermo Fisher Scientific) were used to investigate the functional groups on the TA–FeIII/PES composite NF membrane surfaces. The FTIR absorption spectroscopy was obtained in the region of 400–4000 cm−1 with resolution 4 cm−1 for 64 scans. Raman spectroscopy of the TA–FeIII/PES composite NF membranes was acquired by a He–Ne laser at a wavelength of 633 nm.X-ray photoelectron spectroscopy (XPS, PerkinElmer Phi 1600 ESCA system), using Al Kα (1486.6 eV) as the radiation source, was used to evaluate the surface compositions of the TA–FeIII/PES composite NF membranes. Survey spectra were collected over a range of 0–1100 eV. The takeoff angle of the photoelectron was set at 90°, corresponding to the measured depth of about 10 nm. The static contact angles of the TA–FeIII/PES composite NF membranes were measured by a contact angle goniometer (JC-2000C Contact Angle Meter, Powereach Co., Shanghai, China) at room temperature. At least six measurements at different locations on one surface were averaged to get a reliable value.
Morphological characteristics of the TA–FeIII/PES composite NF membranes were based on their surface images, which were obtained by using field emission scanning electron microscope (FESEM, Nanosem 430). Before taking the SEM image, the membranes were freeze-dried and sputtered gold to increase the sample conductivity. The element mapping was also conducted with Nanosem 430 FESEM equipped with energy-dispersive X-ray spectroscopy (EDX) of ISIS300 (Oxford) to detect the ferric ion (FeIII) distribution profiles on the TA–FeIII/PES composite NF membrane surfaces.
The surface images and roughness of the TA–FeIII/PES composite NF membrane surfaces were measured by atomic force microscopy (AFM, Nanoscope IIIA AFM, Digital Instruments, Santa Barbara, CA, USA). The root mean square roughness values in 5 μm × 5 μm area of the membrane surfaces were calculated from high profiles of three dimensional AFM images.
2.4 Separation property of TA–FeIII/PES composite NF membranes
The separation performance of the TA–FeIII/PES composite NF membranes was evaluated by a dead end filtration system, consisting of a filtration cell (model 8200, Millipore Co., USA, effective membrane area was 28.7 cm2) with a volume capacity of 200 mL and a solution reservoir. The operation pressure of the system was maintained by pressurized nitrogen gas. All the experiments were carried out at a stirring speed of 400 rpm and a temperature of 25 ± 2 °C. Each membrane was initially compacted at 0.25 MPa for 0.5 h by pure water before measurement at 0.20 MPa. The water flux Jw (L m−2 h−1) was calculated by the equation:
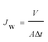 | (1) |
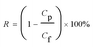 | (2) |
2.5 Stability of TA–FeIII/PES composite NF membranes
The stability of the TA–FeIII/PES composite NF membranes at different pH values (pH = 2, 6, and 10 for acidic, neutral, and alkaline aqueous solutions, respectively) was first investigated. After immersion into feed solutions over 24 h, the fluxes and dye rejection of the TA–FeIII/PES composite NF membranes were measured, respectively.The oxidation resistant ability of the TA–FeIII/PES composite NF membranes was then investigated in detail. The TA–FeIII/PES composite NF membranes were immersed into NaClO solution containing 100 ppm available chlorine for a certain time. The NaClO solution was replaced with the freshly prepared solution every two days. After washing to remove the remaining NaClO solution, water contact angle, permeation and rejection of the TA–FeIII/PES composite NF membranes were measured again. The functional groups on the TA–FeIII/PES membrane surfaces after oxidation were also characterized with FTIR spectroscopy.
