3D lanthanide metal–organic frameworks constructed from lanthanide formate skeletons and 3,5-bis(4′-carboxy-phenyl)-1,2,4-triazole connectors: synthesis, structure and luminescence†
Abstract
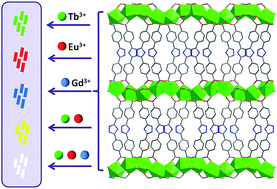
Three isomorphic lanthanide metal–organic frameworks (Ln-MOFs) have been constructed from lanthanide formate skeletons and 3,5-bis(4′-carboxy-phenyl)-1,2,4-triazole (H2bct) connectors. Further, by adjusting the co-doping ratio of different Ln3+ ions into the framework, two doped Ln-MOFs are synthesized and show tunable luminescence emission including white-light emission.

 Please wait while we load your content...
Please wait while we load your content...