Up/down conversion, tunable photoluminescence and energy transfer properties of NaLa(WO4)2:Er3+,Eu3+ phosphors
Abstract
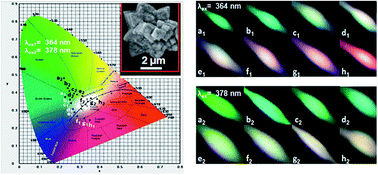
Er3+ or/and Eu3+ codoped NaLa(WO4)2 down conversion (DC) and up conversion (UC) phosphors were prepared by a facile hydrothermal process. For NaLa(WO4)2:Er3+ phosphors, the WO42− group can efficiently absorb ultraviolet (UV) light, and emit bright blue and green emissions by the f–f transitions of Er3+ through the host sensitization effect. The critical distance of the Er3+ ions in NaLa(WO4)2 is calculated and the energy quenching mechanism is proven to be a resonant type dipole–dipole interaction. More significantly, in the Er3+ and Eu3+ codoped NaLa(WO4)2 phosphors, the bright green emissions of Er3+ ions and the red characteristic emissions of Eu3+ ions can be observed, and the Er3+–Eu3+ energy migration has been demonstrated to be a resonant type of a dipole–dipole mechanism. Color-tunable emissions of NaLa(WO4)2:Er3+,Eu3+ microcrystals are realized under different UV radiation, and this could make them good candidates for use as full-color DC phosphors for near UV-LEDs. More practically, under near infrared (NIR) laser excitation at 980 nm, these phosphors also exhibit intense green and red emissions from the Er3+–Eu3+ energy transfer process, which causes the observed UC of Eu3+. The mechanism of UC luminescence is proposed by the observed dependence of integral intensity on the power of the pumping laser.

 Please wait while we load your content...
Please wait while we load your content...