A low-temperature sintered heterostructure solid film of coordination polymer nanoparticles: an electron-rectifier function based on partially oxidised/reduced conductor phases of Prussian blue†
Kenta Ono,
Manabu Ishizaki*,
Shinobu Soma,
Katsuhiko Kanaizuka,
Takanari Togashi and
Masato Kurihara*
Yamagata University, 1-4-12 Kojirakawa-machi, Yamagata, 990-8560 Japan. E-mail: kurihara@sci.kj.yamagata-u.ac.jp; manabu-ishizaki@sci.kj.yamagata-u.ac.jp
First published on 3rd November 2015
Abstract
Inks of surface-modified nanoparticles (NPs) are a promising fluid for fabricating thin-film electronic devices; however, such NPs are still functionally discrete from each other. Therefore, reconstituting electronic communication among the NPs has been a challenging subject for their nanoscale interfacial assembly. Here, we demonstrate the reconstitution (sintering) ability of surface-modified Prussian blue (PB) and its Ni-replaced analogue (Ni-PBA) NPs that lead to multilayer electronic functions. A double-layer (DL) rigid solid film of the PB (bottom layer) and the Ni-PBA (top layer) NPs has been successfully fabricated on indium-tin-oxide (ITO) substrates by a spin-coating technique using their water inks and a low-temperature sintering process between 120 and 150 °C to immobilise the spin-coated NPs without serious mismatch on the heterostructure DL interface. The DL solid films act as an electron-rectifier device, which is entirely characterised by a PB bottom layer with a minimum nanoscale thickness of ∼20 nm. In the mechanism of the electron-rectifier phenomenon, we reveal the appearance of theoretically predicted partially oxidised/reduced conductor phases of PB.
Introduction
Coordination polymers (CPs) are well-known high-performance functional crystals,1,2 and innumerable CP crystals have been synthesised via various types of infinite bonding chains composed of metal ions and organic/inorganic bridging molecules. Prussian blue (PB), FeIII4[FeII(CN)6]3·xH2O, is a historic blue pigment and the first artificial CP crystal with lattice spaces surrounded by mixed-valence Fe(II)–CN–Fe(III) 3-D frameworks (Fig. 1a).3 So far, PB and its metal-replaced analogue (PBA) crystals have been intensively investigated as a representative CP family that shows versatile functionalities such as electronic, photonic, magnetic, and their hybridization.2,4–7 In particular, the inherent natures originating from the extended d–π electron bonding networks throughout the crystals are anticipated to be advantageously utilised to create a new class of thin-film device functions. Actually, from the advent of electro-deposited PB and PBAs,5–7 the crystalline thin film specifically shows electrochemically reversible redox couples on electrodes, e.g., Fe(II)–CN–Fe(II) (Prussian white (PW)) ↔ Fe(II)–CN–Fe(III) (PB) and PB ↔ Fe(III)–CN–Fe(III) (Prussian yellow (PY)). These redox states include fascinating spin-conjugated electronic properties for PB-based electronic/spintronic devices;4 however, electro-deposition technology generally contains inconvenient processability for the industrial and scientific production of various types of thin-film devices.Currently, the importance of printed electronics (PE) for producing electronic devices using various substrates has increased globally because such solution-based technology with higher processability strongly contributes to a low-consumption industry with respect to saving energy, resources, and time.8–12 Inks and pastes of surface-modified nanoparticles (NPs) are promising candidates for printable fluids; nevertheless, NPs are in a functionally discrete state from each other. Therefore, reconstituting electronic communication among such discrete NPs on substrates has been a scientifically challenging subject for their nanoscale interfacial assembly.7,9–12 In addition, the low-temperature reconstitution via sintering among the NPs at ≤150 °C is an indispensable technique to realise flexible and/or lightweight thin-film electronic devices using heat-sensitive commodity plastic substrates such as polyethylene terephthalate (PET).9,13,14 Metal NPs of silver and copper have been widely adopted for printed conductive circuits. Their low-temperature sintering caused by the melting-point drop phenomenon occurs even at room temperature,9–11,15 whereas a similar phenomenon is intrinsically unavailable in the case of CPs due to the poor mobility of surface atoms, even with the nanoscale downsizing of their crystals.
In a series of reports, we have focused on greener synthetic methodologies of PB and PBA NPs suitable for industrial applications.16,17 In conventional synthesis, PB has been obtained as an insoluble solid immediately precipitated from a concentrated aqueous mixture of Fe3+/2+ and [FeII/III(CN)6]4−/3−. We have introduced the conclusion that the insoluble PB solid is a physically aggregated form of ∼10 nm NPs,16,17 and noticed that in the surface active reaction sites of each PB NP, the 3-D network with cyano-bridged d–π bonding is discontinuous, and each PB NP inevitably bears active surface reaction sites of FeIII–OH2 (Fig. 1a).16,17 By partially modifying the surface reaction sites using [Fe(CN)6]4− (Fig. S1†), PB NPs can be stably redispersed into water directly from the insoluble PB solid to prepare a dense PB NP ink. Dense water inks of PBA NPs have been prepared using a synthetic strategy similar to PB. Furthermore, a low-temperature sintering method suitable for the partially surface-modified PB and PBA NPs has been developed whose mechanism is completely different from the melting-point drop of metal NPs.9–11,15
In this study, we demonstrate high-processability features focusing on PB and PBA NP inks, to construct high-performance multilayer solid thin films18 (Fig. 1): (1) single-layer (SL) thin films are prepared on various substrates by a simple spin-coating solution process (Fig. 1b); (2) a low-temperature heating process transforms the spin-coated film of the NPs into a densely packed rigid solid film via our proposed sintering mechanism (Fig. 1d); (3) heterostructure multilayer thin films of the NPs with different metal compositions can be produced by combination of successive spin-coating and low-temperature sintering (Fig. 1c). Here, we construct low-temperature sintered heterostructure double-layer (DL) films on indium-tin-oxide (ITO) glass and PET substrates (Fig. 1c, photograph) using water inks of the PB NPs and its Ni-replaced analogue (Ni-PBA) NPs. The film thickness is successfully adjusted in nanoscale, depending on the concentrations of their NP inks. The as-prepared DL rigid solid films composed of the PB (bottom layer) and the Ni-PBA (top layer) NPs exhibit an electron-rectification phenomenon,19 which is controlled by the nanoscale thickness of the bottom PB layer. An essential question still remains as to why the PB insulator phase20 can transport holes/electrons from the electrodes to change into the PY/PW phase. Our elucidation of the electron-rectification mechanism will shed light on the long-standing question regarding the theoretically predicted half-metallic conductivity of the partially oxidised/reduced PB phases, i.e., hole-/electron-injected PB phases.21
Results and discussion
Characterisation of PB and Ni-PBA NP solids and inks
Scherrer's particulate sizes of the insoluble PB and Ni-PBA NP solids prepared by conventional synthesis were estimated to be 8.9 and 13 nm, respectively, based on the half-height widths of significantly broadening powder X-ray diffraction (XRD) signals (Fig. S2†). According to the fluorescent X-ray (XRF) analysis, the metal composition ratio of the insoluble Ni-PBA NP solid was estimated to be Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.43. The metal composition ratio deviates slightly from the ideal ratio of Fe
1.43. The metal composition ratio deviates slightly from the ideal ratio of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (Ni3[Fe(CN)6]2), and the deficient positive charge is compensated by potassium ions as a formula of K0.28Ni2.86[Fe(CN)6]2, in accordance with earlier reports.16 PB and Ni-PBA NPs were well dispersed into water by partial displacement between H2O and [Fe(CN)6]4− on the surface sites of Fe(III) (Fig. S1†), and number-averaged dynamic light-scattering (DLS) particle sizes in their inks were 17 ± 5 and 49 ± 26 nm, respectively (Fig. S3b†), indicating that the NPs were partially aggregated as secondary particles dispersible in water. The zeta potentials of the PB and Ni-PBA NPs measured in their diluted water inks were −32 and −35 mV, respectively. The NPs underwent surface modification with [Fe(CN)6]4− and were allowed to be dispersed in water by their mutual electrostatic repulsion due to negative surface charges.16 In transmission electron microscopy (TEM) images (Fig. 2a), the surface-modified PB and Ni-PBA NPs were distributed at 8.3 and 12 nm in mean dimensions, respectively, and lattice images of each NP were observed in the high-resolution TEM (Fig. S3a†). As a result, the diluted PB and Ni-PBA NP inks exhibited high transparency of blue and yellow colours with the absorption maximal wavelength, λmax = 683 and 394 nm, respectively (Fig. S3c†).
1.5 (Ni3[Fe(CN)6]2), and the deficient positive charge is compensated by potassium ions as a formula of K0.28Ni2.86[Fe(CN)6]2, in accordance with earlier reports.16 PB and Ni-PBA NPs were well dispersed into water by partial displacement between H2O and [Fe(CN)6]4− on the surface sites of Fe(III) (Fig. S1†), and number-averaged dynamic light-scattering (DLS) particle sizes in their inks were 17 ± 5 and 49 ± 26 nm, respectively (Fig. S3b†), indicating that the NPs were partially aggregated as secondary particles dispersible in water. The zeta potentials of the PB and Ni-PBA NPs measured in their diluted water inks were −32 and −35 mV, respectively. The NPs underwent surface modification with [Fe(CN)6]4− and were allowed to be dispersed in water by their mutual electrostatic repulsion due to negative surface charges.16 In transmission electron microscopy (TEM) images (Fig. 2a), the surface-modified PB and Ni-PBA NPs were distributed at 8.3 and 12 nm in mean dimensions, respectively, and lattice images of each NP were observed in the high-resolution TEM (Fig. S3a†). As a result, the diluted PB and Ni-PBA NP inks exhibited high transparency of blue and yellow colours with the absorption maximal wavelength, λmax = 683 and 394 nm, respectively (Fig. S3c†).
