Enhanced crystallization rate of biodegradable poly(butylene succinate-co-ethylene succinate) by poly(butylene fumarate) as an efficient polymeric nucleating agent
Fan Meng and
Zhaobin Qiu*
State Key Laboratory of Chemical Resource Engineering, MOE Key Laboratory of Carbon Fiber and Functional Polymers, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: qiuzb@mail.buct.edu.cn; Fax: +86-10-64413161
First published on 5th November 2015
Abstract
The crystallization kinetics and morphology of poly(butylene succinate-co-ethylene succinate) (PBES), a biodegradable copolyester with slow crystallization rate, were systematically investigated in this work using poly(butylene fumarate) (PBF) as a novel polymeric nucleating agent. The effects of different PBF contents on the crystallization process of PBES were studied under both the nonisothermal and isothermal melt crystallization conditions. The nonisothermal melt crystallization peak temperature values of PBES were increased by about 18 and 23 °C, respectively, in the presence of only 1 and 2 wt% PBF. Under the isothermal melt crystallization conditions, PBF also accelerated the crystallization process of PBES. When PBES was isothermally crystallized at 90 °C, the crystallization half-time values were obviously reduced from 21.1 min in the absence of nucleating agent to 13.6 and 4.9 min when the PBF contents were 1 and 2 wt%, respectively. The spherulitic morphology study revealed that PBF acted as an efficient nucleating agent for the crystallization of PBES, as the spherulites size became significantly smaller with increasing the PBF content. The crystal structure of PBES remained unmodified in the presence of PBF. The nucleating mechanism was also discussed.
Introduction
The crystallization behavior studies of biodegradable polymers have attracted considerable research interests from both the academic and industrial viewpoints, as they not only affect the crystalline morphology and crystal structure but also influence the physical properties and hydrolytic or enzymatic degradation behaviors of biodegradable polymers.1–6 One of the main issues that restrict the melt processing and practical end use is that some biodegradable polymers do not crystallize or crystallize very slowly, especially under nonisothermal melt crystallization conditions.7–10 To accelerate the crystallization process of biodegradable polyesters with slow crystallization rate, the addition of a nucleating agent is one of the simplest and most convenient methods, because it may lower the crystallization activation energy, provide more additional nucleating sites, reduce the spherulites size, increase the spherulites nucleation density, and enhance the crystallization rate.11–15 In some cases, the addition of nucleating agents may even modify the crystal structure, adjust the polymorphic crystals, enhance the thermal stability, improve the mechanical properties, and favor the transparency of the polymeric materials.16–20 In literature, many inorganic compounds, organic compounds, and nanofillers have been found to act as nucleating agents for the crystallization processes of some biodegradable polymers.21–30 In addition, some polymers may also act as polymeric nucleating agents for biodegradable polymers.31–39 For instance, Tsuji et al. found that polyglycolide (PGA) could accelerate the crystallization process of PLLA by acting as a polymeric nucleating agent.31 Zhang et al. and Li et al. reported that polyoxymethylene (POM) may enhance the crystallization process of PLLA by acting as a polymeric nucleating agent in their separate works.32,33 Yang et al. found that poly(vinyl butyral) (PVB) may enhance the crystallization rate of poly(butylene succinate) (PBS) as a macromolecular nucleating agent.36PBS and poly(ethylene succinate) (PES) are the most important members of aliphatic biodegradable polymers, as they have already been industrialized and commercially available.40,41 To adjust the physical properties and meet the various end use requirements, a series of poly(butylene succinate-co-ethylene succinate) (PBES) samples, the copolyesters of PBS and PES, have been synthesized and investigated.42–45 According to the comonomer composition, PBES may exhibit different glass transition temperature and melting point; moreover, the mechanical properties and hydrolytic degradation rates also vary with the comonomer composition. Unlike the extensive investigations of PBS and PES, only a few works have reported the basic thermal properties, crystalline structure and morphology, mechanical properties, and degradation behaviors of PBES.42–45 In previous work, we studied the influences of the comonomer composition and crystallization temperature on the crystallization rates, crystal structure and spherulitic morphology of a series of PBES with different ethylene succinate (ES) contents. Compared with the homopolymer PBS, the crystallization rates of PBES were obviously reduced, which in turn may affect the practical application from a polymer processing viewpoint.45