3. Results and discussion
3.1 Chemical features of TA–FeIII/PES composite NF membranes
TA was a water-soluble high molecular weight polyphenolic compound containing sufficient galloyl groups, which could provide binding sites for metal ions to chelate. Metal chelation was a salient feature of TA molecule (like many other polyphenols), upon which it acted as a polydentate ligand for metal ion coordination.11,19,20 A green coating method was proposed to prepare composite NF membranes without organic solution and toxic reagent, which was shown in Fig. 1. When PES membranes were immersed into TA aqueous solution. TA molecules would diffuse into the surface and pores of support, eventually formed an active layer attributed to the attraction of galloyl and catechol groups, which had a high affinity for a wide variety of substrates.13,21 After the PES membranes containing TA were subsequently immersed into FeCl3 solution, FeIII ion would be successfully introduced to the resultant membranes, originating from the coordination reaction between TA and FeIII ions. The galloyl groups of TA reacted with FeIII ion to form a stable metal–polyphenol complex coating upon PES support. The visible color of the membranes with the TA–FeIII coating was dark blue at neutral pH value range (Fig. 7A).FTIR spectra of PES substrate and the TA–FeIII/PES composite NF membranes were given in Fig. S1.† It was evident that besides the typical PES bands of the substrate, the TA–FeIII/PES composite NF membranes exhibited additional peaks at 1716 and 3456 cm−1, which corresponded to ester groups and hydroxyl groups of TA.16 It obviously indicated the successful adsorption of TA and coordination between TA and FeIII ion on the PES membrane surfaces. Meanwhile, the relative peak intensities of ester groups and hydroxyl groups were increased with an increase of TA concentration in aqueous solution, which illustrated that the amount of deposited metal–polyphenol complex was increased with the corresponding increase of reaction monomer concentration in aqueous phase.
The characteristic peaks of TA–FeIII coordination complex in the TA–FeIII/PES membrane surfaces clearly appeared in Raman spectra. In Fig. 2, the peaks at 528 and 633 cm−1 were assigned specifically to bidentate chelation of the FeIII ion by the phenolic oxygen of catechol, that is C3 and C4 oxygen of the TA molecules,22 which was used throughout as an indicator of the presence of TA–FeIII coordination. Meanwhile, the peaks in the 1100–1600 cm−1 region (1229, 1335 and 1478 cm−1 peaks) arose from catechol ring vibrations,23,24 specifically originating from the presence of TA on the TA–FeIII/PES composite NF membrane surfaces.
 | ||
| Fig. 2 Raman spectroscopy of PES substrate (0# membrane) and TA–FeIII/PES composite NF membrane (3# membrane). | ||
The successful incorporation of TA–FeIII complex coating could be further confirmed by XPS analysis in Fig. 3. The carbon (C1s), oxygen (O1s) and sulfur (S2s and S2p) peaks appeared in the XPS spectrum of PES support. After treatment with TA and FeCl3, the intensities of characteristic peaks (S2s and S2p) weakened, and new peaks for iron (Fe2p) emerged in the TA–FeIII/PES composite NF membranes. Quantitative analysis of the surface chemical composition also supported the successful deposition of TA–FeIII coating. The amount of Fe (Fe2p) reached to 0.36%, and O (O1s) was increased from 20.48% to 24.59%, respectively, with a concurrent decrease in the amount of S (S2s and S2p) from 4.18% to 1.38%. The presence of S element was also demonstrated that the TA–FeIII coating was a thin layer within 10 nm, corresponding to the measured depth of about 10 nm. In Fig. 4, the EDS image also showed that Fe element was relatively evenly distributed on the membrane surfaces. All these results indicated that the TA–FeIII coating was successfully formed on the support.
 | ||
| Fig. 3 XPS spectra of PES substrate (0# membrane) and TA–FeIII/PES composite NF membrane (3# membrane) surfaces. | ||
The surface and cross section morphologies of the TA–FeIII/PES composite NF membranes were showed in Fig. 4 and S2.† SEM images showed that the membrane surfaces became more compact and smooth with an increase of TA concentration (1#, 3#, and 5# membranes), corresponding to the AFM results that the Rms of TA–FeIII/PES composite NF membranes were decreased from 4.46 ± 0.15 to 3.44 ± 0.20 nm (Fig. 5). Furthermore, the AFM images showed a grainy surface with higher roughness (Rms = 5.25 ± 0.20 nm) with an increase of FeCl3 concentration. The galloyl and catechol groups from TA had the potential to attach on different substrate surface with different charges, as well as coordinate with FeIII to form a stable metal–polyphenol complex.25,26 With the increase of TA concentration, more TA molecules attached on the PES support and coordinated with FeIII, consequently forming a more compact and smooth coating. But when the FeIII concentration were further increased, the excess FeIII induced aggregation of TA–FeIII complex on the membrane surface and sequentially increased roughness.27 It revealed that sufficient TA could provide more binding sites for iron ions to chelate, which could avoid the complex aggregation and obtain more uniform and smooth surfaces of the TA–FeIII/PES composite NF membranes.