Characterisation of PB and Ni-PBA films
Inks of the PB NPs could be uniformly spread on indium-tin-oxide (ITO) glass substrates by simple spin-coating (Fig. 1b). Coated NP films were scratched by atomic force microscopy (AFM) probes; the NP images became obscure in the first contact-mode scan (Fig. S4a†), and the scanning area of the film surface was extensively scratched by the AFM probe within the third scan (Fig. S4b†). This scratch test suggests that spin-coated NP films are essentially fragile. To overcome the fatal fragility of the NP films, a low-temperature sintering process has been adopted to create new chemical bonds among the NPs.17 We have focused on active coordination sites exposed on the surface of each PB and PBA NP, in which the 3-D network of metal-cyano-bridged d–π bonds is discontinuous (Fig. 1a). The active surface sites, M–OH2 (M = FeIII or NiII), are allowed to be modified by the addition of [FeII(CN)6]4− to generate M–[NC–FeII(CN)5]4− moieties on the NP surfaces.17 To achieve low-temperature sintering among NPs, a partial surface modification has been performed. In this study, 70% of the surface M–OH2 sites are covered with [FeII(CN)6]4− as an ideal cubic NP dimension of 10 nm (Fig. S1†). The partially surface-modified NPs had a potential ability to connect to each other in the spin-coated films; functionally discrete NPs are allowed to connect via a new metal-cyano-bridged d–π bonding network of M–[NC–FeII(CN)4–CN]4−–M from M–[NC–FeII(CN)5]4− and M–OH2 with the release of H2O ligands (Fig. 1d).Based on the thermogravimetric (TG) weight loss, the coordination and crystal water molecules of the PB and Ni-PBA NPs were released at between 50 and 200 °C (Fig. S5†). The 3-D framework structure of the NPs was maintained against heating of up to 150 °C because the powder XRD pattern was unchanged after heating (Fig. S2†).22 Through the heating process at 150 °C or lower, the spin-coated films of the PB NPs adhered well to the ITO substrates. The fragility of the films was surprisingly improved; low-temperature sintered film with a thickness of approximately 20 nm sustained almost no damage under the same AFM contact-mode scanning conditions as compared with the spin-coated films before sintering (Fig. S4†). In addition, PB NPs were densely packed throughout a similarly sintered film with a 340 nm thickness without any serious voids or cracks from the scanning electron microscopy (SEM) cross-section image (Fig. S6†). The fragility of the spin-coated Ni-PBA NP films was similarly improved by heating at 150 °C or lower. The densely packed Ni-PBA NPs were observed in the SEM cross-section image (Fig. S6†), and the Ni-PBA NP film with a 410 nm thickness was robust based on the AFM scratch test (Fig. S7†). As a result, the PB and Ni-PBA NPs apparently never re-eluted into water from their rigidly sintered films on the ITO substrates.
Electrochemical properties of PB and Ni-PBA NP films
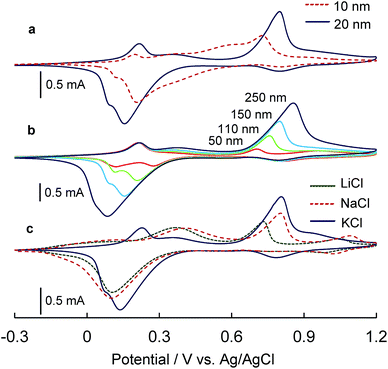
The as-sintered PB NP films showed characteristic reversible redox properties in their cyclic voltammograms (CVs) (Fig. 2c).5 The K+ ions size-selectively contributed to the reversibility to afford two sharp CV redox wave couples at E1/2 = 0.16 and 0.84 V of PW ↔ PB ↔ PY. The redox response occurred selectively in cations (Fig. S8†). The Stokes radii fit better with the crystal lattice spaces of PB in the order of K+ ≫ Na+ > Li+.5 In the sintered film of the spin-coated Ni-PBA NPs, the CVs were also influenced by the counter cations (Fig. S8†). The redox waves were essentially derived from FeIII–CN–NiII ↔ FeII–CN–NiII, while two divided redox couples at E1/2 = 0.44 and 0.60 V were apparently observed in the case of the K+ ions (Fig. 2c). The division is probably caused by the different surroundings of FeIII–CN–NiII moieties due to their defect sites, i.e., sites deficient in [FeIII(CN)6]3−. The redox potentials between FeIII–CN–NiII ↔ FeII–CN–NiII were negatively shifted in the order of Li+ ≈ Na+ > K+.The relationship between the thickness of sintered films and the concentrations of PB and Ni-PBA NP inks was investigated using a stylus profiler (Fig. S9†). The sintered films are adjustable from ten or several tens of nanometres over a submicrometre range of thickness by simple spin-coating techniques. Under the present conditions, the thickness of PB and Ni-PBA NP films was almost linearly controlled up to 100 and 250 nm, respectively, depending on the concentrations of the NPs. The deviation from linearity in the high concentration range is probably caused by complicated factors such as increased viscosity related to thixotropy.
