Synthesis and characterization of a polyamide thin film composite membrane based on a polydopamine coated support layer for forward osmosis
Abstract
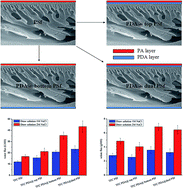
In this study, a facile method has been developed to prepare high performance thin film composite (TFC) forward osmosis (FO) membranes, which was conducted by coating the surface of a polysulfone (PSf) substrate with polydopamine (PDA) prior to the interfacial polymerization of trimesoyl chloride and m-phenylenediamine. The PDA coating layer was investigated by ATR-FTIR, XPS, FESEM and contact angle measurements. Results showed that the PDA layer played the following roles: (1) endowing the PSf substrate with catechol and ethylamino groups to enhance the hydrophilicity, which resulted in a significant increase in water flux; (2) facilitating the formation of a denser selective polydopamine layer during the interfacial polymerization process to enhance salt rejection. The TFC membrane based on PDA coated dual PSf surfaces showed a water flux of 43.4 LMH and a salt flux of 6.46 gMH in pressure retarded osmosis (PRO) mode using 2 M NaCl as draw solution.

 Please wait while we load your content...
Please wait while we load your content...