 | ||
| Fig. 5 SEM and AFM images of the top surface morphologies of TA–FeIII/PES composite NF membranes (1, 3, 5 and 9# membranes). | ||
The surface hydrophilicity of the membranes has a significant impact on the membrane permeability. Water contact angles of TA–FeIII/PES composite NF membranes prepared in different concentration of TA and FeCl3 were given in Table 1. The water contact angle of PES substrate was 74.1 ± 3.2° (0# membrane). For TA–FeIII/PES composite NF membranes at a fixed FeCl3 concentration of 1.0 g L−1, the water contact angles were changed apparently from 53.2 ± 2.1° to 36.3 ± 1.5° when the TA concentration was increased from 0.5 to 3.0 g L−1 (from 1# to 6# membrane). However, when the concentration of TA was fixed at 1.5 g L−1, the water contact angles were changed slightly with an increase of FeCl3 concentration from 0.5 to 2.0 g L−1 (from 7# to 9# membrane). Therefore, it implied that the concentration of TA rather than FeCl3 played a dominant factor in affecting the surface hydrophilicity of membranes. The hydroxyl groups from galloyl and catechol of TA, providing hydrogen bonding sites to adsorb water molecule,28 took the main contribution in enhancing the surface hydrophilicity of TA–FeIII/PES composite NF membranes.
| Membrane | The concentration of monomers (g L−1) | Water contact (°) | ||
|---|---|---|---|---|
| TA | FeCl3 | Ratio (TA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FeIII) FeIII) |
||
| 0# | 0.0 | 0.0 | 0 | 74.1 ± 3.2 |
| 1# | 0.5 | 1.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
53.2 ± 2.1 |
| 2# | 1.0 | 1.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45.7 ± 1.8 |
| 3# | 1.5 | 1.0 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
43.1 ± 2.0 |
| 4# | 2.0 | 1.0 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
42.5 ± 1.2 |
| 5# | 2.5 | 1.0 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
37.1 ± 0.9 |
| 6# | 3.0 | 1.0 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
36.3 ± 1.5 |
| 7# | 1.5 | 0.5 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40.2 ± 1.3 |
| 8# | 1.5 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40.3 ± 1.9 |
| 9# | 1.5 | 2.0 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
43.3 ± 2.1 |
3.2 Separation performance of TA–FeIII/PES composite NF membranes
The preparation conditions (such as monomer concentration, reaction time) were investigated to optimize the separation performance of TA–FeIII/PES composite NF membranes. Firstly, the reaction time had little influence on the water flux and rejection of the membranes (Fig. S3†), which indicated that the coating formation process was completed instantaneously, not enhanced with the extension of time. It was consistent to traditional interfacial polymerization.29 Once the coordination complex formed a thin active layer, it would block excess monomers into active layer for reaction no matter how the contact time extended. The reaction time for TA–FeIII coating of subsequently test was fixed at 1 min. Fig. 6 presented the effect of TA concentration on fluxes and rejection of the TA–FeIII/PES composite NF membranes measured at operation pressure of 0.20 MPa, while the FeCl3 concentration was fixed at 1.0 g L−1. With an increase of TA concentration from 0.5 to 1.5 g L−1, water flux was obviously decreased from 161.4 to 45.6 L m−2 h−1, the orange GII rejection ratio was increased from 71.5% to 94.8%, and Na2SO4 rejection ratio was dramatically increased from 20.6% to 62.1%. The separation performance of the TA–FeIII/PES composite membranes was really in the NF range. The TA–FeIII/PES composite NF membranes acquired similar pure water flux (both about 46 L m−2 h−1) to our previous study of TA–TMC composite NF membranes prepared by interfacial polymerization,16 but lower dye rejection and salt rejection, probably resulting from the slightly large pore size in the active layer of the TA–FeIII/PES composite NF membranes.It was claimed that the concentration of monomer had significant effect on the growth of coating during the coordination reaction.10,30 A higher TA concentration would increase the degree of coordination reaction to generate a thicker and more compact coating. It could be definitely demonstrated by the result that the water flux was decreased to 34.3 L m−2 h−1, and the orange GII rejection was elevated to 95.5% when the concentration of TA was increased from 0.5 to 3.0 g L−1. In complete contrast to these observations based on TA, FeIII ions had minimal impact upon coating assembly and separation performance of the TA–FeIII/PES composite NF membranes (Fig. S4†). When FeIII ions concentration was elevated from 0.5 to 2.0 g L−1, the change of orange GII rejection was in the range of 10% and water flux was within 15%. Based on the above results, the optimum preparation condition for the TA–FeIII/PES composite NF membranes were as follows: TA concentration 1.5 g L−1, FeCl3 concentration 1.0 g L−1, and reaction time 1 min.
