Graphene/polyurethane composites: fabrication and evaluation of electrical conductivity, mechanical properties and cell viability
Abstract
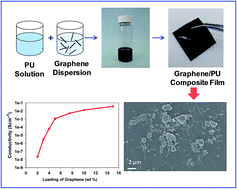
Recently conducting and electroactive polymers have received the attention of researchers to explore their potential in biomedical applications. Polyurethanes (PUs) are of particular interest to make conductive polymer composites by the incorporation of conductive particles because of their inherent biocompatibility, biostability, excellent processability and good mechanical properties. In the present work, conductive composites of graphene and a siloxane polyurethane (Elast-Eon™) were prepared. The graphene/Elast-Eon™ composites were prepared using different methods i.e. solution mixing, melt processing and in situ polymerisation in order to compare the effect of the processing method on the conductivity of resulting composites. The composites were prepared with varying content of graphene and the electrical conductivity of the resulting composites was determined using a two point probe method. In order to improve the conductivity, effect of cooling rate during compression moulding as well as annealing of composite films was examined. Both of these approaches were found to significantly improve the conductivity of composites with lower graphene content (≤5 wt%). A conductivity of 1.12 × 10−3 S cm−1 was achieved with 5 wt% loading of graphene and a maximum conductivity of 5.96 × 10−2 S cm−1 was achieved with 15 wt% of graphene content. The composites were further characterised using scanning electron microscopy (SEM), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and tensile testing methods. The tensile and TGA results showed that the composites have good mechanical properties and showed that composites retain the thermal properties of parent PU material. Furthermore, the cytotoxicity assay tests found that composites were not cytotoxic to living cells in vitro and potentially useful in biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...