Poly(butylene fumarate) (PBF) is a type of unsaturated aliphatic polyester.46–49 As PBF and PBS have the similar conformational structures, chemical structures, and crystal volumes, they may exhibit the similar basic thermal properties and crystal lattice matching.46–49 PBF has the superior mechanical properties, high melting point, and good thermal stability, because of the rigid carbon–carbon double bonds of the aliphatic chains; moreover. PBF is also a biodegradable polyester due to the presence of ester bonds in the main chains.46–49 PBF may also act as a polymeric nucleating agent of biodegradable polymers.50,51 For instance, Guo et al. found that PBF was an efficient polymeric nucleating agent not only for PBS but also for poly(butylene succinate-co-10 mol% butylene adipate) and poly(butylene succinate-co-10 mol% propylene succinate), which were both the random copolyesters of PBS.50,51
Consequently, PBF is expected to act as the nucleating agent of PBES, novel biodegradable copolyesters of PBS and PES with slow crystallization rate; thus, the crystallization process of PBES may be accelerated, which is greatly essential for its melt processing and practical application. However, such research has never been reported in literature till now. Therefore, in this research, we first prepared the PBES/PBF blends with low PBF contents via a solution and casting process, further studied the effect of PBF on the crystallization behavior of PBES to confirm whether it could act as a polymeric nucleating agent, and also discussed the nucleating mechanism. The research of this work is interesting and important from the viewpoints of polymer crystallization and practical application of biodegradable polyesters, because it may promote their wider practical application after enhancing slow crystallization rate.
Experimental section
The PBES and PBF samples were synthesized by our laboratory. The PEBS sample was poly(BS-co-13 mol% ES), which had an average molecular weight (Mw) of 3.4 × 104 g mol−1 and a melting point (Tm) of 114.9 °C. The PBF sample had an Mw value of 1.7 × 104 g mol−1 and a Tm of 123.3 °C. Fig. 1 shows their chemical structures.The PBES/PBF blends were prepared using a solution and casting method. Chloroform was used as the mutual solvent. In this research, two samples containing 1 and 2 wt% of PBF were prepared and were named as PBES/PBF1 and PBES/PBF2, respectively. For comparison, PBES was also prepared through a similar procedure.
The nonisothermal melt crystallization behavior and overall isothermal melt crystallization kinetics were studied with a TA instruments differential scanning calorimeter (DSC) Q100. The samples were first heated to 140 °C at 20 °C min−1, held for 3 min to erase any thermal history, and reached the crystal-free melt state. For the nonisothermal melt crystallization behavior study, the samples were cooled from the crystal-free melt at a cooling rate of 10 °C min−1 to 20 °C and then heated to 140 °C at 20 °C min−1 to study the subsequent melting behavior. For the isothermal melt crystallization kinetics study, the samples were cooled from the crystal-free melt to the desired crystallization temperature at 60 °C min−1, held for a period of time to ensure complete crystallization, and heated to 140 °C at 20 °C min−1 to study the subsequent melting behavior. All operations were performed under nitrogen purge.
The spherulitic morphology was investigated with a polarized optical microscope (POM) (Olympus BX51) equipped with a first-order retardation plate and a temperature controller (Linkam THMS 600). The samples were first melted at 140 °C for 3 min to erase any thermal history and then quenched to 90 °C at 60 °C min−1.
The crystal structure of all the samples was investigated with a Rigaku d/Max2500 VB2+/PC X-ray diffractometer at room temperature at 40 kV and 200 mA with a rate of 4° min−1. The samples were isothermally crystallized at 80 °C for 24 h after first erasing any previous thermal history.
