Enhancement in membrane performances of a commercial polyamide reverse osmosis membrane via surface coating of polydopamine followed by the grafting of polyethylenimine
Abstract
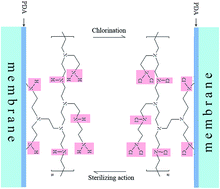
Membrane fouling and chlorine degradation are two of the major challenges the reverse osmosis (RO) membrane industry is facing in recent years. In the present study, a commercial aromatic polyamide RO membrane (XLE-400, DOW Co., Ltd.) was modified via surface coating of polydopamine (PDA) followed by the grafting of polyethylenimine (PEI). The successful modification was confirmed by X-ray photoelectron spectroscopy (XPS). Membrane surface properties were characterized through scanning electron microscopy (SEM), atomic force microscopy (AFM), zeta potential and contact angle. The results showed that modification enhanced the surface hydrophilicity, moved the surface charge towards the positive side and made the surface slightly rougher without damaging the surface peak-and-valley substrate. The influence of modification on the permselectivity of the membrane was also examined. The modified membrane had a higher salt rejection and a slightly lesser water flux than the unmodified membrane. Furthermore, the chlorination, fouling and simulated biofouling experiments were done. The results showed that, due to the abundant presence of grafted amino groups coming from PEI, the modified membrane exhibited higher chlorine resistance, anti-fouling and antibacterial properties.

 Please wait while we load your content...
Please wait while we load your content...