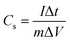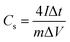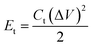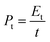Oxygen-enriched hierarchical porous carbon derived from biowaste sunflower heads for high-performance supercapacitors†
Di Ma,
Guang Wu,
Jiafeng Wan*,
Fangwei Ma*,
Weidan Geng and
Shijiao Song
Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education of China, School of Chemistry and Material Science, Heilongjiang University, Harbin 150080, China. E-mail: wanjiafeng@hlju.edu.cn; Fangwei_ma@hotmail.com; Tel: +86 451 8660 8616
First published on 14th December 2015
Abstract
Sunflower heads, a biowaste from sunflower crop, was first explored as a sustainable precursor to synthesize porous carbon (PC) as a supercapacitor electrode material. Here we report the preparation of oxygen-enriched PC by the simple and low-cost carbonization of sunflower heads followed by KOH activation. The resulting carbon has a hierarchical pore structure comprised of abundant micropores and mesopores, a specific surface area of 1032 m2 g−1 and a high oxygen content of 21 wt%. Due to the above unique porous nature and oxygen enrichment, the sunflower head carbon (SFHCs) exhibits a high specific capacitance of 345 F g−1 at 1 A g−1 in 6 M KOH aqueous electrolyte and shows considerable rate capability with a retention of 81% at 20 A g−1. Moreover, the SFHCs symmetric supercapacitor delivers a high energy density of 9.2 W h kg−1 at a power output of 482 W kg−1. This study first introduces the utilization of biowaste sunflower heads as a renewable precursor to prepare high value-added porous carbon for high performance supercapacitors.
1 Introduction
Supercapacitors have received increasing attention for decades due to their high power density,1,2 fast charge–discharge rate and long cycling stability and have been attractive for various applications, including consumer electronics, renewable energy, hybrid electrical vehicles and other successive power supplies.3–5 Generally, on the basis of the energy storage mechanism and the nature of the electrode material, supercapacitors can be classified into two categories: electrical double layer capacitors (EDLC), where the electrostatic charge accumulates at the electrode/electrolyte interface, and pseudocapacitors with fast and reversible redox reactions taking place between the electrolyte and electrode's functional groups.6–8 Porous carbon (PC), especially activated carbon (AC), is one of the most widely researched electrode materials for EDLCs due to its stable thermal and chemical properties, high specific surface area, high electronic conductivities, environmental friendliness, and abundance of raw material.9,10 In addition, heteroatom species (such as oxygen and nitrogen atoms) involved in carbon-based EDLCs can effectively improve the surface wettability and bring in pseudocapacitance contribution by participating in various reversible faradaic reactions, which is beneficial to enhance the performance of supercapacitors.11–13PC with higher specific capacitance, long cycle life and low cost has long been regarded as the most promising candidate electrode materials for EDLCs. However, the commercial porous carbon electrode materials for supercapacitor are primarily composed of micropores with majority of pore size below 2.0 nm, which leads to a low charge storage capability and poor capacitance retention especially at very high discharge rate, hampering their utilization for high power and energy density supercapacitor to a great extent. To overcome the above dilemma, hierarchical PC consisted of micro-, meso- and macropores are currently attracting a great degree of interest as supercapacitor electrodes.14,15 Among them, the mesopores can act as fast ion-transportation channels to enhance ion diffusion kinetics, and the micropores serve as a high loading of accessible ion storage sites to enhance the capacitance.
Recently, various porous carbons derived from biomass have been attracting much attention and widely used as supercapacitor electrodes.16–18 Biomass has exhibited several advantages for carbon sources to access PCs. Biomass is not only generally renewable, inexpensive, and environmentally benign, but also possesses inherent architecture and abundant heteroatoms. Up to now, the two most simple, economic, and efficient techniques to prepare PC from agricultural and forest biomass are physical activation with different oxidizing gases, such as air, O2, CO2, steam or their mixtures, and chemical activation with KOH, NaOH, H3PO4 or ZnCl2.9,19 A small portion of biomass such as broad beans,20 bamboos,21 seaweed biopolymers,22 rice husk,23 etc. have been successfully developed as carbon precursors for supercapacitor electrodes. Many biomass-derived doped PCs have presented superior performance for supercapacitor. For example, oxygen-rich PC derived from artemia cyst shells possessed the high capacitance of 330 F g−1 at 1 A g−1.24 The rich oxygen-containing AC prepared from shiitake mushroom delivered the capacitance of 306 F g−1 at 1 A g−1.25 Similarly, nitrogen-rich carbons derived from egg white and Broussonetia papyrifera were also utilized as effective electrode materials and achieved the specific capacitance of 325 F g−1 and 300 F g−1, respectively.26,27 Therefore, exploring new precursors those are cheap, accessible and renewable, especially agricultural wastes, is a very imperative work.
Sunflower, as one of the world's four major oil crops, is widely planted all over the world. In China, sunflower is mainly cultivated in northeast, northwest and north of China. Sunflower heads, byproducts of sunflower industry, are very abundant and readily available, and tons of sunflower heads can be heavily generated from sunflower farm in China every year. However, sunflower heads have not been fully utilized so far. Only a small portion of sunflower heads were used to extract pectin,28,29 a great amount of sunflower heads were discarded as agricultural wastes. Hence, it is a very valuable work to explore techniques of producing high value-added porous carbon materials from sunflower heads as low-cost, renewable carbon precursors. Here, we reported a simple approach to produce hierarchical PC with abundant oxygen functional groups from the waste sunflower heads by means of pre-carbonization followed by KOH activation. The SFHCs possess a high specific surface area of 1032 m2 g−1, hierarchical porous structure and enriched oxygen groups (21 wt%). The SFHCs exhibit a high capacitance of 345 F g−1 at a current density of 1 A g−1, good rate capability in a three-electrode cell. For a two-electrode system, the SFHCs deliver a high energy density of 9.2 W h kg−1 at the power density of 482 W kg−1.
2 Experimental section
2.1 Preparation of sunflower head derived carbons
The sunflower heads, stripped of seeds and sepals, were collected from farmland of Harbin suburbs, China. Fresh sunflower heads were washed with deionized water to remove the impurities and cut into small pieces, and then dried at 80 °C for 12 h. The dry samples were pre-carbonized in ceramic crucible at 500 °C with a heating rate of 5 °C min−1 for 1 h under N2 flow. Afterwards, the pre-carbonized materials were mixed with KOH solution at different concentrations (weight ratio of materials/KOH is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1
1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Then the samples were pyrolyzed at 800 °C for 1 h under N2 flow. The temperature was ramped from room temperature to 800 °C at 5 °C min−1. Finally, the as-obtained activated sample was grounded to powder, washed with 1 M HCl solution to remove any inorganic impurities, and then washed with deionized water until neutral pH of the filtrate. The residue was finally dried at 120 °C for 12 h in air. The resultant sunflower head derived carbon materials are denoted as SFHC-1:1, SFHC-1:1.5, SFHC-1:2 when the weight ratio of pre-carbonization sample with KOH solid is 1
2). Then the samples were pyrolyzed at 800 °C for 1 h under N2 flow. The temperature was ramped from room temperature to 800 °C at 5 °C min−1. Finally, the as-obtained activated sample was grounded to powder, washed with 1 M HCl solution to remove any inorganic impurities, and then washed with deionized water until neutral pH of the filtrate. The residue was finally dried at 120 °C for 12 h in air. The resultant sunflower head derived carbon materials are denoted as SFHC-1:1, SFHC-1:1.5, SFHC-1:2 when the weight ratio of pre-carbonization sample with KOH solid is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, respectively.
1.5, respectively.
2.2 Sample structure and morphology characterizations
The prepared carbon materials were tested with field emission scanning electron microscopy (SEM, Hitachi S-4800) and transmission electron microscope (TEM, JEOL-JEM2100) at an acceleration voltage of 200 kV and the structure was examined using Raman spectra (JY Horiba HR800). X-ray diffraction (XRD) patterns were collected with a Bruker D8 X-ray diffractometer using Cu-Kα (λ = 1.5406 Å) at step scan 0.02° from 10° to 80°. Raman spectra were recorded by a LabRAM HR Raman spectrometer with a 514.5 nm laser excitation. Nitrogen sorption analysis was carried out at 77 K using an ASAP 2420 surface area and porosimetry instrument. The surface area was calculated by Brunauer–Emmett–Teller (BET) method and the pore size distribution plots were recorded from the desorption branch of the isotherms based on the Barrett–Joyner–Halenda (BJH) model. The elemental analysis and surface states were characterized by X-ray photoelectron spectrometer (XPS) measurement was performed on a VG ESCALAB MK II (VGScientific, UK).2.3 Electrochemical performance
To obtain the electrochemical properties of the prepared samples, both three-electrode and two-electrode configurations were used. All electrochemical characterizations were carried out on a CHI660E electrochemical workstation (Shanghai Chenhua Instruments Co.) at room temperature. 10 wt% polytetrafluoroethylene, 10 wt% acetylene black and 80 wt% SFHC were fully mixed and grounded in a mortar. Then the slurry was pressed onto nickel foam current collector at 6 MPa (the active mass is about 1.6 mg) to form the working electrode. In three-electrode system, the above loaded nickel foam (1.0 × 1.0 cm2), a platinum foil and an Ag/AgCl were used as the working, counter and reference electrodes, respectively. As for the basic electrolyte (6 M KOH), the cyclic voltammetry curves were obtained with a potential range from −1.0 V to 0 V. Galvanostatic charge–discharge measurements were done at 1–30 A g−1 over a voltage range of −1.0 V to 0 V. Electrochemical impedance spectroscopy (EIS) was conducted over a frequency range from 100 kHz to 10 mHz at open circuit potential with the amplitude of 5 mV. The specific gravimetric capacitances in two kinds of electrolyte were all calculated from the discharge process according to:where I (A) is the charge/discharge current, m (g) is the mass of active material, and ΔV (V) is the voltage change within the discharge time Δt (s) excluding the IR drop during the discharge process.
For a two-electrode system, the two symmetrical electrodes and a porous polypropylene separator were sandwiched together in a poly(tetrafluoroethylene) cell. Charge–discharge measurements were done galvanostatically in 6 M KOH at a current density of 0.5–10 A g−1 between −1.0 V and 0 V and the cyclic voltammetry tests for these devices were performed in the potential range of −1.0 V to 0 V by varying the scan rate from 10 to 200 mV s−1.
The specific capacitance of a single electrode in the supercapacitor cell can be calculated according to the following equation:
where Et (W h kg−1) is the specific energy density, Pt (W kg−1) is the specific power density, Ct (F g−1) is the specific capacitance of the total symmetrical system, ΔV (V) is the cell voltage for charging and discharging excluding the IR drop during the discharge process, and Δt (s) is the discharge time.
3 Results and discussion
Fig. 1 illustrates a schematic diagram for the preparation of PC from sunflower heads. Sunflower heads were obtained from the fully ripened sunflower plants by removing the sepals and seeds. The cleaned and dried heads were cut into fine debris and pre-carbonized at 500 °C for 60 min. Then soaked the pre-carbon with KOH aqueous solution and further carbonized at 800 °C to obtain sunflower heads-derived hierarchical PC. It is noted that KOH activation plays an important role in the development of the micropore structure in SFHCs.Fig. 2a shows a representative SEM micrograph of SFHC-1:1.5. The carbonized and activated sample has morphologically micro-monolithic structure, with a small number of macropores in rough surface. The higher magnification SEM image of the sample (Fig. 2b) displays a rough surface composed of massive tiny nano-particles. The SFHC-1:1 and SFHC-1:2 specimens also possess an analogous macroscopic morphology (Fig. S1†). These numerous irregular particles assembled each other may generate larger mesopores, which can be clearly observed in TEM image. Further information about the microstructure and morphology of SFHC was obtained from transmission electron microscopy (TEM) shown in Fig. 2c and d. Large numbers of light spots are observed representing the existence of plentiful small-mesopores (Fig. 2c), which can be attributed to the collapse and connection of micropores during the activation process. Moreover, several light areas marked by circle lines indicate the presence of moderate mesopores with pore size about 20 nm in the carbon framework, which confirms the deduction from the above SEM observation. In addition, the disordered slit-like micropores can be clearly seen in a high-resolution TEM image, as shown in Fig. 2d. This result also indicates that the micropore structure is distributed all over the skeleton of the carbon due to sufficient KOH activation.
 | ||
| Fig. 2 (a and b) Scanning electron microscopy (SEM) image and (c and d) transmission electron microscopy (TEM) images of SFHC-1:1.5 at different magnifications. | ||
The surface area and pore size distribution were analyzed on the basis of nitrogen physisorption measurements. As evidently shown in Fig. 3a, the SFHCs exhibit the combined type I/IV isotherm, which comprises of a rapid and distinct adsorption at low relative pressure (P/P0 < 0.1) and a type-H4 hysteresis loop at a relative pressure P/P0 ranging from 0.5 to 1.0, indicating the coexistence of abundant micropores and mesopores, which is well consistent with the TEM results. Micropores mainly contribute to a high surface area for the samples, and mesopores not only contribute to the large surface area, but also provide high adsorbate accessibility by providing wider transport channels to micropores. Such a hierarchical porous texture with coexistence of micropores and mesopores is desirable for high rate performance supercapacitor.30 The pore size calculated from the BJH model reveals that the pore size of the SFHCs is centered at less than 2.0 nm and 4.0 nm with a range of around 3.0–6.0 nm (Fig. 3b). It also can be seen that the peak centered at around 4.0 nm steadily upshifts to larger mesopore, resulting from KOH deeper activation. The porous properties of the SFHCs are summarized in Table 1. The specific surface areas of SFHC-1:1, SFHC-1:1.5 and SFHC-1:2 decrease from 1101, 1032 to 970 m2 g−1, and the pore volumes decrease from 0.62, 0.60 to 0.59 cm3 g−1 with increasing KOH dosage. Meanwhile, the mesopore surface area, mesopore volume and average pore size gradually increase. These results can be attributed to the intensive activation generated at high KOH dosage, leading to the etching wall of micropores and the collapse of pores, which may widen the pore size and reduce the fraction of micropore areas.
| Samples | SBET [m2 g−1] | Smicro [m2 g−1] | Smeso [m2 g−1] | Vpore [cm3 g−1] | Vmicro [cm3 g−1] | Vmeso [cm3 g−1] | Daver [nm] |
|---|---|---|---|---|---|---|---|
| SFHC-1:1 | 1101 | 906 | 195 | 0.62 | 0.47 | 0.15 | 2.25 |
| SFHC-1:1.5 | 1031 | 826 | 205 | 0.60 | 0.43 | 0.17 | 2.34 |
| SFHC-1:2 | 954 | 741 | 213 | 0.59 | 0.38 | 0.21 | 2.56 |
Fig. 3c shows powder X-ray diffraction (XRD) in the wide-angle region of these SFHC materials. There are two broad peaks at 2θ = 22.3°, and 43.8°, which correspond to the (002) and the (100) reflection of the graphitic stacks and the interlayer condensation, respectively.31 The broadness of the XRD peaks reflects the serious defective nature of SHPCs resulting from vast oxygen doping (confirmed by XPS results) and their amorphous carbon structure. Raman spectroscopy can be further elucidated the specific nature of the SFHCs (Fig. 3d). Two peaks located at around 1320 cm−1 and 1590 cm−1 are assigned to the characteristic D (defects and disorder carbon) and G (graphitic carbon) bands of carbon materials, respectively.32 The intensity ratio (ID/IG) of the D- and G-bands reflects the degree of disorder structure in SFHCs. Here, the ID/IG ratios of SFHC-1:1, SFHC-1:1.5, and SFHC-1:2 were determined to be 0.83, 0.85, and 0.8, respectively. This represents a high degree of disorder, amounts of edges, and lots of defects.
X-ray photoelectron spectroscopy (XPS) data were recorded to determine the surface elemental composition and chemical status. Four elements of C, O, N and S were detected in SFHC samples, while only two obvious peaks with binding energy at 284 eV, 532 eV and a weak peak at 400 eV were found in Fig. 4a, which are characteristic of C 1s, O 1s and N 1s orbital, respectively. The content of various elements present in the samples prepared under different activation conditions was calculated in Table 2. It is found that a large amount of oxygen heteroatoms are doped in the carbon framework and the O content in SFHCs-1:1.5 can reach as high as 21.25%. A small quantity of N heteroatoms were also introduced into these carbon samples, and the mass concentration of N element gradually decreases with enhancing the KOH dosage, meaning that the intense alkali activation has removed some nitrogen heteroatoms. Detailed C 1s peak of SFHC-1:1.5 (Fig. 4b) by deconvolution analysis shows four peaks at 284.1 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds), 285.1 eV (C–C bonds), 286.3 eV (C–O bonds) and 289.1 eV (O–C
C bonds), 285.1 eV (C–C bonds), 286.3 eV (C–O bonds) and 289.1 eV (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds).33 The fitted nitrogen XPS spectra (Fig. 4c) show the presence of pyridinic N (398.4 eV), pyrrolic N (400.3 eV) and quaternary N (401.3 eV).34,35 Four distinct peaks at around 531.5 eV, 532.1 eV, 533.7 eV and 535.1 eV in the O 1s spectra (Fig. 4d) respectively reveal the presence of C
O bonds).33 The fitted nitrogen XPS spectra (Fig. 4c) show the presence of pyridinic N (398.4 eV), pyrrolic N (400.3 eV) and quaternary N (401.3 eV).34,35 Four distinct peaks at around 531.5 eV, 532.1 eV, 533.7 eV and 535.1 eV in the O 1s spectra (Fig. 4d) respectively reveal the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O quinone type groups, C–OH phenol groups and/or C–O–C ether groups, carbonyl groups in anhydrides or carboxyls, and chemisorbed oxygen and/or water, which participate in faradaic reactions to generate pseudocapacitance and improve wettability to enhance exposed surface for charge storage.36–38
O quinone type groups, C–OH phenol groups and/or C–O–C ether groups, carbonyl groups in anhydrides or carboxyls, and chemisorbed oxygen and/or water, which participate in faradaic reactions to generate pseudocapacitance and improve wettability to enhance exposed surface for charge storage.36–38
 | ||
| Fig. 4 (a) XPS survey spectra of SFHC-1:1, SFHC-1:1.5 and SFHC-1:2; (b)–(d) high resolution XPS of C 1s, N 1s and O 1s peaks of SFHC-1:1.5, respectively. | ||
| Samples | C 1s [mass%] | O 1s [mass%] | N 1s [mass%] | S 2p [mass%] |
|---|---|---|---|---|
| SFHC-1:1 | 81.22% | 14.12% | 3.13% | 0.74% |
| SFHC-1:1.5 | 75.55% | 21.25% | 2.02% | 0.51% |
| SFHC-1:2 | 77.36% | 19.81% | 1.41% | 0.47% |
Electrochemical measurements were first performed in a three-electrode system to evaluate the supercapacitor performance of SHPCs in 6 M KOH aqueous electrolyte. The comparative CV plots of these ACs prepared at various dosage of KOH at a scan rate of 20 mV s−1 are shown in Fig. 5a. All the CV curves exhibit approximately rectangular shape (typical capacitive behavior) with weakly broadened humps at −1 V to −0.5 V, indicating that the capacitive behavior comes from the combination of EDLC and pseudocapacitance caused by redox reactions from oxygen functional groups in the carbon frameworks.39 We observed that the CV curve encircled area of SFHC-1:1.5 is the largest comparing with that of two other samples (SFHC-1:1 and SFHC-1:2), indicating that SFHC-1:1.5 possesses the highest specific capacitance. Fig. 5b shows the CVs of SFHC-1:1.5 within scan rates of 10–200 mV s−1. It can be observed that the quasi-rectangular shape and the redox peaks can be largely maintained even at a high potential scan rate of 200 mV s−1, indicating a good rate performance of the electrode attributed to hierarchical pores structure. Usually, the shape of the CV curves of PC electrodes should tilt and departure from the well rectangular shape at a high scan rate, mainly due to the unavoidable electrode and electrolyte resistances on account of large proportion of micropores.
The galvanostatic charge/discharge curves of the SFHCs at current density of 1 A g−1 (Fig. 5c) show nearly linear and symmetric triangular shapes with a gradual slope change between −1.0 V and −0.4 V, which are analogous to quasi-rectangular shape from CV results. This result might be attributed to the doping of oxygen atom, which improved the hydrophilicity and induced pseudocapacitive behaviour. Fig. 5d shows the GCD curves of SFHC-1:1.5 at different current densities from 1 to 10 A g−1, all GCD curves are almost symmetrical with slight distortion. The IR drop at current density below 2 A g−1 is negligible while increases gradually at higher current densities due to an inadequate time for electrolyte diffusion into the inner pores.
Rate capability is another key factor for the application of carbon-based electrode materials. The specific capacitance values of all the samples were calculated at different current densities ranged from 1 A g−1 to 30 A g−1 (Fig. 5e). For samples of SFHC-1:1, SFHC-1:1.5 and SFHC-1:2, their specific capacitances at the current density of 1 A g−1 are 317 F g−1, 345 F g−1 and 297 F g−1, respectively. Even at high current density of 20 A g−1, SFHC-1:1, SFHC-1:1.5 and SFHC-1:2 still maintained high specific capacitances of 255 F g−1, 280 F g−1 and 240 F g−1. When the current density increases from 1 to 20 A g−1, the capacitance retention can reach as high as 80%, 81% and 81%, respectively, revealing the good energy storage output capacity at high current density. The specific capacitance slightly decreases owing to the insufficient electrolyte ions diffusion kinetics across the micropores at higher operating current densities. It can be seen that SFHC-1:1.5 possesses the highest specific capacitance at all tested current densities, indicating its superior capacitor performance compared to the other two samples. This result probably attributes to appropriate allocation proportion of mesopores/microporous and abundant in oxygen functional groups, which are favorable for providing a convenient ion transfer pathway to enhance electrolyte accessibility to the microporous area.
The specific capacitance of SFHC-1:1.5 is compared to that of recently reported biomass derived carbon materials in Table 3. It is found that the hierarchical PC synthesized from sunflower heads by us shows an excellent performance than many PCs prepared from other biomass, such as broad bean shells, shiitake mushroom and pomelo peel.
| Precursor | Electrolyte | Electrode system | Current density | Cg/(F g−1) | Reference |
|---|---|---|---|---|---|
| Broad bean shells | 6 M KOH | 3-electrode | 0.5 A g−1 | 202 | 20 |
| Shiitake mushroom | 6 M KOH | 3-electrode | 1 A g−1 | 306 | 25 |
| Pectin | 6 M KOH | 3-electrode | 1 A g−1 | 231 | 40 |
| Willow catkins | 6 M KOH | 3-electrode | 1 A g−1 | 279 | 41 |
| Human hair | 6 M KOH | 3-electrode | 1 A g−1 | 180 | 42 |
| Cellulose | 6 M KOH | 3-electrode | 0.5 A g−1 | 253 | 43 |
| Glucose | 6 M KOH | 3-electrode | 1 A g−1 | 237 | 44 |
| Pomelo peel | 6 M KOH | 3-electrode | 1 A g−1 | 289 | 45 |
| Sunflower head | 6 M KOH | 3-electrode | 1 A g−1 | 345 | This work |
Electrochemical impedance spectroscopy (EIS) is a powerful tool to understand the various electrochemical reactions/processes involved in the supercapacitor electrode. The Nyquist plots of SFHCs electrodes in the frequency range from 100 kHz to 10 mHz are shown in Fig. 5f. The SFHCs display good capacitive behavior with nearly vertical slope at the low-frequency region, indicating their low diffusion resistance of the electrolyte ion in the pore. At very high frequency, the intercept of plot with real axis represents the equivalent series resistance (ESR) Rs, which is a combination of the ionic resistance of the electrolyte, the intrinsic resistance of the activate materials and contact resistance with the current collector. From the inset of the magnified high-frequency region of Fig. 5f, it is found that the internal resistances are 0.63 Ω, 0.61 Ω and 0.65 Ω for SFHC-1:1, SFHC-1:1.5 and SFHC-1:2, respectively, indicating good conductivity of the test device in aqueous electrolytes. At medium–high frequencies, a distinct semicircle loop is observed, which represents charge transfer resistance (Rct) at the interface between electrolytes and electrode. The Rcts of SFHC-1:1, SFHC-1:1.5 and SFHC-1:2 are 0.19 Ω, 0.13 Ω and 0.17 Ω, respectively. The length of the 45° segment (Warburg impedance) is related to the resistance caused by ion diffusion into the bulk of the electrode particles. Warburg impedance of SFHC-1:1.5 is smaller than other samples, showing a lower resistance attributed to the ions rapid transport into the small micropores.
To confirm oxygen functional groups really exerting an important role for enhancing the overall capacitance due to pseudocapacitance, the SFHC-1:1.5 sample was heat-treated at 800 °C for one hour at vacuum degree ranged from 0.07–0.08 MPa to deprive oxygen functional groups in SFHC-1:1.5. The comparison of capacitance performance of SFHC-1:1.5 and deoxidized sample in three-electrode cells is shown in Fig. 6. The CV curve (Fig. 6a) of deoxidized SFHC-1:1.5 is obviously different from that of SFHC-1:1.5 and presents a perfect rectangular shape with complete disappearance of the redox peak at −0.4 V to −1 V, showing its typical electric double layer capacitance behavior.46,47 The CV encircling area of deoxidized SFHC-1:1.5 is much lower than O-enriched sample, suggesting that it possesses smaller capacitance. The galvanostatic charge/discharge curves at a current density of 1 A g−1 were also comparably given in Fig. 6b, and the curve profile of deoxidized SFHC-1:1.5 is close to a linear line within the potential window, which is agree well with the CV result (no redox peaks). The charge–discharge time of deoxidized SFHC-1:1.5 is far shorter than that of SFHC-1:1.5, which is consistent with the smaller encircled area in CV measurements. The specific capacitance of deoxidized SFHC-1:1.5 (124 F g−1) is about only one-third of that in KOH (345 F g−1). Therefore, the pseudocapacitance due to the presence of abundant oxygen functional groups in the PC framework has made a great contribution to the overall specific capacitance in KOH solution.
To further determine the actual supercapacitor performance of SFHC-1:1.5, a symmetrical cell was assembled with two equal sized and/or effective massed electrodes in 6 M KOH aqueous electrolyte. The CVs of SFHC-1:1.5 obtained at various scan rates from 10 mV s−1 to 200 mV s−1 are shown in Fig. 7a. The CV curves exhibit typical rectangular shaped characteristics even at a high scan rate (200 mV s−1), suggesting the excellent rate performance of SFHC-1:1.5. The triangular shape of the charge/discharge plots (Fig. 7b) indicate good charge propagation behaviour of ions in the PC electrode. Moreover, the transition periods can be easily observed between −1.0 V and −0.6 V, indicating the redox reactions from oxygen functional groups performed in the charge–discharge process.
The gravimetric capacitances at different current densities for the single electrode were calculated on the basis of the charge–discharge curves, and the results are given in Fig. 7c. The specific capacitance is 290 F g−1 at a current density of 0.5 A g−1, which is higher than that of bean dregs derived carbon materials (210 F g−1 at 1 A g−1) and the carbons derived from celtuce leaves (273 F g−1 at 0.5 A g−1).48,49 When the current density increases up to 10 A g−1, the specific capacitance of 217 F g−1 is still retained, which is superior to the PC microflakes (200 F g−1 at 10 A g−1) and the chestnut shell (92 F g−1 at 10 A g−1).50,51 Furthermore, the cycling performance of the SFHC-1:1.5 (Fig. 7d) was investigated in two-electrode system, SFHC-1:1.5 shows good cycling stability that retains about 83% of the initial capacitance after 5000 cycles under a current density of 5 A g−1. The decrease of capacitance with cycle could be mainly attributed to the inactivation of partly oxygen surface functional groups.
The power density and energy density of the symmetric supercapacitor is shown in a Ragone plot (Fig. 8). Benefitting from a high specific capacitance of up to 70 F g−1 (obtained at a current density of 0.5 A g−1) and an operating voltage of 1 V, the assembled symmetric supercapacitor displayed a high energy density of 9.2 W h kg−1 and a power density of 482 W kg−1, which is higher than those of reported carbon-based symmetric supercapacitors in aqueous electrolytes. It can be attributed to its hierarchically interconnected pores structure with high specific surface area and enriched oxygen functional groups.
 | ||
| Fig. 8 The Ragone plots of our symmetric supercapacitor and other previously reported carbon-based symmetric supercapacitors. | ||
4 Conclusions
The sunflower head was first utilized as renewable biomass source to prepare an oxygen-enriched hierarchical PC for advance supercapacitor. The SFHCs retains a high oxygen percentage of 21 wt% after pyrolysis at 800 °C in the presence of an activating agent. The material offers a capacitance of 345 F g−1 at 1 A g−1, a capacitance of 280 F g−1 at a large current density of 20 A g−1, as well as a good cycling stability with capacitance retention of 83% after 5000 charge–discharge cycles. We also found that oxygen functional groups play a crucial role on the improvement of the excellent performance. More importantly, a high power density of 9.2 W h kg−1 for 1 V working voltage was achieved at a power density of 482 W kg−1 in aqueous electrolytes. These results show that the oxygen-enriched hierarchical PC from sunflower head displays great applied potential for high-performance supercapacitors.Acknowledgements
This work was supported by National Natural Science Foundation of China (21406056).Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS PubMed.
- T. Chen and L. Dai, J. Mater. Chem. A, 2014, 2, 10756–10775 CAS.
- H. Jiang, P. S. Lee and C. Li, Energy Environ. Sci., 2013, 6, 41–53 CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- H. Zhu, J. Yin, X. Wang, H. Wang and X. Yang, Adv. Funct. Mater., 2013, 23, 1305–1312 CrossRef CAS.
- J. Zhang and X. Zhao, ChemSusChem, 2012, 5, 818–841 CrossRef CAS PubMed.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 CAS.
- A. G. Pandolfo and A. F. Hollenkamp, J. Power Sources, 2006, 157, 11–27 CrossRef CAS.
- D. Hulicova-Jurcakova, A. M. Puziy, O. I. Poddubnaya, F. Suarez-Garcia, J. M. D. Tascon and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 5026–5027 CrossRef CAS PubMed.
- W. Shen and W. Fan, J. Mater. Chem. A, 2013, 1, 999–1013 CAS.
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie, G. O. Shitta-Bey, G. Wilson, A. Cruden and R. Carter, Energy Environ. Sci., 2010, 3, 1238–1251 CAS.
- S. Dutta, A. Bhaumik and K. C.-W. Wu, Energy Environ. Sci., 2014, 7, 3574–3592 CAS.
- B. Fang, J. H. Kim, M. Kim and J. Yu, Acc. Chem. Res., 2013, 46, 1397–1406 CrossRef CAS PubMed.
- L. Chen, T. Ji, L. Brisbin and J. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 12230–12237 CAS.
- M. Genovese, J. Jiang, K. Lian and N. Holm, J. Mater. Chem. A, 2015, 3, 2903–2913 CAS.
- Z. Li, W. Lv, C. Zhang, B. Li, F. Kang and Q. Yang, Carbon, 2015, 92, 11–14 CrossRef CAS.
- L. L. Zhang, Y. Gu and X. S. Zhao, J. Mater. Chem. A, 2013, 1, 9395–9408 CAS.
- G. Xu, J. Han, B. Ding, P. Nie, J. Pan, H. Dou, H. Li and X. Zhang, Green Chem., 2015, 17, 1668–1674 RSC.
- W. Tian, Q. Gao, Y. Tan, K. Yang, L. Zhu, C. Yang and H. Zhang, J. Mater. Chem. A, 2015, 3, 5656–5664 CAS.
- E. Raymundo-Piñero, F. Leroux and F. Béguin, Adv. Mater., 2006, 18, 1877–1882 CrossRef.
- Y. Gao, L. Li, Y. Jin, Y. Wang, C. Yuan, Y. Wei, G. Chen, J. Ge and H. Lu, Appl. Energy, 2015, 153, 41–47 CrossRef CAS.
- Y. Zhao, W. Ran, J. He, Y. Song, C. Zhang, D. Xiong, F. Gao, J. Wu and Y. Xia, ACS Appl. Mater. Interfaces, 2015, 7, 1132–1139 CAS.
- P. Cheng, S. Gao, P. Zang, X. Yang, Y. Bai, H. Xu, Z. Liu and Z. Lei, Carbon, 2015, 93, 315–324 CrossRef CAS.
- Z. Li, Z. Xu, X. Tan, H. Wang, C. M. B. Holt, T. Stephenson, B. C. Olsenab and D. Mitlin, Energy Environ. Sci., 2013, 6, 871–878 CAS.
- T. Wei, X. Wei, Y. Gao and H. Li, Electrochim. Acta, 2015, 169, 186–194 CrossRef CAS.
- J. Kang, X. Hua, R. Yang, Y. Chen and H. Yang, Food Chem., 2015, 180, 98–105 CrossRef CAS PubMed.
- X. Hua, K. Wang, R. Yang, J. Kang and J. Zhang, Food Hydrocolloids, 2015, 44, 122–128 CrossRef CAS.
- A. B. Fuertes and M. Sevilla, ACS Appl. Mater. Interfaces, 2015, 7, 4344–4353 CAS.
- S. T. Senthilkumar, R. K. Selvan, J. S. Melo and C. Sanjeeviraja, ACS Appl. Mater. Interfaces, 2013, 5, 10541–10550 CAS.
- F. Su, C. K. Poh, J. S. Chen, G. Xu, D. Wang, Q. Li, J. Lin and X. W. Lou, Energy Environ. Sci., 2011, 4, 717–724 CAS.
- C. L. Chen, B. Liang, A. Ogino, X. K. Wang and M. Nagatsu, J. Phys. Chem. C, 2009, 113, 7659–7665 CAS.
- M. Y. Song, H. Y. Park, D. Yang, D. Bhattacharjya and J. Yu, ChemSusChem, 2014, 7, 1755–1763 CrossRef CAS PubMed.
- Y. Tan, C. Xu, G. Chen, Z. Liu, M. Ma, Q. Xie, N. Zheng and S. Yao, ACS Appl. Mater. Interfaces, 2013, 5, 2241–2248 CAS.
- D. H. Jurcakova, M. Seredych, G. Q. Lu and T. J. Bandosz, Adv. Funct. Mater., 2009, 19, 438–447 CrossRef.
- Z. Zhang, Z. Zhou, H. Peng, Y. Qin and G. Li, Electrochim. Acta, 2014, 134, 471–477 CrossRef CAS.
- S. Biniak, G. Szymanski, J. Siedlewski and A. Swiatkowski, Carbon, 1997, 35, 1799–1810 CrossRef CAS.
- C. Long, L. Jiang, X. Wu, Y. Jiang, D. Yang, C. Wang, T. Wei and Z. Fan, Carbon, 2015, 93, 412–420 CrossRef CAS.
- Y. Fan, P. Liu, Z. Yang, T. Jiang, K. Yao, R. Han, X. Huo and Y. Xiong, Electrochim. Acta, 2015, 163, 140–148 CrossRef CAS.
- K. Wang, N. Zhao, S. Lei, R. Yan, X. Tian, J. Wang, Y. Song, D. Xu, Q. Guo and L. Liu, Electrochim. Acta, 2015, 166, 1–11 CrossRef CAS.
- W. Si, J. Zhou, S. Zhang, S. Li, W. Xing and S. Zhuo, Electrochim. Acta, 2013, 107, 397–405 CrossRef CAS.
- J. Deng, T. Xiong, F. Xu, M. Li, C. Han, Y. Gong, H. Wang and Y. Wang, Green Chem., 2015, 17, 4053–4060 RSC.
- F. Gao, G. Shao, J. Qu, S. Lv, Y. Li and M. Wu, Electrochim. Acta, 2015, 155, 201–208 CrossRef CAS.
- Q. Liang, L. Ye, Z. Huang, Q. Xu, Y. Bai, F. Kang and Q. Yang, Nanoscale, 2014, 6, 13831–13837 RSC.
- B. You, J. Yang, Y. Sun and Q. Su, Chem. Commun., 2011, 47, 12364–12366 RSC.
- F. Ma, L. Sun, H. Zhao, Q. Li, L. Hou, T. Xia and S. Gao, Chem. Res. Chin. Univ., 2013, 29, 735–742 CrossRef CAS.
- C. Ruan, K. Ai and L. Lu, RSC Adv., 2014, 4, 30887–30895 RSC.
- R. Wang, P. Wang, X. Yan, J. Lang, C. Peng and Q. Xue, ACS Appl. Mater. Interfaces, 2012, 4, 5800–5806 CAS.
- F. Ma, J. Wan, G. Wu and H. Zhao, RSC Adv., 2015, 5, 44416–44422 RSC.
- L. Cheng, P. Guo, R. Wang, L. Ming, F. Leng, H. Lia and X. S. Zhao, Colloids Surf., A, 2014, 446, 127–133 CrossRef CAS.
- H. Zhu, X. Wang, F. Yang and X. Yang, Adv. Mater., 2011, 23, 2745–2748 CrossRef CAS PubMed.
- X. Li, W. Xing, S. Zhuo, J. Zhou, F. Li, S. Qiao and G. Lu, Bioresour. Technol., 2011, 102, 1118–1123 CrossRef CAS PubMed.
- W. Qu, Y. Xu, A. Lu, X. Zhang and W. Li, Bioresour. Technol., 2015, 189, 285–291 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24401c |
| This journal is © The Royal Society of Chemistry 2015 |