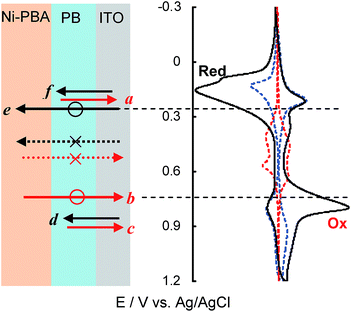
In preparing the DL films, the Ni-PBA NP ink was spin-coated on the sintered bottom layer of the PB NPs on ITO (Fig. 1c), and the as-hybridised films were further sintered. In a typical case, the top layer was formed with a thickness of ∼150 nm on the bottom layer with a thickness of ∼20 nm (Fig. S10†). The heterostructure DL film exhibited an intriguing electronic property (Fig. 3a) that was definitely different from those of the single-layer (SL) films (Fig. 2c). In the CVs of the DL films, two reversible redox couples appeared at the same applied potentials, E1/2 = 0.16 and 0.84 V, as those of the PB SL film. A new rectified current couple was observed at Epa = 0.80 and Epc = 0.16 V. The redox couples at the potentials, E1/2 = 0.44 and 0.60 V, derived from the SL film of Ni-PBA NPs disappeared in the DL film. To elucidate the evolution mechanism of the rectification phenomenon, the thickness dependency of its bottom and top layers was investigated. The bottom layer was formed by two different thicknesses of 10 and 20 nm using adjusted concentrations of the PB NP ink, and then the Ni-PBA layer was overlaid at a constant thickness of ∼150 nm. The redox currents at E1/2 = 0.16 and 0.84 V were increased by thickening the bottom layer (Fig. 3a); therefore, the two redox couples were attributed to the electron transfer between the PB layer and the ITO electrode. The rectified current couple became obscure in the case of the 10 nm thickness. The rectified peak current intensities (ipa and ipc) depended on the thickness of the top layer fabricated on the 20 nm bottom layer (Fig. 3b), indicating that the rectified charge density of the DL films can be accumulated as the top layer increases in thickness. The rectification response of the DL film appeared more clearly in the case of K+ than in Na+ and Li+ (Fig. 3c). When the 20 nm bottom layers were sintered at 100 °C, the electron-rectification phenomenon resulted in the appearance of weak leak currents in the CV (Fig. S11†). This is probably caused by incomplete connections (incomplete sintering) among the bottom PB NPs. If the bottom and top layers were heated to between 120 and 150 °C, the rectification phenomena could be reproducibly observed (Fig. S11†). These results imply that the top layer electrochemically united with the bottom layer via the low-temperature reconstitution of the electron-transferable d–π bonding networks among the NPs. The bottom layer of the sintered PB NPs behaves as a high-performance blocking (insulator) solid layer for electron transfer synchronised with K+ migration between the ITO electrode and the top layer.
Electron-rectification mechanism and partially oxidised/reduced conductor phases of PB
In the rectification phenomenon, the redox sequence of potentials a to f is shown in Fig. 4; the bottom layer is oxidised from PW to PB at potential a, corresponding to the redox couple PW ↔ PB of the PB SL film. The FeII–CN–NiII state cannot be oxidised to the FeIII–CN–NiII state up to a starting potential, b, for oxidation from PB to PY, meaning that the PB state of the bottom layer closes the electron-transfer pathway between the top layer and the electrode as an insulator. At the starting potential, b, i.e., the negative potential edge of the redox wave of PB ↔ PY, the bottom layer can open the electron-transfer pathway for the top layer. Thus, the oxidation current flow from the top layer to the electrode is observed around potential b as the rectification phenomenon. The redox wave of PB ↔ PY appears at the same potentials, c and d, as in the PB SL film. In the reverse potential scan from d to f, the FeIII–CN–NiII state is not subject to reduction to FeII–CN–NiII up to the starting potential, e, for the reduction from PB to PW. At the starting potential, e, i.e., the positive potential edge of the redox wave of PB ↔ PW, the bottom layer can open the electron transfer pathway for the top layer. The rectification current flow from the electrode to the top layer is observed around potential e. The redox wave of PB ↔ PW appears at the same potentials, a and f, as in the PB SL film.According to the proposed mechanism (Fig. 4), three different phases appeared in the bottom PB layer within the potential window between the two redox couples corresponding to PW ↔ PB (a and f) and PB ↔ PY (c and d). The bottom PB layer initially behaves as an insulator phase within potentials e and b. If some electrons and holes are injected into the PB insulator layer near potentials e and b, respectively, they are allowed to migrate from the ITO electrodes into the top Ni-PBA layer. After the completion of the redox reaction, i.e., electron and hole accumulation of the Ni-PBA layer, the redox state of PB can be changed into PW and PY. These results strongly indicate that the PB insulator layer is transformed into partially oxidised/reduced conductor phases, i.e., hole-/electron-injected conductor phases.