Results and discussion
Enhanced nonisothermal melt crystallization behavior
The nonisothermal melt crystallization behavior of PBES and its blends with small contents of PBF was first studied with DSC. Fig. 2 depicts the crystallization exotherms of PBES and the PBES/PBF blends, which were nonisothermally crystallized from the crystal-free melt at 10 °C min−1 after being annealed at 150 °C for 3 min. For comparison, the crystallization exotherm of PBF is also included in Fig. 2. PBES displayed a nonisothermal melt crystallization peak temperature (Tp) of 53.9 °C, while PBF showed a higher Tp of 88.3 °C, suggesting that the latter was easier and stronger to crystallize than the former. After the blending with small contents of PBF, the crystallization exotherms of the PBES/PBF blends obviously shifted upward to a high temperature range, indicating the enhanced nonisothermal melt crystallization behavior of PBES induced by PBF. From Fig. 2, PBES/PBF1 presented a Tp of 72.0 °C. Relative to PBES, the Tp value was enhanced by 18.1 °C in PBES/PBF1, suggesting that a small amount of PBF may significantly enhance the nonisothermal melt crystallization behavior of PBES. With further increasing the PBF content, the Tp value was increased to be 76.9 °C for PBES/PBF2. With respect to PBES/PBF1, the increase in Tp of PBES/PBF2 was not so significant, indicating that the enhancement of Tp may level off after the blending with a certain amount of PBF. Fig. 2 also clearly demonstrates that the crystallization exotherms became narrower in the blends than in PBES, suggesting again the enhanced nonisothermal melt crystallization behavior. In addition, the lamellar thickness should also be greater in the blends than in PBES, because they were crystallized at higher temperature range. The smaller degree of supercooling may result in thicker crystals and narrower lamellar thickness distribution in the nucleated samples, with respect to PBES. In brief, the blending with small contents of PBF apparently promoted the nonisothermal melt crystallization behavior of PBES, indicating that PBF was an efficient polymeric nucleating agent for the nonisothermal melt crystallization behavior of PBES. | ||
| Fig. 2 The nonisothermal melt crystallization exotherms of PBES, PBF, and their blends at 10 °C min−1. | ||
Enhanced isothermal crystallization rate
The overall isothermal melt crystallization kinetics studies of PBES and its blends with PBF were further performed with DSC in this work. All the samples were cooled quickly to the desired crystallization temperature (Tc) after erasing any previous thermal history. Fig. 3 illustrates the plots of relative crystallinity (Xt) versus crystallization time (t) for each sample after crystallizing different Tc values. For all of the samples, the time for finishing crystallization became longer with increasing Tc, suggesting a reduction of crystallization rate with an increase in Tc. When they were crystallized at the same Tc of 88 or 90 °C, the crystallization time of PBES became shorter in the presence of PBF than in the absence of PBF, suggesting the nucleating agent effect of PBF. For example, at a given Tc of 90 °C, PBES finished the isothermal melt crystallization within around 55 min, whereas PBES/PBF1 and PBES/PBF2 completed the crystallization within around 37 and 12 min, respectively. The blending with small contents of PBF may supply more additional nucleating sites and thus dramatically accelerate the isothermal melt crystallization process of the blends. | ||
| Fig. 3 The plots of relative crystallinity against crystallization time of (a) PBES, (b) PBES/PBF1, and (c) PBES/PBF2 at indicated Tc values. | ||
We further analyzed the overall isothermal melt crystallization kinetics using the well-known Avrami equation as follows for all of the samples:
| 1 − Xt = exp(−ktn) | (1) |
 | ||
| Fig. 4 The Avrami plots for (a) PBES, (b) PBES/PBF1, and (c) PBES/PBF2 after crystallizing at indicated Tc values. | ||
The n and k values were acquired from the Avrami plots illustrated in Fig. 4 and are also shown in Table 1 for comparison. Table 1 clearly indicates that the n values were almost the same or only varied slightly for each sample, regardless of Tc, suggesting the same crystallization mechanism. The average n values were slightly varied from 2.4 for PBES to around 2.7 and 3.0 for the blends with increasing the PBF content from 1 to 2 wt%, respectively. The slight variation of the n values indicated that all the samples may crystallize through the same three-dimensional spherulitic growth with athermal nucleation mechanism, despite the PBF content.54 For the samples showing the same n values, the k values became greater with decreasing Tc, indicate of faster crystallization rate.
| Samples | Tc (°C) | n | k (min−n) | t0.5 (min) |
|---|---|---|---|---|
| PBES | 84 | 2.4 | 9.30 × 10−3 | 5.8 |
| 86 | 2.4 | 5.68 × 10−3 | 7.6 | |
| 88 | 2.4 | 1.73 × 10−3 | 12.6 | |
| 90 | 2.4 | 4.82 × 10−4 | 21.1 | |
| PBES/PBF1 | 86 | 2.5 | 1.98 × 10−2 | 4.4 |
| 88 | 2.8 | 3.92 × 10−3 | 7.6 | |
| 90 | 2.8 | 7.55 × 10−4 | 13.6 | |
| 92 | 2.6 | 1.02 × 10−4 | 25.1 | |
| PBES/PBF2 | 88 | 2.9 | 4.39 × 10−2 | 2.6 |
| 90 | 3.0 | 5.19 × 10−3 | 4.9 | |
| 92 | 3.0 | 6.56 × 10−4 | 10.3 | |
| 94 | 3.0 | 7.66 × 10−5 | 21.1 |
Table 1 clearly demonstrates that the variations of Tc and the PBF content may result in a slight difference in the n values; therefore, it brought a difficulty in comparing the crystallization rate by still using the k values, because of their different units (min−n). In this case, we used the crystallization half-time (t0.5) values to compare the crystallization rates, which were calculated through the following equation and are also shown in Table 1.
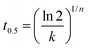 | (2) |
From Table 1, the t0.5 values of each sample became gradually greater with increasing Tc, suggesting slower crystallization rates. At the same Tc of 88 or 90 °C, the blends exhibited the smaller t0.5 values than PBES; moreover, the t0.5 values became gradually smaller with increasing the PBF content, indicating faster crystallization rate.
Fig. 5 illustrates the variation of 1/t0.5 with Tc for all of the samples, because 1/t0.5, the reciprocal of t0.5, may conveniently represent the crystallization rate. On one hand, the 1/t0.5 values obviously increased with a decrease in Tc for all of the samples, indicating that the greater supercooling at lower Tc favored a faster crystallization rate. The enhanced crystallization rate with decreasing Tc also suggested that the nucleation process dominated the overall isothermal crystallization rates of the samples, as the greater supercooling made the nucleation process easier. On the other hand, the 1/t0.5 values gradually became greater with increasing the PBF content, when all the samples were crystallized at the same Tc. Such increment in the 1/t0.5 value indicated the enhanced crystallization rate, which should arise from the nucleation effect of PBF. Therefore, the PBF content presented the similar effect on the variation of both the nonisothermal and isothermal melt crystallization processes of the blends.
It is also interesting to study the effect of PBF on the melting characteristics of the nucleated samples after the isothermal melt crystallization process. After they were crystallized at the same Tc of 88 or 90 °C, all the samples exhibited a main endothermic peak. For brevity, the results were not shown here. The Tm values of the nucleated samples were slightly greater than that of PBES. For instance, PBES displayed a Tm value of 99.5 °C, while the nucleated samples presented the Tm value of 99.9 °C, when they were isothermally crystallized at 88 °C. Moreover, PBES displayed a Tm value of 100.9 °C, while the nucleated samples presented the Tm value of 101.4 °C, when they were isothermally crystallized at 90 °C. The slightly increased Tm in the nucleated samples indicated that the crystal thickness should be slightly thicker in the blends than in PBES.
Spherulitic morphology and crystal structure studies
In the above sections, the crystallization processes of the blends were accelerated under different crystallization conditions, which should arise from the nucleating agent effect of PBF. In this section, the crystalline morphologies of all of the samples were investigated with POM to directly explore the nucleating agent effect of PBF. Fig. 6 illustrates the POM images of PBES and the blends with different PBF contents after crystallizing at 90 °C and filling the entire space. Fig. 6a clearly indicates that several large PBES spherulites were observed for PBES with the diameter in a range of 150–200 μm; moreover, the clear boundaries were obviously seen between neighboring spherulites. Parts b and c of Fig. 6 suggested that the blends also presented spherulites morphology, although the size of these spherulites was extremely small, relative to PBES. The diameter of the spherulites was only around ten to several tens micrometers in the blends; furthermore, the clear boundaries could not be observed between neighboring spherulites. The crystalline morphologies results from Fig. 6 clearly demonstrated that the spherulites nucleation density was apparently enhanced by the blending with PBF; moreover, increasing the PBF content further enhanced the nucleation density, especially in the case of PBES/PEF2. As a result, PBF actually acted as an efficient polymeric nucleating agent for the crystallization of PBES. The nucleation mechanism may be attributed to the similar crystal lattice parameters between PBF and PBES, as PBS had a monoclinic crystal structure of a = 0.523 nm, b = 0.906 nm, c = 1.090 nm, and β = 123.87°, and PBF also had a monoclinic crystal structure of a = 0.501 nm, b = 0. 926 nm, c = 1.093 nm, and β = 120.90°.50,51 The mismatching of the lattice parameters of PBF and PBS was rather small; therefore, an epitaxial nucleation mechanism may account for the enhanced nucleation density and overall crystallization rate of the blends.55 | ||
| Fig. 6 Spherulites of (a) PBES, (b) PBES/PBF1, and (c) PBES/PBF2 after crystallizing at 90 °C and filling the entire space. | ||
The crystal structures of PBES and the PBES/PBF blends were further investigated. Fig. 7 displays the wide-angle X-ray diffraction (WAXD) patterns of all of the samples, which were isothermally crystallized at 80 °C for 24 h after first erasing any previous thermal histories. PBES exhibited three typical diffraction peaks located at 2θ = 19.5°, 21.7°, and 22.6°, which were attributed to the (020), (021), and (110) planes of PBS, respectively, as PBES had the same crystal structure as PBS.50,51 As far as the PBES/PBF blends were concerned, they presented almost the similar WAXD profiles as PBES, despite the PBF content. Consequently, they both should crystallize and pack through the same crystal structure as PBES.
Conclusions
The effects of PBF as a novel polymeric nucleating agent on the crystallization kinetics and morphology of PBES were extensively studied in this research with DSC, POM, and WAXD. During the nonisothermal melt crystallization process at a cooling rate of 10 °C min−1, the nonisothermal melt crystallization peak temperature values of PBES were increased by about 18 and 23 °C when the PBF contents were only 1 and 2 wt%, respectively, indicating that PBF was an efficient nucleating agent of enhancing the nonisothermal melt crystallization behavior of PBES. The overall isothermal crystallization process of PBES was also accelerated by PBF and influenced by the different PBF contents. When PBES was isothermally crystallized at 90 °C, the crystallization half-time values were obviously reduced from 21.1 min in the absence of nucleating agent to 13.6 and 4.9 min in the presence of 1 and 2 wt% PBF, respectively. The overall isothermal crystallization kinetics of PBES in the presence of different PBF contents at different crystallization temperature values was analyzed by the Avrami equation. On the basis of the spherulites study, the nucleating agent role of PBF on the crystallization process of PBES was further confirmed, as the spherulites nucleation density values became significantly smaller with increasing the PBF content. On the basis of the WAXD study, the addition of PBF and its content did not change the crystal structure of PBES. The nucleating mechanism was also discussed.Acknowledgements
Part of this work was supported by the National Natural Science Foundation, China (51573016, 51373020 and 51221002).References
- Z. Gan, H. Abe and Y. Doi, Biomacromolecules, 2000, 1, 713 CrossRef CAS PubMed.