3.3 Stability of the TA–FeIII/PES composite NF membranes
After 24 hours' filtration experiment, the water flux and the dyes rejection ratio of composite membrane all kept stable values (less than 4.4% decline), indicating that the membranes possessed good durability (Fig. S5†), the coordination effect between catechol groups and metal ion was still relevant to pH value, generally presenting different color or color depth. The pH value was also found to be the major factor governing the formation of different types of metal–polyphenol complexes.19,22 The complexes of TA and FeIII ion were undoubtedly pH-dependent, similarly the obtained TA–FeIII/PES composite NF membranes exhibited pH-dependent property. The influence of pH on the membrane performance was investigated by changing the pH values (from 2 to 6 and 10) of feed solutions, which resulted in a visible change in color from colorless to blue and brown (Fig. 7A). It was claimed that mono(catecholato)-FeIII, bis(catecholato)-FeIII, and tris(catecholato)-FeIII were known to present colorless, blue and brown, respectively.14,19 Therefore, color changes implied that mono-, bis-, and tris(catecholato)-FeIII co-contributed to the coating assembled on the PES supports (Fig. 7B). Meanwhile, the water permeability and rejection of dyes and salts of TA–FeIII/PES composition NF membranes were changed at different pH values (Fig. 7C).Compared with feed solution of pH 6, the water flux of feed solution at pH 2 was dramatically increased from 40.1 to 73.4 L m−2 h−1 and the rejection ratio of orange GII was decreased from 70.7% to 41.6%. It illustrated that at low pH value, most of the hydroxyl groups were protonated, which led to rapid and slight destabilization of cross-links, sequentially enlarged the pore size in the active layer of the TA–FeIII/PES composite NF membranes and resulted in a high water flux but a low dye rejection. But the structure of membranes was still stable, disassembly and peeling of the coating were not observed. When the feed solution with water at pH 10, tris-type complexes was a dominant species, might give rise to more compact membrane structure and better separation performance compared with that of lower pH value. But in fact, the water flux and the rejection of orange GII were similar with that of feed solution of pH 6, originating from the generation of ferric hydroxide which might interfere with the coordination between TA and FeIII.13–15 Therefore, the TA–FeIII/PES composition NF membranes could be used in a relatively wide pH range in practical application.
3.4 Antioxidant ability of the TA–FeIII/PES composite NF membranes
Free chloride was generally employed as a disinfectant to kill microorganisms in the practical application of NF process. But for most of the NF membranes prepared by interfacial polymerization, the traditional polyamide active layer might be destroyed inevitably by free chloride, inducing to deprive the separation performance of polyamide active layer and shorten the membrane lifespan. In this study, the oxidation resistance for the TA–FeIII/PES composite NF membranes was investigated to verify the possibility in long-term service. The TA–FeIII/PES composite NF membranes were immersed in NaClO solution containing 100 ppm available chlorine for a certain time. The separation performance of the TA–FeIII/PES composite NF membranes was shown in Fig. 8. With extension of immersing time, the water flux was decreased from 45.6 to 28.3 L m−2 h−1 and the orange GII rejection still remained stable after immersing in NaClO solution for 12 days. Additionally, the water contact angle of TA–FeIII/PES composite NF membrane was elevated from 43.1° to 59.5° (Fig. S6A†).According to FTIR spectra, a new peak at 1610 cm−1 appeared on the spectrum of the immersed membrane (Fig. S6B†), corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in quinone bonds,31 which indicated the existence of polyphenol oxide compounds on the membrane surface. These results attributed that the TA are polyphenols with abundant OH groups in ortho positions, which could be oxidized into ortho-quinone via the semiquinone radicals in sodium hypochlorite solution.32 Incompletely complex OH groups of TA were sacrificed in the process of immersing in NaClO solution, which slightly decreased the hydrophilicity of membranes. The strong relationship between hydrophilicity and permeability of membranes correspondingly caused water flux decreasing.33 But the immersion into oxidizing solution didn't influence the compact structure of TA–FeIII coating. The TA–FeIII complex coating kept consistent pore size, which made the membranes obtain stable orange GII rejection. Therefore, the TA–FeIII/PES composite NF membranes possessed good chemical stability and oxidation resistance ability into NaClO solution, which is also benefit to practical application.
O stretching vibration in quinone bonds,31 which indicated the existence of polyphenol oxide compounds on the membrane surface. These results attributed that the TA are polyphenols with abundant OH groups in ortho positions, which could be oxidized into ortho-quinone via the semiquinone radicals in sodium hypochlorite solution.32 Incompletely complex OH groups of TA were sacrificed in the process of immersing in NaClO solution, which slightly decreased the hydrophilicity of membranes. The strong relationship between hydrophilicity and permeability of membranes correspondingly caused water flux decreasing.33 But the immersion into oxidizing solution didn't influence the compact structure of TA–FeIII coating. The TA–FeIII complex coating kept consistent pore size, which made the membranes obtain stable orange GII rejection. Therefore, the TA–FeIII/PES composite NF membranes possessed good chemical stability and oxidation resistance ability into NaClO solution, which is also benefit to practical application.
Compared to our previous work of TA–TMC composite NF membranes prepared by interfacial polymerization,16 the chemical stability experiment strongly demonstrated that the TA–FeIII coordination coating was much stable than the polyester thin film of TA–TMC composite NF membrane and polyamide active layer of PIP–TMC composite NF membrane.16 After immersing in NaClO solution for 12 days, the TA–FeIII/PES composite NF membranes still possessed stable dye rejection of orange GII and congo red, keeping more than 92% and 97%, respectively. But the orange GII rejection of TA–TMC composite NF membrane was decreased by 20% after immersing for 10 days, and that of PIP–TMC composite NF membrane was even decreased by 70% after immersing for 4 days. As for traditional and widely used thin-film composite polyamide membrane prepared by m-phenylenediamine (MPD) and TMC, after NaClO treatment for 2 days, the water flux increased to fifty times and the NaCl rejection declined to almost 0 compared with the initial membranes. The membrane structure was obviously destroyed, correspondingly showing bad oxidation resistance ability.34 The green coating by coordination of polyphenol and metal irons possessed excellent chemical stability and oxidation resistance, which could significantly develop the preparation method of composite NF membranes.
4. Conclusions
TA–FeIII/PES composite NF membranes were successfully prepared by green coating method, which was not only facile and simple, but also had no harmful impact on environment to the best of our knowledge. The coating was formed by the coordination of hydroxyl groups from TA and FeIII ions upon porous PES support. TA concentration had significant influence on the separation performance of the TA–FeIII/PES composite NF membranes, salt and dye rejection ratios were increased, and water flux was decreased with the increase of TA concentration. TA–FeIII/PES composite NF membranes possessed good structural stability without disassembly and peeling under the condition of using in acid or alkaline solution. TA–FeIII/PES composite NF membranes had excellent chemical stability and antioxidant ability. After immersing into NaClO solution for a long term, the TA–FeIII/PES composite NF membranes still remained initial rejection property.Acknowledgements
This research is supported by Tianjin Natural Science Foundation (No. 13JCYBJC20500, 14JCZDJC37400), and National Science Fund for Distinguished Young Scholars (21125627).References
- A. I. Schäfer, A. G. Fane and T. D. Waite, Nanofiltration – Principles and Applications, Elsevier Advanced Technology, New York, 2005 Search PubMed
.
- A. El-Hashani, A. Toutianoush and B. Tieke, J. Membr. Sci., 2008, 318, 65–70 CrossRef CAS
.
- M. V. Tres, H. C. Ferraz, R. M. Dallago, M. Di Luccio and J. V. Oliveira, J. Membr. Sci., 2010, 362, 495–500 CrossRef CAS
.
- G. Székely, J. Bandarra, W. Heggie, B. Sellergren and F. C. Ferreira, J. Membr. Sci., 2011, 381, 21–33 CrossRef
.
- M. H. Wu and Y. Q. Zhang, RSC Adv., 2014, 4, 4140–4145 RSC
.
- Y. P. Zheng, S. C. Yu, S. Shuai, Q. Zhou, Q. B. Cheng, M. H. Liu and C. J. Gao, Desalination, 2013, 314, 89–95 CrossRef CAS
.
- V. Freger, Langmuir, 2003, 19, 4791–4797 CrossRef CAS
.
- M. B. Schenker and J. A. Jacobs, Tuber. Lung Dis., 1996, 77, 4–18 CrossRef CAS PubMed
.
- A. Muezzinoglu, M. Odabasi and L. Onat, Atmos. Environ., 2001, 35, 753–760 CrossRef CAS
.
- M. A. Rahim, H. Ejima, K. L. Cho, K. Kempe, M. Müllner, J. P. Best and F. Caruso, Chem. Mater., 2014, 26, 1645–1653 CrossRef CAS
.
- S. Kim, D. S. Kim and S. M. Kang, Chem.–Asian J., 2014, 9, 63–66 CrossRef CAS PubMed
.
- S. W. Taylor, D. B. Chase, M. H. Emptage, M. J. Nelson and J. H. Waite, Inorg. Chem., 1996, 35, 7572–7577 CrossRef CAS
.
- H. Ejima, J. J. Richardson, K. Liang, J. P. Best, M. P. van Koeverden, G. K. Such, J. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed
.
- Q. Wei, K. Achazi, H. Liebe, A. Schulz, P. L. Noeske, I. Grunwald and R. Haag, Angew. Chem., Int. Ed., 2014, 53, 11650–11655 CrossRef CAS PubMed
.
- S. Kim, T. Gim and S. M. Kang, ACS Appl. Mater. Interfaces, 2015, 7, 6412–6416 CAS
.
- Y. Zhang, Y. L. Su, J. M. Peng, X. T. Zhao, J. Z. Liu, J. J. Zhao and Z. Y. Jiang, J. Membr. Sci., 2013, 429, 235–242 CrossRef CAS
.
- W. Zhao, Y. L. Su, C. Li, Q. Shi, X. Ning and Z. Y. Jiang, J. Membr. Sci., 2008, 318, 405–412 CrossRef CAS
.
- Y. Q. Wang, T. Wang, Y. L. Su, F. B. Peng, H. Wu and Z. Y. Jiang, Langmuir, 2005, 21, 11856–11862 CrossRef CAS PubMed
.
- H. Xu, J. Nishida, W. Ma, H. Wu, M. Kobayashi, H. Otsuka and A. Takahara, ACS Macro Lett., 2012, 1, 457–460 CrossRef CAS
.
- J. Guo, Y. Ping, H. Ejima, K. Alt, M. Meissner, J. J. Richardson, Y. Yan, K. Peter, D. von Elverfeldt, C. E. Hagemeyer and F. Caruso, Angew. Chem., Int. Ed., 2014, 53, 5546–5551 Search PubMed
.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed
.
- N. Holten-Andersen, M. J. Harrington, H. Birkedal, B. P. Lee, P. B. Messersmith, K. Y. C. Lee and J. H. Waite, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2651–2655 CrossRef CAS PubMed
.
- B. A. Sommer and D. W. Margerum, Inorg. Chem., 1970, 9, 2517–2521 CrossRef
.
- I. Michaud-Soret, K. K. Andersson and L. Que, Biochemistry, 1995, 34, 5504 CrossRef CAS PubMed
.
- M. Andjelkovic, J. Vancamp, B. Demeulenaer, G. Depaemelaere, C. Socaciu, M. Verloo and R. Verhe, Food Chem., 2006, 98, 23–31 CrossRef CAS
.
- T. Mori, K. Rezai-Zadeh, N. Koyama, G. W. Arendash, H. Yamaguchi, N. Kakuda, Y. Horikoshi-Sakuraba, J. Tan and T. Town, J. Biol. Chem., 2012, 287, 6912–6927 CrossRef CAS PubMed
.
- M. J. Harrington, A. Masic, N. Holten-Andersen, J. H. Waite and P. Fratzl, Science, 2010, 328, 216–220 CrossRef CAS PubMed
.
- H. J. Kim, D. G. Kim, H. Yoon, Y. S. Choi, J. Yoon and J. C. Lee, Adv. Mater. Interfaces, 2015, 1500298, DOI:10.1002/admi.201500298
.
- P. G. Ingole, H. C. Bajaj and K. Singh, RSC Adv., 2013, 3, 3667–3676 RSC
.
- S. Zhang, Z. Jiang, X. Wang, C. Yang and J. Shi, ACS Appl. Mater. Interfaces, 2015, 7, 19570–19578 CAS
.
- J. Breton, C. Boullais, J. Burie, E. Nabedryk and C. Mioskowskis, Biochemistry, 1994, 33, 14378–14386 CrossRef CAS PubMed
.
- J. Gust and J. Suwalski, Corros. Sci., 1994, 50, 355–365 CrossRef CAS
.
- Q. Shi, Y. L. Su, S. P. Zhu, C. Li, Y. Y. Zhao and Z. Y. Jiang, J. Membr. Sci., 2007, 303, 204–212 CrossRef CAS
.
- T. Shintani, H. Matsuyama, N. Kurata and T. Ohara, J. Appl. Polym. Sci., 2007, 106, 4174–4179 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23490e |
| This journal is © The Royal Society of Chemistry 2015 |