In 2009, Illas et al. predicted by the periodic density function calculation that PY and PW behave as half-metals.21 In the PB oxidation process to PY, the Fermi level was gradually stabilised into the topmost occupied band due to the t2g orbital electrons. A partially oxidised form, (K0.75Fe[Fe(CN)6] = K0.75FeIII[FeIII(CN)6]0.25[FeII(CN)6]0.75), of PB (KFeIII[FeII(CN)6]) theoretically exhibited a very limited electron density at the Fermi level, and the top of the conduction band is less than 0.05 eV above it. In the subsequent oxidation of the PB to PY, the top of the conduction band changed linearly within a range of 0.05 and 0.75 eV. In 2011, Einaga et al. synthesised PB and its partially oxidised form, Berlin green (BG) powders, and they concluded on the basis of the experimental conductivities and theoretical calculations that PB was reaffirmed as a band insulator and BG as a conductor.20 The previously mentioned theoretical and experimental results about the partially oxidised form of PB bearing a half-metallic nature are consistent with our mechanism for this rectification phenomenon.
Electrochemically or synthetically obtained films of the PW state are unstable and return to the PB state.23 Therefore, the theoretically predicted half-metallic nature of partially reduced PB states is experimentally unclear. In addition, the reduction process from PB to PW is more theoretically complicated than that from PB to PY because of the large changes involved in the structural (lattice expansion) and electronic properties in the former process.21 In this study, we have successfully obtained experimental evidence that the partially reduced state of PB is defined as a conductor phase electron-transferable into the Ni-PBA top layer from the ITO electrode.
Conclusions
With progress in the miniaturisation of electronic devices, the functional units are anticipated to become increasingly smaller. Therefore, molecule-10,13,24 and NP-based25 electronic devices have been competitively developed for actual applications. From the perspective of an NP-based technology, the electron-rectifier device can be entirely characterised by a bottom layer with a minimum thickness of approximately 20 nm, that is, a stack layer of only two or three PB NPs. In the SEM cross-section image (Fig. 2b), the sintered PB NPs are uniformly adhered as a film of approximately 20 nm thickness on the ITO granular layer, and the film surface is surprisingly flat against the roughness of the ITO substrate. In the SEM top-view image, the PB NPs homogeneously cover the ITO granular surface (Fig. 2b) without any serious inter-particulate gaps or vacant domains. The low-temperature sintering successfully prevents the functional disorder of the bottom stack layer of two or three PB NPs when the Ni-PBA NP inks are overlaid. In fact, cracks due to mismatches in the DL heterostructure interface are never observed in the film adhered to the ITO substrate in the SEM cross-section image of the electron-rectifier film (Fig. S10†). In a preliminary attempt to produce PE devices using heat-sensitive commodity plastics and solid films of CP NPs, DL films are successfully prepared on an ITO substrate made of PET, and it reproduces a similar electrochemical response due to electron rectification on the curved substrate, which demonstrates its flexibility (Fig. 1c, S12†).In the electro-rectification phenomenon, we can experimentally visualise both of the partially oxidised/reduced conductor phases along with an insulator phase in the PB NP solid film within its redox-potential window. The conductor phases are a new class for designing PB- and PBA-based electronic and spintronic devices. Inks of surface-modified NPs are advantageously available as a printable fluid; nevertheless, the NPs are in a functionally discrete state from each other. Reconstituting electronic communication among the discrete NPs will be a key technology in CP electronics (Fig. 1d). For future applications, further investigations of the low-temperature sintering (reconstitution) processes among CP NPs will lead to a variety of high-performance printed electronic/spintronic devices that originates from the extended d–π bonding networks.
Experimental
Materials
Guaranteed-grade Fe(NO3)3·9H2O, Ni(NO3)2·6H2O, K4[Fe(CN)6]·3H2O, and K3[Fe(CN)6] and extra-pure grade Na4[Fe(CN)6]·10H2O were purchased from Kanto Chemicals. All reagents used to prepare the insoluble PB and Ni-PBA NP solids were used without further purification. Guaranteed-grade LiCl was supplied from Nacalai Tesque. Guaranteed-grade NaCl and KCl were supplied by Kanto Chemicals. ITO glass (10 Ω sq−1) and ITO PET (60 Ω sq−1) substrates were purchased from Furuuchi Chemical and Sigma-Aldrich, respectively.Apparatuses
Powder X-ray diffraction (XRD) analyses were performed using a Rigaku MiniFlex II desktop X-ray diffractometer (Cu Kα1 radiation). The metal compositions of the Ni-PBA NPs were determined by fluorescent X-ray (XRF) analyses using a Rigaku Primini wavelength dispersive X-ray spectrometer. The dynamic light-scattering (DLS) particle sizes and the zeta potentials of the PB and Ni-PBA NPs were measured on an Otsuka ELSZ-1000ZS particle-size analyser. Thermogravimetric analysis (TG) was performed using a TA Instruments SDT Q600 under a stream of He, and the liberated gasses from the PB and Ni-PBA NPs synchronised with the TG weight loss were analysed using a JEOL JEM-Q1050 mass spectrometer. Thermogravimetric-differential thermal analysis (TG-DTA) was performed using a Rigaku Thermo plus EVO2 under a stream of air. Spin-coated thin films were fabricated on ITO glass and ITO PET substrates using Mikasa MS-A100 and Kyowa Riken K359 S-1 spin-coaters, respectively. The thicknesses of spin-coated and low-temperature sintered films of the PB and Ni-PBA NPs were determined using a Bruker Dektak XT stylus profiler. Scanning electron microscope (SEM) and transmission electron microscope (TEM) images were recorded on a JEOL JSM-7600F and JEOL JEM-2100F, respectively. The fragility of the NP film surfaces was evaluated by probe scratching via contact-mode images of atomic force microscopy (AFM) using a Shimadzu SPM-9700 scanning probe microscope. An ALS electrochemical analyser, model 1100P, was used to measure the cyclic voltammetry. ITO glass and ITO PET substrates were plasma treated using Meiwafosis SEDE.Preparation of PB NP inks
The insoluble PB NP solid was prepared in accordance with the previous literature.16 An aqueous solution (7.50 mL) of Fe(NO3)3·9H2O (3.23 g, 8.00 mmol) was added to an aqueous solution (12.0 mL) of Na4[Fe(CN)6]·10H2O (2.90 g, 6.00 mmol). The reaction mixture was vigorously stirred for 30 min. The resulting blue precipitate of PB was centrifuged at 2740 × g for 10 min, washed with water three times and with methanol once, and dried under reduced pressure. The as-obtained insoluble PB NP solid with a yield of 1.84 g (84%) was FeIII4[FeII(CN)6]3·xH2O (x = 10–15) in the formula, where the number of waters of crystallization, x, was determined using TG-DTA.The insoluble PB NP solid (1.53 g, 1.40 mmol as a formula of FeIII4[FeII(CN)6]3·13H2O) was stirred with water (10 mL) for 30 min. Into the resulting blue suspension, an aqueous solution (10 mL) of K4[Fe(CN)6]·3H2O (0.414 g, 0.980 mmol, 10% based on the total number of Fe ions of the PB) was added, and the mixture was then stirred for 2 days. After centrifugal removal of aggregated large particles of the PB NPs at 2600 × g for 10 min, the ink of the PB NPs (68.2 mM as the formula of FeIII4[FeII(CN)6]3·13H2O) was diluted with water to adjust to various concentrations.
Preparation of Ni-PBA NP inks
The insoluble Ni-PBA NP solid was prepared in accordance with previous studies.16 An aqueous solution (12 mL) of Ni(NO3)2·6H2O (3.49 g, 12.0 mmol) was added to an aqueous solution (24 mL) of K3[Fe(CN)6] (2.62 g, 8.00 mmol). The reaction mixture was vigorously stirred for 30 min. The precipitated Ni-PBA, NiII3[FeIII(CN)6]2·11H2O, was separated in a procedure similar to that in the case of the insoluble PB NP solid with a yield of 3.13 g (90%), where the hydration number was similarly estimated using TG-DTA.The insoluble Ni-PBA NP solid (3.19 g, 4.00 mmol as the formula of NiII3[FeIII(CN)6]2·11H2O) was stirred with water (10 mL) for 2 h. Into the resulting yellow suspension, an aqueous solution (16.7 mL) of K4[Fe(CN)6]·3H2O (0.845 g, 2.00 mmol, 10% based on the total number of metal (Ni and Fe) ions of the Ni-PBA) was added, and the mixture was then stirred for 2 weeks. After the centrifugal removal of aggregated large particles of the Ni-PBA NPs at 2600 × g for 10 min, the ink of Ni-PBA NPs (Ni-PBA NP ink) (148.5 mM as the formula of NiII3[FeIII(CN)6]2·11H2O) was diluted with water to adjust to various concentrations.
Fabrication of single-layer (SL) and double-layer (DL) thin films using PB NP and Ni-PBA NP inks
To fabricate SL films composed of PB NPs or Ni-PBA NPs (Fig. 1b), PB NP (5–60 mM) or Ni-PBA (5–100 mM) NP inks (150 μL) were developed on the plasma-treated hydrophilic surface of an ITO glass substrate (2 × 2 cm2) using a spin-coating technique (first step: accelerate from 0 to 2000 rpm for 15 s; second step: 2500 rpm for 5 s), and spin-coated NP SL films were sintered at a low temperature between 100 and 150 °C for 1 h in a constant-temperature oven. To prepare DL films of the PB NPs and the Ni-PBA NPs with different thicknesses, the as-sintered PB NP SL film received a topcoat of the Ni-PBA NP inks with various concentrations via a similar spin-coating process, and the hybridised films were further sintered at 100–150 °C for 1 h.Electrochemical measurements
The electrochemical properties of SL and DL films of PB and Ni-PBA NPs were investigated in a 0.1 M LiCl, NaCl, or KCl electrolyte aqueous solution controlled at pH 3 by the addition of a diluted HCl aqueous solution under applied potentials between −0.3 and 1.2 V vs. Ag/AgCl using an ALS plate material evaluating cell (measurement area: 47.8 mm2).Fabrication of DL film on an ITO PET substrate
An electrochemical measurement area (2 × 2 cm2) was exposed on an ITO PET substrate (5 × 6 cm2) while covering the other area with masking tape. A similar DL film composed of the PB NPs and the Ni-PBA NPs was fabricated on the as-masked ITO PET substrate using the same procedures as for the spin-coating and the low-temperature sintering. After removing the masking tape, the electrochemical properties of the as-prepared DL film were investigated using a column-shaped glass cell with a diameter of 25.2 mm (Fig. S12†).Acknowledgements
This work was partially supported by a Grants-in-Aid for Scientific Research (C) (No. 24550069), (B) (No. 15H03783), and Young Scientists (B) (No. 26810030) of the Japan Society for the Promotion of Science (JSPS). This work was funded by the Mukai Science and Technology Foundation.Notes and references
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed; A. Corma, H. García and F. X. Llabrés i Xamera, Chem. Rev., 2010, 110, 4606 CrossRef PubMed; H. Furukawa, K. E. Cordova, M. O'keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed; W. Zhong and R.-G. Xiong, Chem. Rev., 2013, 112, 1163 CrossRef PubMed; A. Carné, C. Carbonell, I. Imaz and D. Maspoch, Chem. Soc. Rev., 2011, 40, 291 RSC; A. Morozan and F. Jaouen, Energy Environ. Sci., 2012, 5, 9269 Search PubMed; M. D. Allendorf, A. Schwartzberg, V. Stavila and A. A. Talin, Chem.–Eur. J., 2011, 17, 11372 CrossRef PubMed.
- V. Stavila, A. A. Talin and M. D. Allendorf, Chem. Soc. Rev., 2014, 43, 5994 RSC; E. Cornado and G. M. Espallargas, Chem. Soc. Rev., 2013, 42, 1525 RSC.
- A. Kraft, Bull. Hist. Chem., 2008, 33, 61 CAS.
- O. Sato, J. Photochem. Photobiol., C, 2004, 5, 203 CrossRef CAS; R. Lescouëzec, L. M. Toma, J. Vaissermann, M. Verdaguer, F. S. Delgado, C. Ruiz-Pérez, F. Lloret and M. Julve, Coord. Chem. Rev., 2005, 249, 2691 CrossRef; H. Tokoro and S. Ohkoshi, Dalton Trans., 2011, 40, 6825 RSC; S. Ohkoshi, H. Tokoro and K. Hashimoto, Coord. Chem. Rev., 2005, 249, 1830 CrossRef; S. Ohkoshi, S. Takano, K. Imoto, M. Yoshikiyo, A. Namai and H. Tokoro, Nat. Photonics, 2014, 8, 65 CrossRef.
- K. Itaya, T. Ataka and S. Toshima, J. Am. Chem. Soc., 1982, 104, 4767 CrossRef CAS.
- V. D. Neff, J. Electrochem. Soc., 1978, 125, 886 CrossRef CAS; K.-C. Cheng, F.-R. Chen and J.-J. Kai, Electrochim. Acta, 2007, 52, 3330 CrossRef; W. Chen, J. Tang and X.-H. Xia, J. Phys. Chem. C, 2009, 113, 21577 CrossRef; M. Takachi, T. Matsuda and Y. Moritomo, Jpn. J. Appl. Phys., 2013, 52, 044301 CrossRef.
- B. Kong, C. Selomulya, G. Zheng and D. Zhao, Chem. Soc. Rev., 2015, 44, 7997 RSC.
- M. Singh, H. M. Haverinen, P. Dhagat and G. E. Jabbour, Adv. Mater., 2010, 22, 673 CrossRef CAS PubMed; R. R. Søndergaard, M. Hösel and F. C. Krebs, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 16 CrossRef; H. Minemawari, T. Yamada, H. Matsui, J. Tsutsumi, S. Haas, R. Chiba, R. Kumai and T. Hasegawa, Nature, 2011, 475, 364 CrossRef PubMed; P. H. Lau, K. Takei, C. Wang, Y. Ju, J. Kim, Z. Yu, T. Takahashi, G. Cho and A. Javey, Nano Lett., 2013, 13, 3864 CrossRef PubMed; T. Someya, T. Sekitani, S. Iba, Y. Kato, H. Kawaguchi and T. Sakurai, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 9966 CrossRef PubMed.
- K. Fukuda, Y. Takeda, Y. Yoshimura, R. Shiwaku, L. T. Tran, T. Sekine, M. Mizukami, D. Kumaki and S. Tokito, Nat. Commun., 2014, 5, 4147 CAS.
- J. Perelaer, P. J. Smith, D. Mager, D. Soltman, S. K. Volkman, V. Subramanian, J. G. Korvink and U. S. Schubert, J. Mater. Chem., 2010, 20, 8446 RSC.
- A. Kamyshny, J. Steinke and S. Magdassi, Open Appl. Phys. J., 2011, 4, 19 CrossRef CAS.
- J. Perelaer and U. S. Schubert, J. Mater. Res., 2013, 28, 564 CrossRef CAS.
- S. Park, G. Wang, B. Cho, Y. Kim, S. Song, Y. Ji, M.-H. Yoon and T. Lee, Nat. Nanotechnol., 2012, 7, 438 CrossRef CAS PubMed.
- M. S. White, M. Kaltenbrunner, E. D. Głowacki, K. Gutnichenko, G. Kettlgruber, I. Graz, S. Aazou, C. Ulbricht, D. A. M. Egbe, M. C. Miron, Z. Major, M. C. Scharber, T. Sekitani, T. Someya, S. Bauer and N. S. Sariciftci, Nat. Photonics, 2013, 7, 811 CrossRef CAS.
- B. T. Anto, S. Sivaramakrishnan, L.-L. Chua and P. K. H. Ho, Adv. Funct. Mater., 2010, 20, 296 CrossRef CAS; K. Fukuda, T. Sekine, Y. Kobayashi, Y. Takeda, M. Shimizu, N. Yamashita, D. Kumaki, M. Itoh, M. Nagaoka, T. Toda, S. Saito, M. Kurihara, M. Sakamoto and S. Tokito, Org. Electron., 2012, 13, 3296 CrossRef.
- A. Gotoh, H. Uchida, M. Ishizaki, T. Satoh, S. Kaga, S. Okamoto, M. Ohta, M. Sakamoto, T. Kawamoto, H. Tanaka, M. Tokumoto, S. Hara, H. Shiozaki, M. Yamada, M. Miyake and M. Kurihara, Nanotechnology, 2007, 18, 345609 CrossRef.
- M. Ishizaki, K. Kanaizuka, M. Abe, Y. Hoshi, M. Sakamoto, T. Kawamoto, H. Tanaka and M. Kurihara, Green Chem., 2012, 14, 1537 RSC.
- L. Britnell, R. V. Gorbachev, R. Jalil, B. D. Belle, F. Schedin, A. Mishchenko, T. Georgiou, M. I. Katsnelson, L. Eaves, S. V. Morozov, N. M. R. Peres, J. Leist, A. K. Geim, K. S. Novoselov and L. A. Ponomarenko, Science, 2012, 335, 947 CrossRef CAS PubMed; N. Huo, J. Kang, Z. Wei, S.-S. Li, J. Li and S.-H. Wei, Adv. Funct. Mater., 2014, 24, 7025 CrossRef; R. Cheng, D. Li, H. Zhou, C. Wang, A. Yin, S. Jiang, Y. Liu, Y. Chen, Y. Huang and X. Duan, Nano Lett., 2014, 14, 5590 CrossRef PubMed; J. Meng, J.-J. Chen, L. Zhang, Y.-Q. Bie, Z.-M. Liao and D.-P. Yu, Small, 2015, 11, 1660 CrossRef PubMed; T. Niu and A. Li, Prog. Surf. Sci., 2015, 90, 21 CrossRef; S. Burnside, S. Winkel, K. Brooks, V. Shklover, M. Grätzel, A. Hinsch, R. Kinderman, C. Bradbury, A. Hagfeldt and H. Pettersson, J. Mater. Sci.: Mater. Electron., 2000, 11, 355 CrossRef.
- H. D. Abruna, P. Denisevich, M. Umana, T. J. Meyer and R. W. Murray, J. Am. Chem. Soc., 1981, 103, 1 CrossRef CAS; P. Denisevich, K. W. Willman and R. W. Murray, J. Am. Chem. Soc., 1981, 103, 4727 CrossRef; R. M. Metzger, Chem. Rev., 2003, 103, 3803 CrossRef PubMed; C. H. W. Cheng, S. W. Boettcher, D. H. Johnston and M. C. Lonergan, J. Am. Chem. Soc., 2004, 126, 8666 CrossRef PubMed; K. Miecznikowski, M. Chojak, W. Steplowska, O. Makowski, P. J. Kulesza, L. Adamczyk, M. A. Malik, M. Gałkowski and H. Bala, Pol. J. Chem., 2004, 78, 1183 Search PubMed.
- D. M. Pajerowski, T. Watanabe, T. Yamamoto and Y. Einaga, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 153202 CrossRef.
- J. C. Wojdeł, I. P. R. Moreira, S. T. Bromley and F. Illas, J. Mater. Chem., 2009, 19, 2032 RSC.
- A. Takahashi, H. Tanaka, N. Minami, M. Kurihara and T. Kawamoto, Bull. Chem. Soc. Jpn., 2015, 88, 69 CrossRef.
- E. Reguera, J. Fernández-Bertrán, A. Dago and C. Díaz, Hyperfine Interact., 1992, 73, 295 CrossRef CAS.
- V. Panchal, A. Lartsev, A. Manzin, R. Yakimova, A. Tzalenchuk and O. Kazakova, Sci. Rep., 2014, 4, 5881 CrossRef CAS PubMed; C. Joachim, J. K. Gimzewski and A. Aviram, Nature, 2000, 408, 541 CrossRef PubMed; W. Lee, K. Kim, W. Jeong, L. A. Zotti, F. Pauly, J. C. Cuevas and P. Reddy, Nature, 2013, 498, 209 CrossRef PubMed; J. V. Barth, G. Costantini and K. Kern, Nature, 2005, 437, 671 CrossRef PubMed.
- J. Li, Y. Zhang, S. To, L. You and Y. Sun, ACS Nano, 2011, 5, 6661 CrossRef CAS PubMed; Y. Z. Long, M. Yu, B. Sun, C. Z. Gu and Z. Fan, Chem. Soc. Rev., 2012, 41, 4560 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18678a |
| This journal is © The Royal Society of Chemistry 2015 |