Enhanced photocatalytic activity for the degradation of rhodamine B by integrating salinity gradient power into a photocatalytic fuel cell†
Mingrui Sui‡
ab,
Yue Dong‡ab and
Hong You*ab
aState Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, No 73 Huanghe Road, Nangang District, Harbin 150090, China. E-mail: youhonghit@sina.com; Fax: +86-451-86283118; Tel: +86-451-86283118
bSchool of Municipal and Environmental Engineering, Harbin Institute of Technology, No 73 Huanghe Road, Nangang District, Harbin 150090, China
First published on 20th October 2015
Abstract
Photocatalytic fuel cells (PFCs) are an energy-sustainable system concentrating on the degradation of refractory pollutants; however, their degradation efficiency still needs to be improved. Herein, a three-chamber photocatalytic fuel cell (PFC) was constructed based on the in situ utilization of junction potential created by a salt concentration gradient across the ion exchange membrane (IEM) for better degradation of rhodamine B (RhB). The system displays degradation efficiency as a linear function of NaCl concentration (y = 4.3089 × 10−4x + 0.0312, R2 = 99.58%), with a maximum first-order kinetic constant (k) of 0.0505 min−1 at a NaCl concentration of 45 g L−1. In addition, the maximum power density increased rapidly from 170.00 to 319.70 mW m−2 with an increase in NaCl concentration. Moreover, the salt solution was reduced in salinity by 19.70%, 41.69%, and 58.25% under an operation time of 12 h, 24 h, and 36 h. Furthermore, the photoanode exhibited greater degradation performance over time, which ensured the stable operation of the system. These results show that integrating salinity gradient power into the PFC system is an effective method to accelerate the degradation of refractory pollutants and to achieve the additional function of salinity removal.
Introduction
With an ever-decreasing cost and improving performance, photocatalytic fuel cells (PFCs) mediated by semiconductors are increasingly gaining popularity for persistent and hazardous wastewater remediation and simultaneous electricity production over recent years.1,2 PFCs offer several advantages over conventional catalytic oxidation technology, in terms of energy recovery and the unselective oxidation for almost all organic compounds.3–5 PFCs use highly-ordered TiO2 nanotubes as the anode due to their low cost, chemical inertness, and photostability.6,7 The performance of electricity generation and degradation efficiency of refractory pollutants is heavily dependent on the sum of the photogenerated electrons and holes on the surface of TiO2. However, a high degree of recombination between the photogenerated electrons and holes in TiO2 still presents a challenge for wider applications of PFCs.8Many approaches have been used to reduce the adverse effects of the recombination of electron–hole pairs for the enhancement of organic wastewater treatment. This includes metal doping,9,10 coupling other semiconductors with TiO2 (ref. 11) and changing the fabrication methods to optimize the microstructure of TiO2.12 In addition, the application of an external positive anodic bias has been utilized.13 A PFC-MFC system was constructed to utilize MFC electricity for the effective degradation of organic pollutants.14 On the other hand, a PFC was also used as the power source for another PEC to enhance its wastewater treatment.15 This utilization of external positive anodic bias was shown to effectively improve the performance of organic matter degradation in PFCs, but the electricity generation was sacrificed, thus shadowing this feature of PFCs.
It is important to note that salinity gradient power (SGP) is considered as a clean and sustainable form of energy, generated from the reversible mixing of solutions with high and low salt concentrations.16,17 Using reverse electrodialysis (RED) as the main membrane-based technology converts potential energy into useful electricity.18 A typical potential difference of 0.1–0.2 V per cell pair in a RED stack system can be created across the membrane.19 This potential difference named as junction potential was shown to effectively enhance the electrode reaction and current generation. For example, previous studies of three-chamber microbial desalination cells (MDCs) modified with a microbial fuel cell to contain a middle desalination chamber has demonstrated improved anode and cathode performance when compared to an individual MFC.20 The system used a lower salinity wastewater stream in the anode chamber and normal seawater in the middle chamber to create a significant concentration gradient across the ion exchange membrane (IEM), which forms the junction potential, and thereby improves the desalination performance.21–23
Tapping into the advantages of junction potential created by a salinity difference into a PFC to enhance its performance and reduce the recombination of electron–hole pairs for better photoanode performance remains crucial and vital. Herein, a three-chamber PFC system containing a middle chamber fed with a high salt concentration solution (NaCl) was investigated. The system utilized the salinity gradient between the middle chamber and photoanode chamber for junction potential generation to enhance the photoanode performance. Such a unique process might contribute to a synergistically-enhanced system for both power generation and organic wastewater treatment in two ways: (1) PFC electrodes with a middle salt chamber provide the appropriate electrode reactions and salinity gradient power can be effectively extracted to increase the power output of the system and (2) the inclusion of the RED stack improves the performance of the PFC photoanode enhancing the degradation efficiency of refractory pollutants. This study investigates the systems performance in terms of RhB degradation and power production, and speculates how the recombination of the photogenerated electrons and holes can be reduced.
Materials and methods
Preparation of the TiO2/Ti photoanode
Titanium mesh (2 × 10 cm, nominal aperture 0.19 mm, wire diameter 0.23 mm, wires per inch 6060, open area 20%, purity > 99.6%) was cleaned with an acid mixture (HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3
HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4 = 1
H2SO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and then degreased by sonication in acetone for 15 min. This was followed by rinsing with deionized water for 5 min and drying at ambient temperature. Electrochemical anodization was performed in a two-electrode configuration using cleaned Ti mesh as the working electrode and a same-size graphite plate as the counter electrode (the distance of the two electrodes was 3 cm). The electrolyte contained 0.3 wt% NH4F and 2 vol% H2O in ethylene glycol. The anodization was carried out at a constant voltage of 60 V for 40 min using a direct current power supply. The as-prepared TiO2 nanotube array was prepared by annealing in a muffle furnace at 500 °C for 3 h with heating and cooling rates of 1 °C min−1 for crystallization.
4) and then degreased by sonication in acetone for 15 min. This was followed by rinsing with deionized water for 5 min and drying at ambient temperature. Electrochemical anodization was performed in a two-electrode configuration using cleaned Ti mesh as the working electrode and a same-size graphite plate as the counter electrode (the distance of the two electrodes was 3 cm). The electrolyte contained 0.3 wt% NH4F and 2 vol% H2O in ethylene glycol. The anodization was carried out at a constant voltage of 60 V for 40 min using a direct current power supply. The as-prepared TiO2 nanotube array was prepared by annealing in a muffle furnace at 500 °C for 3 h with heating and cooling rates of 1 °C min−1 for crystallization.
Reactor design and operation
The PFC reactor was made of polymethyl methacrylate consisting of three chambers: an anode chamber (15 mL), middle chamber (17 mL), and cathode chamber (17 mL). These chambers were assembled by three same modules and the net liquid volume of the anode chamber was smaller because of the TiO2/Ti mesh and magnetic bar in it. The chambers were clamped together with gaskets and separated using an anion exchange membrane (AEM, DF120, Tianwei) and cation exchange membrane (CEM, Ultrex CMI7000, Membrane International). All chambers were cylindrically drilled on the block with a cross-sectional area of 7 cm2. A quartz window was placed in the anode chamber to allow the transmission of UV light. TiO2/Ti mesh was folded and woven onto a titanium lead wire. An air cathode was made via a “rolling-press” method using activated carbon and PTFE.The PFC functioned with ultraviolet illumination provided by a 150 W xenon lamp (GY-10A, Tuopu Co. Ltd., China. Table. S1, ESI†) installed 15 cm away from it. A model pollutant of rhodamine B (RhB, 30 mg L−1) dissolved in 0.05 M Na2SO4 was used as the anode solution. Prior to use, the RhB solution in the anode chamber was stirred in the dark for 30 min to achieve an adsorption/desorption equilibrium. The electrolyte that was used for the cathode compartment was phosphate buffer (50 mM, pH 7.0). Moreover, tests were conducted with different NaCl concentrations (45 g L−1, 35 g L−1, 25 g L−1, 15 g L−1, 5 g L−1, and 0 g L−1) in the middle chamber. To gain further insight into the effect of the junction potential created by salinity difference on the PFC performance, a control dual-chamber PFC with no middle chamber was adopted. An AEM and CEM were placed between the anode and cathode chamber, respectively. An external resistance (1000 Ω) was used in all the experiments carried out. A saturated calomel reference electrode (SCE, +242 mV vs. standard hydrogen electrode, SHE) was used for calculating the anode and cathode potentials.
Analysis and calculation
The surface morphology of the TiO2/Ti mesh was characterized by field emission scanning electron microscopy (SEM, FEI Quanta 200F). The current–voltage properties of the TiO2/Ti electrode were investigated using an electrochemical workstation (Metrohm Autolab 85061) in a three-electrode system with Pt sheet as the counter electrode and saturated calomel electrode (SCE) as the reference electrode (at a scan rate of 10 mV s−1). The voltages of the PFCs were continuously recorded using a data acquisition system (PISO-813) every 1 min. The polarization curves were obtained by recording the current response to a linear potential decrease using the electrochemical workstation (Metrohm Autolab 85061) at a scan rate of 0.1 mV s−1. Power density was calculated by normalizing the power by the cathode surface area (7 cm2). The performance of the PFCs was also evaluated in terms of the fill factor (FF) using eqn (1).
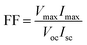 | (1) |
Ion concentrations (Na+, Cl−) in the three chambers were determined using inductively coupled plasma atomic emission spectrometry (ICP-AES, IRIS Intrepid II XSP, Thermal), with pH measurements obtained immediately after sampling using a probe. The concentration of RhB was determined by measuring the solution absorbance using an UV-vis spectrophotometer (UV-722S, PuXiTongYong, China) at a wavelength of 552 nm. At the scheduled sampling interval, the sample (2 mL) was withdrawn from the anode for analysis, before injecting it back immediately.
Results
Characterization of TiO2/Ti electrodes
Fig. 1 shows the well-aligned tube-like morphology of the TiO2 electrode prepared by electrochemical anodization with an average diameter of 0.5 μm and length of 5 μm (Fig. 1). SEM images show the Schottky-type contact naturally formed between the highly-ordered TiO2 nanotubes and Ti substrate, which can provide a unidirectional electric channel for the transport of photogenerated electrons. These nanotubes can absorb reflected and refracted light due to their geometric features, thus minimizing the loss of photons resulting from scattering effects in the liquid. TiO2 nanotubes grown vertically on the Ti wire present a well-radial-3D array. Irregular fissures observed among the different TiO2 nanotube bundles can add more active adsorption sites for RhB and contribute to their higher photocatalytic efficiency.The photoelectric properties of the TiO2 electrode were well demonstrated by LSV (Fig. 2). In the absence of light irradiation, the photocurrent value rapidly increased to zero as the external bias was increased from −0.5 V to −0.3 V and kept constant when the applied bias was larger than −0.3 V. However, upon introduction of UV irradiation, the photocurrent increased sharply with an increase in the external bias compared to that without irradiation. A maximal current of 3.1 mA was achieved at an applied bias of 0.4 V, suggesting that a positive bias facilitated the separation of the photogenerated charges. This difference in photocurrent response of the TiO2 electrode in the presence and absence of UV irradiation suggests that there was considerable photocatalytic activity on the electrode surface.
 | ||
| Fig. 2 Linear sweep voltammetric curves for the TiO2/Ti electrode in 0.05 M Na2SO4 solution at a scan rate of 10 mV s−1 in the three-electrode system with or without illumination. | ||
Degradation of RhB
The degradation of RhB was employed to investigate and compare the photocatalytic performance of the PFC systems with different concentrations of salt solution in the middle chamber, as shown in Fig. 3. The linear relationship of ln(Ct/C0) vs. t showed first-order kinetics during RhB degradation with an normalized R2 ranging from 0.9750 to 0.9995 (Table 1). The photocatalytic degradation of RhB under open-circuit conditions was 91.45% with a k value of 0.0201 min−1. The use of a resistance of 1000 Ω significantly enhanced the degradation ratio. The RhB degradation using PFCs with distilled water in the middle chamber realised 90.23% with a k value of 0.0183 min−1. An increase in the NaCl concentration from 5 mg L−1 to 45 mg L−1, consequently increased the decoloration rate of RhB from 0.0333 min−1 to 0.0505 min−1. Further insight on the reinforcement effect in the decoloration of RhB using a dual-chamber reactor was investigated. A cation exchange membrane (CEM) and anion exchange membrane (AEM) were used to separate the two chambers, respectively. The decoloration rate of RhB was registered at 0.0313 min−1 and 0.0324 min−1 for AEM and CEM, respectively, comparable to that of 5 g L−1 NaCl in the middle chamber. The difference in the decoloration rate of the AEM and CEM might have been induced by the heterogeneity in the transfer efficiency of different ionic species. These results indicate that the PFC with a middle salt chamber has better degradation efficiency than a simple dual-chamber PFC. The presence of a higher NaCl concentration in the middle chamber may have contributed to the better performance during RhB removal.| PFC systems | k (min−1) | R2 | Decoloration ratio (%) |
|---|---|---|---|
| TiO2 | 0.0201 | 0.9948 | 91.45 |
| 45 g L−1 NaCl | 0.0505 | 0.9995 | 99.76 |
| 35 g L−1 NaCl | 0.0460 | 0.9783 | 99.67 |
| 25 g L−1 NaCl | 0.0427 | 0.9750 | 99.52 |
| 15 g L−1 NaCl | 0.0374 | 0.9882 | 99.06 |
| 5 g L−1 NaCl | 0.0333 | 0.9930 | 98.49 |
| 0 g L−1 NaCl | 0.0183 | 0.9805 | 90.23 |
| AEM-PFC | 0.0313 | 0.9989 | 97.76 |
| CEM-PFC | 0.0324 | 0.9995 | 97.97 |
Power generation of the PFCs
The voltage and power performance of the PFCs showed similar tendencies as RhB decoloration (Fig. 4 and 5). The PFC systems with faster RhB degradation rates presented higher energy production. The voltage output of the PFC with distilled water in the middle chamber was ∼50 mV with a maximum power density of 3.33 mW m−2, indicating poor electric activity in the absence of a salt solution. An increase in the NaCl concentration from 5 to 45 g L−1, consequently, increased the maximum power density rapidly from 170.00 to 319.70 mW m−2 (Table 2). The voltage change with different NaCl concentrations was highly correlated to the power output, with mean values ranging from ∼270 to ∼500 mV. The dual-chamber PFC with AEM and CEM yielded a comparable power output than that of the PFC with 5 g L−1 NaCl in the middle chamber, suggesting the important role of the NaCl solution in the power output of the PFCs. These voltage outputs changed in a similar tendency with time for all the PFCs and fluctuated in small scale, which resulted from the beating of irradiation light. For example, a gradual increase in the first 30 min, which resulted from the formation of a photoelectrochemical equilibrium in the anode, was then followed by a stable stage. The increase in power output with increased NaCl concentration resulted in a large improvement in the anode performance (Fig. 4b), a likely indication of reaction kinetics at the anode-electrolyte interface. On the other hand, the cathode potentials were nearly stable and barely affected by the change in NaCl concentration (Fig. 4c). | ||
| Fig. 4 Voltage generation (a), anode (b) and cathode potentials (c) of the PFC systems with different NaCl concentrations. | ||
 | ||
| Fig. 5 (a) Power generation and (b) polarization curves for the PFC systems with different NaCl concentrations. | ||
| PFC systems | Pmax (mW m−2) | Voc (V) | Isc (mA m−2) | FF (%) |
|---|---|---|---|---|
| 45 g L−1 NaCl | 319.70 | 0.454 | 2519.01 | 27.93 |
| 35 g L−1 NaCl | 305.90 | 0.442 | 2559.11 | 27.07 |
| 25 g L−1 NaCl | 269.48 | 0.412 | 2372.09 | 27.59 |
| 15 g L−1 NaCl | 239.66 | 0.389 | 2227.34 | 27.63 |
| 5 g L−1 NaCl | 170.00 | 0.358 | 1729.04 | 27.43 |
| 0 g L−1 NaCl | 3.33 | 0.192 | 75.47 | 22.98 |
| AEM-PFC | 186.88 | 0.328 | 2248.71 | 25.33 |
| CEM-PFC | 208.66 | 0.341 | 2185.93 | 27.99 |
FF relates to the energy conversion efficiency in PFC systems and shows a deviation in the real maximum power density produced by the PFC system (Table 2) from the theoretical value. The FF of all the PFCs were similar (around 26.74%) except the PFC with distilled water in the middle chamber (22.98%).
Long-time performance of the PFCs
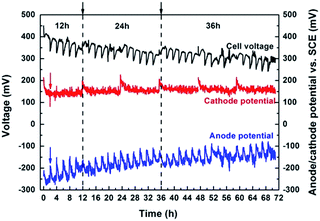
The PFC was operated in batch mode for 72 h to examine its sustainability by recording the voltage and electrode potential (NaCl concentration of 35 g L−1) (Fig. 6). The anolyte and catholyte were replaced every 2 h to refresh the substrate and eliminate pH limitations.while the salt solution was replaced after 12 h and 36 h. The maximum voltage produced by the PFC remained at ∼400 mV (Fig. 6). With the refreshment of the anolyte, the anode potential displayed a regular change, which decreased quickly in the first hour and then became stable. This changed trend in the anode potential was different from that of a previous study in which the anode potential showed a rising trend with the lack of substrate.24 This was probably due to the oxidation of Cl− moving through the AEM into the anode chamber by the highly oxidizing valence band holes in aqueous solutions, thus performing as another fuel. However, there was little change in cathode potential when the solutions were replaced in any of the three chambers, demonstrating the current generation was not affected by the cathode.
The HRT of the middle chamber was essential for the voltage output and desalting efficiency. The slight drop in voltage output was likely due to the decrease in NaCl concentration, which increased the internal resistance and decreased the junction potential. A NaCl removal efficiency of 19.70% was obtained when the HRT of the middle chamber was 12 h. With an increase in HRT (24 h), the NaCl removal increased to 41.69%. A further increase in HRT to 36 h realised a salt removal rate of 58.25% (Fig. S1, ESI†). Unlike a traditional desalting reactor, the low current density in this system can partly address the perplexity comprising water splitting to compensate for the limiting current and back-diffusion of ions from electrode chambers to the middle chamber.25
Discussion
The combined system comprised a photocatalytic process in the anode chamber, desalination process in the middle chamber and electron-consumed process in cathode chamber (Fig. 7). The half reactions in the photoanode chamber are illustrated as follows: As an electron transfer carrier, TiO2 can effectively separate the photogenerated electrons and holes. Upon UV irradiation (Fig. S2, ESI†), the photoelectrons were excited from their resting valence band to the conduction band, generating holes (h+) in the valence band. With photoelectrons transferring to the cathode through an external circuit, the flux of holes arriving at the surface of the photoanode reacted with H2O/OH− to form hydroxyl radicals (˙OH), which have an oxidizing power on RhB.An obvious photoanode potential fluctuation was observed during the operation, this fluctuation was due to macroscopic beating of irradiation light. The electron-participating cathode oxygen reduction reaction was affected by the photoanode because of the direct connection between the photoanode and cathode. This rolling cathode does not smooth the potential fluctuation, due to the relatively limited cathode performance in terms of catalytic activity and conductivity compared to noble metal catalysts such as Pt.
The photocurrent did not decrease with the consumption of RhB, which was different from that observed in previous studies. This stable photocurrent generation over time was likely due to the excellent catalytic properties of TiO2 using Ti mesh as the matrix. Titanol (Ti–OH) on the TiO2 surface is amphoteric with an isoelectric point of 6.25 (eqn (2) and (3)).26 The anolyte pH decreased from 5.15 ± 0.09 to 3.49 ± 0.04 during a cycle in this study (Fig. S3, ESI†), which was lower than the isoelectric point, thus promoting more TiOH2+ deposition on the surface of the photoanode. Moreover, a steady stream of chloride ions moving into the photoanode chamber through the AEM gathered at the electrode surface attributed to the strong absorption properties to anions of TiOH2+. The chloride ions can serve as a substitute fuel for RhB because they can be oxidized to chlorate or perchlorate by holes (h+), off-setting the negative effect of substrate deletion.27 This process also contributed to the stable current of the system.
| TiOH + H+ → TiOH2+ (pH < 6.25) | (2) |
| TiOH → TiO− + H+ (pH > 6.25) | (3) |
The electric potential gradient created by the electrode reactions was mostly responsible for the directional migration of negative and positive ions in the middle chamber, providing favourable electric power for desalination. The inclusion of a desalination module can improve the performance of RhB degradation in the photoanode and electricity production, significantly resulting in synergistically-enhanced power production compared to each individual process. When compared to the salt solution in the middle chamber, the anolyte had a much lower ion concentration. Due to this significant concentration gradient across the IEM, salt ions in the middle chamber were driven to the adjacent electrode chambers. This driving force can be quantified as the junction potential (Δφjct, eqn (4)).28
 | (4) |
Conclusions
In this study, by integrating a PFC with a desalination module, a three-chamber system was constructed for the improved degradation of RhB by a photoanode. The results demonstrate that the combined system is advantageous in several aspects: (1) the formation of a junction potential resulting from the inclusion of the middle chamber can improve the performance of the electrode reactions compared to the simple PFC process; (2) the sustainable photocatalytic reaction resulting from the comparable catalytic activity of TiO2 using Ti mesh as the matrix and favorable oxygen reduction reaction using a cost-effective rolling cathode can establish a stable electric potential difference between the electrodes and provide enough of a driving force for desalination. This synergistically-enhanced trend of power generation and desalination demonstrated the feasible integration of a PFC with a seawater desalination process to strengthen the degradation of RhB, endowing an additional function of the PFC.Acknowledgements
The authors would like to acknowledge the support from “the Fundamental Research Funds for Water Pollution Control and Management Science and Technology major projects” (2009ZX07207-008-5-2) and State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (No. 2013DX09). The authors thank the support from the “State Key Lab of Urban Water Resource and Environment (HIT)” (ES201312).Notes and references
- M. Canterino, I. Di Somma, R. Marotta, R. Andreozzi and V. Caprio, Water Res., 2009, 43, 2710–2716 CrossRef CAS PubMed.
- Y. Liu, J. Li, B. Zhou, X. Li, H. Chen, Q. Chen, Z. Wang, L. Li, J. Wang and W. Cai, Water Res., 2011, 45, 3991–3998 CrossRef CAS PubMed.
- M. Kaneko, H. Ueno, R. Saito and J. Nemoto, Catal. Lett., 2009, 131, 184–188 CrossRef CAS.
- M. Kaneko, H. Ueno, R. Saito, S. Yamaguchi and Y. Fujii, Appl. Catal., B, 2009, 91, 254–261 CrossRef CAS.
- H. Ueno, J. Nemoto, K. Ohnuki, M. Horikawa and M. Hoshino, J. Appl. Electrochem., 2009, 39, 1897–1905 CrossRef CAS.
- Y. Du, Y. Qu, X. Zhou and Y. Feng, RSC Adv., 2015, 5, 25325–25328 RSC.
- J. Miao and B. Liu, RSC Adv., 2013, 3, 1222–1226 RSC.
- Y. Liu, J. Li, B. Zhou, H. Chen, Z. Wang and W. Cai, Chem. Commun., 2011, 47, 10314–10316 RSC.
- D. H. Kim, G. S. Han, W. M. Seong, J.-W. Lee and B. J. Kim, ChemSusChem, 2015, 8, 2392–2398 CrossRef CAS PubMed.
- X. Li, W. Zheng, H. Pan, Y. Yu and L. Chen, J. Catal., 2013, 300, 9–19 CrossRef CAS.
- Z. Jiao, Y. Zhang, T. Chen, Q. Dong and G. Lu, Chem. – Eur. J., 2014, 20, 2654–2662 CrossRef CAS PubMed.
- Y. L. Pang, S. Lim, H. C. Ong and W. T. Chong, Appl. Catal., A, 2014, 481, 127–142 CrossRef CAS.
- J. D. Schuttlefield, J. B. Sambur, M. Gelwicks, C. M. Eggleston and B. A. Parkinson, J. Am. Chem. Soc., 2011, 133, 17521–17523 CrossRef CAS PubMed.
- S.-J. Yuan, G.-P. Sheng, W.-W. Li, Z.-Q. Lin and R. J. Zeng, Environ. Sci. Technol., 2010, 44, 5575–5580 CrossRef CAS PubMed.
- K. Li, H. Zhang, T. Tang, Y. Xu and D. Ying, Water Res., 2014, 62, 1–10 CrossRef CAS PubMed.
- J. Veerman, M. Saakes, S. J. Metz and G. J. Harmsen, Environ. Sci. Technol., 2010, 44, 9207–9212 CrossRef CAS PubMed.
- M. Tedesco, A. Cipollina, A. Tamburini, G. Micale and J. Helsen, Desalin. Water Treat., 2015, 53, 3161–3169 CrossRef CAS.
- J. W. Post, H. V. M. Hamelers and C. J. N. Buisman, Environ. Sci. Technol., 2008, 42, 5785–5790 CrossRef CAS PubMed.
- Y. Kim and B. E. Logan, Desalination, 2013, 308, 122–130 CrossRef CAS.
- A. ElMekawy, H. M. Hegab and D. Pant, Energy Environ. Sci., 2014, 7, 3921–3933 CAS.
- A. Subramani and J. G. Jacangelo, Water Res., 2015, 75, 164–187 CrossRef CAS PubMed.
- H. M. Saeed, G. A. Husseini, S. Yousef, J. Saif and S. Al-Asheh, Desalination, 2015, 359, 1–13 CrossRef CAS.
- B. Zhang and Z. He, RSC Adv., 2012, 2, 3265–3269 RSC.
- Y. Du, Y. Feng, Y. Qu, J. Liu, N. Ren and H. Liu, Environ. Sci. Technol., 2014, 48, 7634–7641 CrossRef CAS PubMed.
- Y. Kim and B. E. Logan, Environ. Sci. Technol., 2011, 45, 5840–5845 CrossRef CAS PubMed.
- C. Kormann, D. W. Bahnemann and M. R. Hoffmann, Environ. Sci. Technol., 1991, 25, 494–500 CrossRef CAS.
- J. D. Schuttlefield, J. B. Sambur, M. Gelwicks, C. M. Eggleston and B. A. Parkinson, J. Am. Chem. Soc., 2011, 133, 17521–17523 CrossRef CAS PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, New York, 2nd edn, 2001 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra20093h |
| ‡ Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |