Hyperlipidemia affects the absorption, distribution and excretion of seven catechins in rats following oral administration of tea polyphenols†
Liwei Xu‡
,
Yuhong Liang‡,
Xin Chen,
Bo Chen,
Yuhui Han and
Liang Zhang*
State Key Laboratory of Tea Plant Biology and Utilization, Anhui Agricultural University, Hefei 230036, China. E-mail: zhli2091@sina.com; Fax: +86-551-65786765; Tel: +86-551-65786765
First published on 5th November 2015
Abstract
To explore the effects of hyperlipidemia on the pharmacokinetics of tea polyphenols, a comparative pharmacokinetics study of seven catechins between normal and obese rats was conducted. Rats were orally administered tea polyphenols (350 mg kg−1) and plasma, stomach, small intestine and colon samples of rats were obtained at 5, 30, 120, 360 and 720 min post administration. The plasma levels of (−)-gallocatechin from obese rats were significantly lower than those of normal rats. During the digestion of tea polyphenols in vivo, compared to normal rats, the levels of seven catechins within the gastric content and tissue of obese rats were significantly increased, in addition to the small intestinal tissue levels of (−)-epigallocatechin gallate and (−)-gallocatechin gallate. On the contrary, the colonic tissue levels of (−)-epigallocatechin, (−)-gallocatechin gallate, (−)-gallocatechin and (+)-catechin in obese rats were significantly decreased compared to the levels in normal rats. Furthermore, the fecal excretion of the seven catechins in obese rats was highly increased. To sum up, hyperlipidemia changed the pharmacokinetics of catechins by increasing their distribution in the stomach and small intestine, but decreasing their distribution in the colon.
Introduction
There have been a large number of studies on the health benefits of tea and tea polyphenols in terms of antioxidant effects, cancer prevention, lipid lowering and neuro-protective effects.1–4 So far, the functions of tea have been confirmed by cell experiments and animal studies. As one of the most reported biological activities, tea polyphenols could prevent hyperlipidemia by decreasing the absorption and biosynthesis of lipids, yet increasing their catabolism.5 Furthermore, green tea has shown promising activities in the prevention of gastro-intestinal cancers, such as gastric, intestinal and colorectal cancers.6,7After oral administration of tea polyphenols, large intestine and other digestive tracts are the tissues in direct contact with ingested tea polyphenols. On the one hand, it was indicated that a large part of ingested (−)-epigallocatechin gallate (EGCG) and (−)-epicatechin (EC) was distributed in the large intestine and esophagus.8,9 On the other hand, it was reported that green tea could reduce cox-2 expression and suppress the formation of colonic preneoplastic lesions and aberrant crypt foci, which is the intermediate step in the development of cancer.10 Moreover, ingestion of 0.02–0.32% EGCG as drinking fluid showed dose-dependent inhibition of tumorigenesis in the small intestine.11 The epidemiological studies also provided evidence that drinking green tea may lower the risk of colorectal cancers.7 It is suggested that pharmacokinetic–pharmacodynamic (PK–PD) modelling for the prevention of gastro-intestinal cancers using tea polyphenols is worthy of investigation.
It has been verified that galloylated polyphenols such as EGCG and (−)-gallocatechin gallate (GCG) were detected in the circulation in a free form, whereas non-galloylated polyphenols circulated mostly in a conjugated form.12 The oral bioavailability of (−)-epigallocatechin (EGC) and EC was 13.7% and 31.2%, which was significantly higher than that of EGCG.13 When rats were orally given EGCG (500 mg kg−1), the highest level of EGCG was detected in the small intestinal mucosa (565 nmol g−1) and colon mucosa (68.6 nmol g−1) of rats. Furthermore, it has been reported that pathological states affected the pharmacokinetics of active compounds,14,15 but widely used tea was less of a concern in this study.
Although there are many reports about the biological activities of tea, green tea didn’t show consistent results for their effects in population-based cohort investigations.16,17 It was concluded that the inconsistency was ascribed to the lower blood and tissue levels of tea polyphenols after green tea consumption. The absorption, distribution, metabolism and excretion (ADME) of tea polyphenols in vivo is critical to explaining their efficacy. In particular, the distribution of tea polyphenols in targeted tissues should be noted, such as the small intestine and colon. So far, the pharmacokinetics of tea polyphenols was mainly studied using healthy animals or humans.13,18 There were few studies about the effects of the physiological and pathological states on the ADME of tea polyphenols.
In this study, we determined the plasma and tissue levels of seven tea catechins in normal and obese rats following single-dose administration of tea polyphenols. The information obtained from the present study will provide insights into the absorption, distribution and excretion of catechins in obese subjects.
Experimental
Chemicals and reagents
EGCG, (+)-catechin (C), EC, ECG, EGC, GCG, and (−)-gallocatechin (GC) (purity > 98%) were purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Methanol and acetonitrile (HPLC-grade) were purchased from Merck (Darmstadt, Germany). HPLC-grade formic acid was obtained from ROE Scientific Inc. (Newark, USA). The tea polyphenols were extracted and refined from unfermented tea. Tea polyphenols contain seven catechins (54.91% of EGCG, 20.04% of EGC, 11.17% of ECG, 3.37% of EC, 2.13% of GCG, 1.98% GC and 0.51% of C) using the UHPLC-MS determination.Animal treatment
Thirty male Sprague-Dawley rats (220–250 g) were purchased from the Nanjing Qinglong Experiment Animal Co., Ltd. (Jiangsu province, China). These animals were maintained on a 12 h light–dark cycle (light on from 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 to 20
00 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 h) at ambient temperature (22–24 °C) and 60% relative humidity. All animal experiments were strictly in accordance with the related guidelines and ethics regulations and approved by the Institutional Animal Care and Use Committee of Nanjing University of Chinese Medicine. All animals had free access to food and water. The food was removed 12 h before collection of blood samples.
00 h) at ambient temperature (22–24 °C) and 60% relative humidity. All animal experiments were strictly in accordance with the related guidelines and ethics regulations and approved by the Institutional Animal Care and Use Committee of Nanjing University of Chinese Medicine. All animals had free access to food and water. The food was removed 12 h before collection of blood samples.
To obtain the hyperlipidemic rats, fifteen rats were given high-fat diets for four weeks according to our published method.19 The serum lipid analysis showed that the serum levels of lipids in the obese rats (low-density lipoprotein cholesterol, total cholesterol and triglycerides) were significantly increased compared to the levels in normal rats (ESI Table 1†). These rats were recruited into the pharmacokinetics study as a model of obesity. In the normal and obese group, rats were orally administered tea polyphenols at a dose of 350 mg kg−1.
After oral administration of tea polyphenols, three rats for each group were sacrificed by inhaling carbon dioxide at 5, 30, 120, 360 and 720 min post administration, and then 2 mL of blood was collected into heparinized tubes. The blood samples were immediately heparinized and centrifuged at 6000 rpm for 5 min. The supernatant was divided into 0.2 mL aliquots and stored in 1.5 mL polypropylene tubes at −20 °C prior to analysis. The digestive tracts were removed and separated into the stomach, small intestine and colon according to an anatomical guide. These samples were stored at −80 °C until analysis.
Instrument and analytical conditions
The quantitation of the catechins in the plasma samples was carried out using a TSQ Vantage UHPLC-MS/MS system (Thermo Fisher Scientific, USA) including the UltiMate 3000 UHPLC, auto-sampler, column compartment and TSQ mass spectrometer. The analytical method and UHPLC-MS/MS conditions are referenced to an established method.20Determination of plasma concentration of catechins
To determine the total catechins (free and conjugated from) in plasma, a 100 μL rat plasma sample was mixed with 10 μL of a mixture of β-glucuronidase and arylsulfatase (Roche Diagnostics GmbH, Mannheim, Germany), and then incubated at 37 °C for 45 min. The reacted mixture was added to 20 μL each of 600 ng mL−1 I.S. and 20% vitamin C solution (preventing oxidation of catechins) and then vortex-mixed for 1 min according to a published method.21 The sample was extracted with 1 mL of ethyl acetate by 3 min of vortex-mixing and then centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. The upper organic phase was transferred into another tube and evaporated until dry using an Integrated SpeedVac concentrator system (Thermo Scientific, USA). The residue was dissolved in 100 μL of 20% acetonitrile aqueous solution and vortex-mixed for 1 min. After centrifuging at 17
000 rpm for 10 min at 4 °C. The upper organic phase was transferred into another tube and evaporated until dry using an Integrated SpeedVac concentrator system (Thermo Scientific, USA). The residue was dissolved in 100 μL of 20% acetonitrile aqueous solution and vortex-mixed for 1 min. After centrifuging at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, 5 μL of the supernatant was injected into an ultra-performance liquid chromatography combined with time-of-flight mass spectrometry (UHPLC-MS/MS) system for analysis.
000 rpm for 10 min at 4 °C, 5 μL of the supernatant was injected into an ultra-performance liquid chromatography combined with time-of-flight mass spectrometry (UHPLC-MS/MS) system for analysis.
Determination of catechins in the circulation solution and tissue of digestive tracts (stomach, small intestine and colon)
The weights of the main digestive organs (stomach, small intestine and colon) were recorded and listed in ESI Table 2.† Then, they were cut longitudinally and washed extensively. The tissues were immediately frozen in liquid nitrogen. Tissues were stored at −80 °C until use. 0.2 g of each organ tissue was accurately weighed and homogenized with 1 mL of methanol. The homogenate was centrifuged at 6000 rpm for 10 min, and then 100 μL of the supernatant was removed. The supernatant was added into 20 μL of I.S., 20 μL of 20% vitamin C solution and then vortex-mixed for 1 min. The sample was extracted with 1 mL of ethyl acetate by 3 min of vortex-mixing and then centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. The upper organic phase was transferred into another tube and evaporated until dry using an Integrated SpeedVac concentrator system (Thermo Scientific, USA). The residue was dissolved in 100 μL of 20% acetonitrile aqueous solution and vortex-mixed for 1 min. After centrifuging at 17
000 rpm for 10 min at 4 °C. The upper organic phase was transferred into another tube and evaporated until dry using an Integrated SpeedVac concentrator system (Thermo Scientific, USA). The residue was dissolved in 100 μL of 20% acetonitrile aqueous solution and vortex-mixed for 1 min. After centrifuging at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, 60 μL of the supernatant was injected into the UHPLC-MS/MS system for analysis.
000 rpm for 10 min at 4 °C, 60 μL of the supernatant was injected into the UHPLC-MS/MS system for analysis.
The circulation solutions were collected by washing the content of the corresponding digestive tract (stomach, small intestine and colon) with 4 mL of methanol, and subsequently extracted by ultrasound for 30 min to obtain the homogenate. The homogenate was prepared by the same method as above. To establish the calibration curves of the individual catechins in the tissue of the stomach, small intestine and colon, the mixed stock solution was added into untreated tissue of the stomach, small intestine and colon, and prepared by the method mentioned above. The calibration curves for the seven catechins were constructed by plotting peak area ratios of the analytes to the concentration of the tissue homogenate (ESI Tables 3–5†).
Data analysis
Results were analyzed using the unpaired Student’s t-test. Differences with p values less than 0.05 were considered to be significant.Results and discussion
Time-dependent changes in plasma levels of catechins in rats
The plasma levels of seven catechins of rats orally administered with tea polyphenols (350 mg kg−1) were plotted against time as shown in Fig. 1. The plasma levels of EGCG, EGC, EC, GCG, C and ECG didn’t show a significant difference between obese and normal rats, but the maximum plasma concentration (Cmax) and the area under the curve (AUC(0–t)) of GC in obese rats were significantly decreased compared to those in normal rats (Table 1).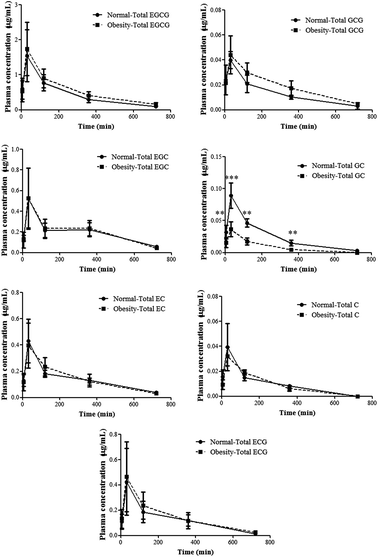 | ||
| Fig. 1 Time-dependent changes of the plasma levels of tea catechins in normal and obese rats given a tea polyphenol solution (350 mg kg−1). **p < 0.01 and ***p < 0.001 compared to normal rats. | ||
| Compounds | EGCG | EGC | EC | GCG | GC | C | ECG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax | AUC(0–t) | Cmax | AUC(0–t) | Cmax | AUC(0–t) | Cmax | AUC(0–t) | Cmax | AUC(0–t) | Cmax | AUC(0–t) | Cmax | AUC(0–t) | ||
| a The units of Cmax and AUC(0–t) for plasma catechins were μg mL−1 and μg min mL−1.b The units of Cmax and AUC(0–t) for stomach, small intestine and colon tissue catechins were μg g−1 and μg min g−1.c The units of Cmax and AUC(0–t) for stomach, small intestine and colon circulation solution catechins were μg mL−1 and μg min mL−1; CS, circulation solution.d *p < 0.05, **p < 0.01, and ***p < 0.001, compared to normal rats. | |||||||||||||||
| Plasmaa | Normal | 1.547 ± 0744 | 333.666 ± 115.826 | 0.524 ± −0.296 | 144.101 ± 50.736 | 0.433 ± 0.166 | 102.999 ± 30.241 | 0.039 ± 0.007 | 9.762 ± 2.668 | 0.089 ± 0.019 | 18.314 ± 3.806 | 0.039 ± 0.019 | 7.460 ± 1.810 | 0.425 ± 0.262 | 95.477 ± 43.704 |
| Obese | 1.741 ± 0.765 | 408.312 ± 135.249 | 0.528 ± 0.290 | 149.466 ± 57.510 | 0.397 ± 0.171 | 105.583 ± 32.192 | 0.044 ± 0.015 | 13.889 ± 4.202 | 0.036 ± 0.012** | 6.619 ± 1.891** | 0.0326 ± 0.008 | 7.011 ± 1.488 | 0.465 ± 0.277 | 107.743 ± 856.036 | |
| Stomachb | Normal | 88.824 ± 3.209 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 885.311 ± 3777.437 885.311 ± 3777.437 |
39.809 ± 2.509 | 6172.108 ± 1324.312 | 10.810 ± 1.218 | 1727.602 ± 344.607 | 6.904 ± 1.522 | 1131.126 ± 224.237 | 8.218 ± 0.909 | 1401.121 ± 214.241 | 1.709 ± 0.340 | 288.403 ± 70.091 | 27.126 ± 2.240 | 6534.711 ± 1602.226 |
| Obese | 86.115 ± 13.328 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 712.524 ± 5194.391* 712.524 ± 5194.391* |
44.826 ± 2.602 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147.741 ± 3021.911*** 147.741 ± 3021.911*** |
13.000 ± 0.800 | 3961.662 ± 1073.517** | 8.221 ± 1.645 | 2097.826 ± 613.755* | 8.609 ± 1.047 | 2836.483 ± 791.425** | 1.907 ± 0.102 | 576.426 ± 144.917* | 30.902 ± 7.114 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210.610 ± 2823.775** 210.610 ± 2823.775** |
|
| Small intestine | Normal | 69.610 ± 20.502 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872.427 ± 4060.509 872.427 ± 4060.509 |
47.811 ± 6.327 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 095.211 ± 3751.439 095.211 ± 3751.439 |
13.226 ± 3.877 | 3921.881 ± 1172.639 | 3.501 ± 1.410 | 743.508 ± 227.412 | 8.901 ± 1.533 | 3277.375 ± 564.723 | 2.487 ± 0.244 | 614.411 ± 156.454 | 29.122 ± 11.776 | 7689.009 ± 2196.551 |
| Obese | 91.322 ± 17.753* | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 166.102 ± 14 166.102 ± 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126.507* 126.507* |
43.973 ± 9.882 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 845.115 ± 4510.542 845.115 ± 4510.542 |
11.004 ± 4.086 | 3205.743 ± 1174.933 | 4.623 ± 1.187* | 996.654 ± 369.032* | 7.323 ± 2.118 | 3006.633 ± 862.935 | 1.522 ± 0.508 | 447.502 ± 178.421 | 29.337 ± 7.334 | 6411.441 ± 2237.576 | |
| Colon | Normal | 81.451 ± 28.033 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515.33 ± 11 515.33 ± 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760.239 760.239 |
33.101 ± 9.723 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303.124 ± 6449.325 303.124 ± 6449.325 |
12.435 ± 5.261 | 5267.821 ± 3005.409 | 7.224 ± 3.355 | 2387.121 ± 1236.337 | 13.446 ± 6.721 | 4646.214 ± 2131.226 | 2.977 ± 1.792 | 1152.922 ± 551.053 | 36.621 ± 13.911 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816.017 ± 4395.119 816.017 ± 4395.119 |
| Obese | 87.881 ± 21.432 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890.215 ± 8394.367 890.215 ± 8394.367 |
22.715 ± 11.323* | 9713.227 ± 3427.325** | 10.792 ± 5.068 | 5209.117 ± 2416.432 | 2.532 ± 0.921** | 1256.261 ± 531.014* | 4.421 ± 2.210** | 2010.002 ± 866.341* | 1.281 ± 0.721** | 622.9.272 ± 266.614* | 27.524 ± 15.003* | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557.257 ± 4638.227 557.257 ± 4638.227 |
|
| Stomachc CS | Normal | 22.826 ± 2.871 | 6045.003 ± 1096.194 | 6.390 ± −0.518 | 1555.382 ± 164.745 | 3.950 ± 0.382 | 986.448 ± 229.193 | 2.096 ± 0.609 | 634.408 ± 187.841 | 1.0066 ± 0.0619 | 333.235 ± 27.373 | 0.3758 ± 0.05709 | 135.301 ± 43.901 | 6.512 ± 2.099 | 1757.326 ± 505.097 |
| Obese | 32.320 ± 1.307 | 8101.872 ± 2136.411 | 7.7193 ± 2.1263 | 2428.096 ± 800.683* | 3.663 ± 0.081 | 1163.522 ± 108.226 | 2.044 ± 0.395 | 831.615 ± 164.323* | 1.4114 ± 0.1109* | 547.611 ± 97.044** | 0.4118 ± 0.1226 | 174.380 ± 44.109 | 9.530 ± 4.258* | 3050.639 ± 864.323* | |
| Small intestine CS | Normal | 104.724 ± 37.336 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 656.759 ± 13 656.759 ± 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 968.505 968.505 |
42.332 ± 18.964 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 913.027 ± 7267.951 913.027 ± 7267.951 |
16.462 ± 1.233 | 7473.449 ± 743.723 | 13.638 ± 2.326 | 6020.351 ± 1005.880 | 7.296 ± 3.206 | 3520.940 ± 1370.592 | 1.713 ± 0.426 | 849.843 ± 213![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 706 |
53.030 ± 27.441 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 363.775 ± 9188.392 363.775 ± 9188.392 |
| Obese | 89.683 ± 9.416 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842.212 ± 6620.901 842.212 ± 6620.901 |
41.674 ± 1.695 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 845.366 ± 2838.202 845.366 ± 2838.202 |
20.131 ± 2.071* | 9536.235 ± 1729.860* | 11.317 ± 2.598 | 5888.427 ± 1142.475 | 7.939 ± 1.118 | 3403.577 ± 502.691 | 2.219 ± 0.377 | 974.829 ± 149.735 | 55.784 ± 6.800 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.148 ± 2767.568 200.148 ± 2767.568 |
|
| Colon CS | Normal | 40.033 ± 16.963 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 397.096 ± 6457.897 397.096 ± 6457.897 |
9.590 ± 2.394 | 4855.868 ± 1378.116 | 5.669 ± 2.968 | 1953.010 ± 727.429 | 4.802 ± 1.792 | 2259.991 ± 452.335 | 3.813 ± 1.541 | 1827.810 ± 439.521 | 0.575 ± 0.239 | 238.717 ± 93.689 | 16.241 ± 3.020 | 6109.774 ± 1305.248 |
| Obese | 47.349 ± 6.406 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 783.274 ± 3834.217 783.274 ± 3834.217 |
11.116 ± 1.287 | 6106.790 ± 824.340 | 6.023 ± 2.299 | 2684.723 ± 1021.665 | 4.041 ± 0.607 | 1826.910 ± 445.002 | 3.051 ± 0.759 | 1622.089 ± 486.267 | 0.724 ± 0.228 | 351.927 ± 147.242 | 17.682 ± 6.918 | 7845.992 ± 2298.411 | |
Tissue distribution of catechins after oral administration of tea polyphenols
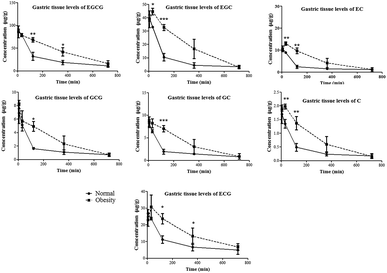
Rats were orally given tea polyphenols at a dose of 350 mg kg−1. In normal and obese rats, the catechin levels of gastric content reached the highest value at 120 min and subsequently decreased. Every catechin showed a similar time-concentration profile, which suggested that the catechin gallate didn’t decompose under the pH condition of the gastric juice (ESI Fig. 1†).The gastric tissue levels of tea polyphenols against time are profiled in Fig. 2. The maximum concentration (Cmax) of gastric tissue levels of the seven catechins didn’t exhibit significant differences between normal and obese rats, but the AUC(0–t) values of the seven catechins in the gastric tissue of obese rats were significantly higher than those in normal rats. The time-concentration profile of catechins of gastric content is profiled in ESI Fig. 1.† As shown in Table 1, the AUC(0–t) values for the catechin levels of gastric content in obese rats were significantly higher than those in normal rats, which may result in the increased uptake of catechins in gastric tissue.
The small intestine is the main organ responsible for the metabolism and absorption of catechins in vivo. As shown in ESI Fig. 2,† an obvious difference was that the highest concentration of catechins in the small intestinal content of obese rats were detected at 120 min post administration, which was earlier than that in normal rats. The Cmax and AUC(0–t) of EC in the small intestinal content of obese rats were significantly higher than those in normal rats. Other catechins didn’t show a statistical difference between the two experimental groups (Table 1).
Except for EGCG and GCG, the small intestinal tissue levels of the other catechins didn’t show a significant difference between the two groups (Fig. 3). Although the AUC(0–t) of EC in the small intestinal content of obese rats was higher than that in normal rats, its distribution in the small intestinal tissue was not increased correspondingly. On the other hand, in the obese rats, the Cmax and AUC(0–t) for the small intestinal tissue levels of EGCG and GCG were significantly increased compared to those in normal rats (Table 1). These compounds are the main galloylated catechins of tea polyphenols, which were reported to have worse oral bioavailability than non-galloylated catechins. In the present study, these results indicated that the distribution of the galloylated catechins was increased in the small intestine of obese rats.
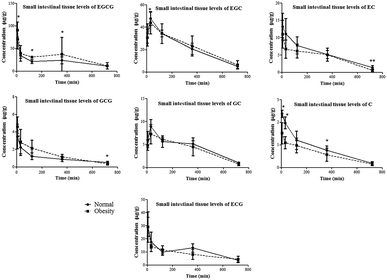 | ||
| Fig. 3 Time-dependent changes in the small intestinal levels of tea catechins in normal and obese rats given tea polyphenol solution (350 mg kg−1). *p< 0.05 and **p < 0.01 compared to normal rats. | ||
When the tea polyphenols entered into the colonic circulation, the decrease of catechin levels in the colonic content was clearly observed (ESI Fig. 3†). For example, the Cmax of EGC in the small intestinal content of normal rats was 42.332 ± 18.964 μg mL−1, but the Cmax in the colonic content was only 9.590 ± 2.394 μg mL−1. Over half of EGC was metabolized and absorbed in the small intestine. The colonic tissue levels of catechins were gradually increased along with the accumulation of catechins in the colonic content.
The colon is the main organ responsible for the microbial metabolism of catechins. The Cmax of EGC, GCG, GC, C and ECG in the colonic tissue of normal rats was significantly higher than that in obese rats (Fig. 4 and Table 1). For example, in the normal rats, the Cmax of EGC was determined to be 33.101 ± 9.723 μg g−1 in the colonic tissue, which was significantly higher than 22.715 ± 11.323 μg g−1 of obese rats. Correspondingly, the AUC(0–t) of EGC was 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303.124 ± 6449.325 μg min g−1 in the colonic tissue of normal rats, which was also significantly higher than that in obese rats (9713.227 ± 3427.325 μg min g−1). Similar results for AUC(0–t) values of GCG, GC and C were observed in the colonic tissue of normal rats. On the other hand, the catechin levels in the colonic content didn’t show a significant difference between the two groups. These results suggested that the distribution of catechins in the colonic tissue did not rely on the systemic circulation. The permeability of the colonic epithelial cells may be affected after a long-term high-fat diet, so the uptake of catechins in the colonic tissue of obese rats was decreased compared to that in normal rats.
303.124 ± 6449.325 μg min g−1 in the colonic tissue of normal rats, which was also significantly higher than that in obese rats (9713.227 ± 3427.325 μg min g−1). Similar results for AUC(0–t) values of GCG, GC and C were observed in the colonic tissue of normal rats. On the other hand, the catechin levels in the colonic content didn’t show a significant difference between the two groups. These results suggested that the distribution of catechins in the colonic tissue did not rely on the systemic circulation. The permeability of the colonic epithelial cells may be affected after a long-term high-fat diet, so the uptake of catechins in the colonic tissue of obese rats was decreased compared to that in normal rats.
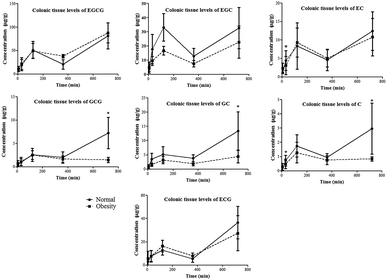 | ||
| Fig. 4 Time-dependent changes in colonic tissue levels of tea catechins in normal and obese rats given tea polyphenol solution (350 mg kg−1). *p < 0.05 compared to normal rats. | ||
Fecal excretion of tea polyphenols in rats
The fecal excretion of tea catechins was determined and is summarized in Table 2. In the obese rats, the total excreted content (μg g−1) of EC, EGC, ECG, EGCG, C, GC and GCG from 0–24 h was increased compared to that in normal rats. In the normal rats, the fecal catechin content only accounted for a small part of the oral administered amount (0.516–1.309%), and the excretion ratios for the seven catechins of the obese group were significantly increased (1.205–3.375%) compared to the ratios in the normal group as shown in Table 2.| Compounds | Fecal amount 0–24 h (μg g−1 body weight) | Excretion ratio 0–24 h (%) | |
|---|---|---|---|
| a *p < 0.05 and **p < 0.01 compared to the normal rat group. | |||
| EGCG | Normal | 2.505 ± 1.430 | 0.747 ± 0.515 |
| Obese | 3.118 ± 0.821 | 1.276 ± 0.336* | |
| EGC | Normal | 0.723 ± 0.445 | 0.588 ± 0.434 |
| Obese | 1.075 ± 0.589 | 1.205 ± 0.661* | |
| EC | Normal | 0.106 ± 0.051 | 0.516 ± 0.317 |
| Obese | 0.187 ± 0.048* | 1.247 ± 0.321* | |
| GCG | Normal | 0.171 ± 0.087 | 1.309 ± 0.844 |
| Obese | 0.196 ± 0.032 | 2.065 ± 0.343* | |
| GC | Normal | 0.129 ± 0.054 | 1.068 ± 0.584 |
| Obese | 0.286 ± 0.117** | 3.375 ± 1.391** | |
| C | Normal | 0.024 ± 0.010 | 0.767 ± 0.399 |
| Obese | 0.036 ± 0.009* | 1.594 ± 0.398** | |
| ECG | Normal | 0.565 ± 0.333 | 0.823 ± 0.591 |
| Obese | 0.740 ± 0.277* | 1.488 ± 0.556* | |
There have been extensive studies about tea polyphenol functions. The health benefits of tea have been verified using various disease models, such as for metabolic syndromes and carcinogenesis. Yet the changes of absorption and distribution of tea polyphenols caused by a disease model have not been clarified. Therefore, the lack of sufficient evidence required a re-evaluation of the pharmacokinetics of tea polyphenols in disease models, which may influence their absorption, metabolism, distribution and excretion and hence reduce their activities. In the present study, although normal and obese rats were orally administered the same dose of tea polyphenols, the results showed a significant decrease in the Cmax and AUC(0–t) of GC in obese rats. For other catechins, there were no statistical differences for absorption between normal and obese rats. These results demonstrated that hyperlipidemia didn’t affect the absorption of the main catechins of tea polyphenols, except for GC.
It has been suggested that the dominant distribution of EGCG and EC in the digestive tract was attributed to the direct contact or involvement in the excretion of tea catechins.9,13 In the present study, the catechin levels in the small intestinal content of obese rats were similar to that of normal rats. When carefully comparing the levels of EGCG and GCG in the small intestinal contents of normal and obese rats, the obese rat data didn’t show statistical differences. The Cmax of EGCG and GCG in the small intestinal content of obese rats was even less than that of normal rats. The most interesting finding of this study is that the small intestinal tissue levels of EGCG and GCG were significantly increased in obese rats compared to those in normal rats. These findings indicated that other important pathological factors affected the uptake and distribution of catechins in the small intestine. It was reported that obese rats showed an alteration in tight junctions and intestinal permeability. Then, the tissue distribution of tea catechins may be subjected to multiple factors.22
By comparing the data of catechin levels in colon circulation solution and feces, we found that hyperlipidemia significantly influenced the excretion of catechins in rats. As shown in Table 2, before metabolism by colonic microflora or uptake by the large intestine, the concentration of catechins in the colon circulation solution didn’t show a difference between normal and obese rats, but after digestion of the tea polyphenols in the large intestine, the fecal excretion of catechins was highly increased. It was reported the catechins were metabolized into low molecular weight phenolics by the gut flora.23–27 So, if high-fat-induced obesity decreases the total bacterial count and increases the relative proportion of Bacteroidales and Clostridiales, the metabolism of tea catechins may be correspondingly affected.28 A reasonable reason for this appearance was the metabolism difference of catechins between normal and obese rats.
Conclusions
The absorption, distribution and excretion of catechins in normal and obese rats showed differences. Firstly, absorption of GC was significantly decreased in obese rats. Secondly, compared to normal rats, the distribution of EGCG and GCG in the gastric and small intestinal tissues of obese rats was significantly increased, but the distribution of EGC, GCG, GC and C in the colonic tissue was significantly decreased. Thirdly, the fecal excretion of catechins was increased in obese rats. To sum up, the absorption, metabolism and distribution of tea catechins were different between normal and obese rats. The distribution of catechins in the digestive tract should get more attention because it may affect their effectiveness.Acknowledgements
Financial support for this research was provided by the National Natural Science Foundation of China (31201335), Anhui Provincial Natural Science Foundation (1308085QC51), and Modern Agro-industry Technology Research System in Tea Industry of Chinese Ministry of Agriculture (nycytx-26) and Chang-jiang Scholars and the Innovative Research Team in University (IRT1101).References
- M. Asim, O. H. Liu, E. Okello, M. Birch-Machin, M. Foltz and G. Lietz, Proc. Nutr. Soc., 2013, 72, E233 CrossRef.
- Y. T. Choi, C. H. Jung, S. R. Lee, J. H. Bae, W. K. Baek, M. H. Suh, J. Park, C. W. Park and S.-I. Suh, Life Sci., 2001, 70, 603–614 CrossRef CAS PubMed.
- S. I. Koo and S. K. Noh, J. Nutr. Biochem., 2007, 18, 179–183 CrossRef CAS PubMed.
- J. M. Yuan, Am. J. Clin. Nutr., 2013, 98, 1676S–1681S CrossRef CAS PubMed.
- H. C. Huang and J. K. Lin, Food Funct., 2012, 3, 170–177 CAS.
- S. M. Henning, P. Wang, N. Abgaryan, R. Vicinanza, D. M. de Oliveira, Y. Zhang, R. P. Lee, C. L. Carpenter, W. J. Aronson and D. Heber, Mol. Nutr. Food Res., 2013, 57, 483–493 CAS.
- S. Sasazuki, A. Tamakoshi, K. Matsuo, H. Ito, K. Wakai, C. Nagata, T. Mizoue, K. Tanaka, I. Tsuji and M. Inoue, Jpn. J. Clin. Oncol., 2012, 42, 335–346 CrossRef PubMed.
- H.-H. S. Chow and I. A. Hakim, Pharmacol. Res., 2011, 64, 105–112 CAS.
- S. Kim, M. J. Lee, J. Hong, C. Li, T. J. Smith, G.-Y. Yang, D. N. Seril and C. S. Yang, Nutr. Cancer, 2000, 37, 41–48 CrossRef CAS PubMed.
- N. Metz, A. Lobstein, Y. Schneider, F. Gosse, R. Schleiffer, R. Anton and F. Raul, Nutr. Cancer, 2000, 38, 60–64 CrossRef CAS PubMed.
- J. Ju, J. Hong, J. N. Zhou, M. Bose, A. M. Carothers and C. S. Yang, Cancer Res., 2005, 65, 582 CrossRef PubMed.
- M.-J. Lee, P. Maliakal, L. Chen, X. Meng, F. Y. Bondoc, S. Prabhu, G. Lambert, S. Mohr and C. S. Yang, Cancer Epidemiol., Biomarkers Prev., 2002, 11, 1025–1032 CAS.
- L. Chen, M. J. Lee, H. Li and C. S. Yang, Drug Metab. Dispos., 1997, 25, 1045–1050 CAS.
- E. Morgan, Clin. Pharmacol. Ther., 2009, 85, 434–438 CrossRef CAS.
- D. Soy, E. Aldasoro, L. Guerrero, E. Posada, N. Serret, T. Mejía, J. Urbina and J. Gascón, Antimicrob. Agents Chemother., 2015, 59, 3342–3349 CrossRef CAS PubMed.
- S. Kuriyama, T. Shimazu, K. Ohmori, N. Kikuchi, N. Nakaya, Y. Nishino, Y. Tsubono and I. Tsuji, JAMA, J. Am. Med. Assoc., 2006, 296, 1255–1265 CrossRef CAS PubMed.
- M. Shimizu, Y. Fukutomi, M. Ninomiya, K. Nagura, T. Kato, H. Araki, M. Suganuma, H. Fujiki and H. Moriwaki, Cancer Epidemiol., Biomarkers Prev., 2008, 17, 3020–3025 CrossRef CAS PubMed.
- L. C. Lin, M. N. Wang, T. Y. Tseng, J. S. Sung and T. H. Tsai, J. Agric. Food Chem., 2007, 55, 1517–1524 CrossRef CAS PubMed.
- J. Zhou, L. Zhang, J. Zhang and X. Wan, BMC Complementary Altern. Med., 2014, 14, 263 CrossRef PubMed.
- L. Zhang, Y. H. Han, L. Xu, Y. H. Liang, X. Chen, J. S. Li and X. Wan, Food Funct., 2015, 6, 2249–2256 CAS.
- M. J. Lee, Z. Y. Wang, H. Li, L. Chen, Y. Sun, S. Gobbo, D. A. Balentine and C. S. Yang, Cancer Epidemiol., Biomarkers Prev., 1995, 4, 393–399 CAS.
- H. Köhler, B. A. McCormick and W. A. Walker, J. Pediatr. Gastroenterol. Nutr., 2003, 36, 175–185 CrossRef.
- C. Li, M. J. Lee, S. Sheng, X. Meng, S. Prabhu, B. Winnik, B. Huang, J. Y. Chung, S. Yan and C.-T. Ho, Chem. Res. Toxicol., 2000, 13, 177–184 CrossRef CAS PubMed.
- C. Li, X. Meng, B. Winnik, M. J. Lee, H. Lu, S. Sheng, B. Buckley and C. S. Yang, Chem. Res. Toxicol., 2001, 14, 702–707 CrossRef CAS PubMed.
- M. R. Meselhy, N. Nakamura and M. Hattori, Chem. Pharm. Bull., 1997, 45, 888–893 CrossRef CAS PubMed.
- M. Schantz, T. Erk and E. Richling, Biotechnol. J., 2010, 5, 1050–1059 CrossRef CAS PubMed.
- A. Takagaki and F. Nanjo, J. Agric. Food Chem., 2013, 61, 4927–4935 CrossRef CAS PubMed.
- C. B. de La Serre, C. L. Ellis, J. Lee, A. L. Hartman, J. C. Rutledge and H. E. Raybould, Am. J. Physiol., 2010, 299, G440–G448 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19699j |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |