Collagen fibril strain, recruitment and orientation for pericardium under tension and the effect of cross links
Abstract
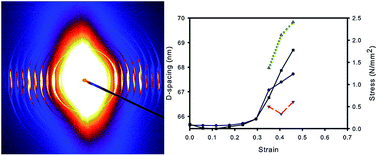
The structural response of collagen fibrils in pericardium and other tissues when subjected to strain and the effect of cross linking on those structural changes are not well understood. Specifically, there is uncertainty about whether natural cross links of glycosaminoglycan (GAG) and synthetic cross links of glutaraldehyde have a mechanical function. Bovine pericardium was treated either with chondroitinase ABC to remove natural cross links or with glutaraldehyde to form synthetic cross links. The collagen fibril orientation index (OI) and D-spacing was measured on pericardium subjected to strain using synchrotron-based small angle X-ray scattering (SAXS). Under strain the collagen fibrils become much more oriented in the direction of the strain, with OI increasing from 0.25 to 0.89 in chondroitinase ABC-treated material, 0.22 to 0.93 in native material, and 0.22 to 0.77 in the glutaraldehyde-treated material. The proportion of fibrils that are recruited during stress varies from 36% in chondroitinase ABC-treated material, 12% in native material, to 45% in the glutaraldehyde-treated material. The increase in D-spacing shows the individual fibrils are strained in chondroitinase ABC-treated material by 2.4% on average or 4.6% for those in the direction of applied strain, in native material, 2.7% and 4.1%, respectively, and in the glutaraldehyde-treated material, 3.2% and 6.4%, respectively. Glutaraldehyde cross links are, therefore, shown to constrain the collagen fibrils and link them together mechanically. GAGs do not have such a marked mechanical effect; contrarily, the nature of internal structural responses to strain suggests that GAGs may have a lubricating rather than a binding effect.

 Please wait while we load your content...
Please wait while we load your content...