- T. Iwata, Y. Doi, K. Isono and Y. Yoshida, Macromolecules, 2001, 34, 7343 CrossRef CAS.
- Z. Gan, K. Kuwabara, H. Abe, T. Iwata and Y. Doi, Polym. Degrad. Stab., 2005, 87, 191 CrossRef CAS.
- G. Papageorgiou and D. Bikiaris, Polymer, 2005, 46, 12081 CrossRef CAS.
- P. Pan and Y. Inoue, Prog. Polym. Sci., 2009, 34, 605 CrossRef CAS.
- J. Xu and B. Guo, Biotechnol. J., 2010, 5, 1149 CrossRef CAS PubMed.
- G. Papageorgiou, D. Bikiaris and D. Achilias, Thermochim. Acta, 2007, 457, 41 CrossRef CAS.
- G. Wang and Z. Qiu, Ind. Eng. Chem. Res., 2012, 51, 16369 CrossRef CAS.
- C. Ha and W. Cho, Prog. Polym. Sci., 2002, 27, 759 CrossRef CAS.
- S. Saeidlou, M. Huneault, H. Li and C. Park, Prog. Polym. Sci., 2012, 37, 1657 CrossRef CAS.
- J. Yu and Z. Qiu, ACS Appl. Mater. Interfaces, 2011, 3, 890 CAS.
- H. Bai, W. Zhang, H. Deng, Q. Zhang and Q. Fu, Macromolecules, 2011, 44, 1233 CrossRef CAS.
- P. Pan, G. Shan, Y. Bao and Z. Weng, J. Appl. Polym. Sci., 2013, 129, 1374 CrossRef CAS.
- Y. Zhao, Z. Qiu and W. Yang, J. Phys. Chem. B, 2008, 112, 16461 CrossRef CAS PubMed.
- H. Pan and Z. Qiu, Macromolecules, 2010, 43, 1499 CrossRef CAS.
- N. Jiang, L. Zhao and Z. Gan, Polym. Degrad. Stab., 2010, 95, 1045 CrossRef CAS.
- T. Dong, W. Kai and Y. Inoue, Macromolecules, 2007, 40, 8285 CrossRef CAS.
- Y. Zhao and Z. Qiu, CrystEngComm, 2011, 13, 7129 RSC.
- M. Weng, Y. He and Z. Qiu, Ind. Eng. Chem. Res., 2012, 51, 13862 CrossRef CAS.
- J. Yang, Y. Chen, S. Qin, J. Liu, C. Bi, R. Liang, T. Dong and X. Feng, Ind. Eng. Chem. Res., 2015, 54, 8048 CrossRef CAS.
- P. Pan, Z. Liang, A. Cao and Y. Inoue, ACS Appl. Mater. Interfaces, 2009, 1, 402 CAS.
- G. Papageorgiou, D. Achilias, S. Nanaki, T. Beslikas and D. Bikiaris, Thermochim. Acta, 2010, 511, 129 CrossRef CAS.
- N. Jacquel, K. Tajima, N. Nakamura, T. Miyagawa, P. Pan and Y. Inoue, J. Appl. Polym. Sci., 2009, 114, 1287 CrossRef CAS.
- P. Pan, Z. Liang, N. Nakamura, T. Miyagawa and Y. Inoue, Macromol. Biosci., 2009, 9, 585 CrossRef CAS PubMed.
- Z. Qiu and Z. Li, Ind. Eng. Chem. Res., 2011, 50, 12299 CrossRef CAS.
- P. Pan, J. Yang, G. Shan, Y. Bao, Z. Weng and Y. Inoue, Macromol. Mater. Eng., 2012, 297, 670 CrossRef CAS.
- M. Weng and Z. Qiu, Thermochim. Acta, 2014, 577, 41 CrossRef CAS.
- Q. Xing, X. Zhang, X. Dong, G. Liu and D. Wang, Polymer, 2012, 53, 2306 CrossRef CAS.
- H. Wang and Z. Qiu, Thermochim. Acta, 2011, 526, 229 CrossRef CAS.
- Z. Qiu and W. Guan, RSC Adv., 2014, 4, 9463 RSC.
- H. Tsuji, K. Tashiro, L. Bouapao and J. Narita, Macromol. Mater. Eng., 2008, 293, 947 CrossRef CAS.
- X. Guo, H. Liu, J. Zhang and J. Huang, Ind. Eng. Chem. Res., 2014, 53, 16754 CrossRef CAS.
- J. Qiu, J. Guan, H. Wang, S. Zhu, X. Cao, Q. Ye and Y. Li, J. Phys. Chem. B, 2014, 118, 7167 CrossRef CAS PubMed.
- J. Zhou, Z. Jiang, Z. Wang, J. Zhang, J. Li, Y. Li, J. Zhang, P. Chen and Q. Gu, RSC Adv., 2013, 3, 18464 RSC.
- P. Pan, G. Shan and Y. Bao, Ind. Eng. Chem. Res., 2014, 53, 3148 CrossRef CAS.
- B. Yang, H. Ni, J. Huang and Y. Luo, Macromolecules, 2014, 47, 284 CrossRef CAS.
- H. Yin, X. Wei, R. Bao, Q. Dong, Z. Liu, W. Yang, B. Xie and M. Yang, CrystEngComm, 2015, 17, 2310 RSC.
- H. Yin, X. Wei, R. Bao, Q. Dong, Z. Liu, W. Yang, B. Xie and M. Yang, CrystEngComm, 2015, 17, 4334 RSC.
- Y. Tang, Y. Gao, J. Xu and B. Guo, CrystEngComm, 2015, 17, 6467 RSC.
- Z. Qiu, M. Komura, T. Ikehara and T. Nishi, Polymer, 2003, 44, 7781 CrossRef CAS.
- K. Chrissafis, K. Paraskevopoulos and D. Bikiaris, Thermochim. Acta, 2005, 435, 142 CrossRef CAS.
- M. Mochizuki, K. Mukai, K. Yamada, N. Ichise, S. Murase and Y. Iwaya, Macromolecules, 1997, 30, 7403 CrossRef CAS.
- Z. Gan, H. Abe and Y. Doi, Biomacromolecules, 2001, 2, 313 CrossRef CAS PubMed.
- A. Cao, T. Okamura, K. Nakayama, Y. Inoue and T. Masuda, Polym. Degrad. Stab., 2002, 78, 107 CrossRef CAS.
- Y. Yang and Z. Qiu, CrystEngComm, 2011, 13, 2408 RSC.
- M. Nikolic, D. Poleti and J. Djonlagic, Eur. Polym. J., 2003, 39, 2183 CrossRef CAS.
- L. Zheng, Z. Wang, C. Li, D. Zhang and Y. Xiao, Ind. Eng. Chem. Res., 2012, 51, 14107 CrossRef CAS.
- L. Zheng, Z. Wang, S. Wu, C. Li, D. Zhang and Y. Xiao, Ind. Eng. Chem. Res., 2013, 52, 6147 CrossRef CAS.
- L. Zheng, Z. Wang, C. Li, Y. Xiao, D. Zhang, G. Guan and W. Zhu, Polymer, 2013, 54, 631 CrossRef CAS.
- H. Ye, R. Wang, J. Liu, J. Xu and B. Guo, Macromolecules, 2012, 45, 5667 CrossRef CAS.
- H. Ye, Y. Tang, J. Xu and B. Guo, Ind. Eng. Chem. Res., 2013, 52, 10682 CrossRef CAS.
- M. Avrami, J. Chem. Phys., 1940, 8, 212 CrossRef CAS.
- M. Avrami, J. Chem. Phys., 1941, 9, 177 CrossRef CAS.
- B. Wunderlich, Macromolecular Physics, Academic Press, New York, 1976, vol. 2 Search PubMed.
- J. Wittmann and B. Lotz, Prog. Polym. Sci., 1990, 15, 909 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |



