Improving thermo-electrochemical cell performance by constructing Ag–MgO–CNTs nanocomposite electrodes†
Abstract
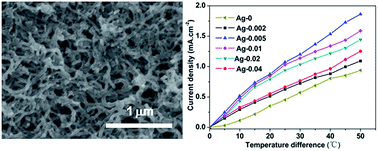
The application of carbon nanotubes (CNTs) as a thermo-electrochemical cell (TEC) electrode is still difficult due to their weak contact with the substrate during the electrophoretic deposition (EPD) method. In this study, by doping the suspension of the CNTs with Mg2+ and Ag powder, Ag–MgO–CNT nanocomposites were successfully prepared on a stainless steel (SS) substrate using the EPD method. The products were confirmed using characterization by scanning electron microscopy, X-ray diffraction, energy-dispersive X-ray and X-ray photoelectron spectroscopy. The TEC performance of the Ag–MgO–CNTs nanocomposite electrodes was significantly improved due to their higher conductivity, thermal conductivity and improved adhesion between the composite film and the SS substrate, depending on the concentrations of the Ag powder. The results suggest that constructing Ag–MgO–CNTs nanocomposite electrodes can effectively enhance the performance of CNTs-based TECs, which might be a promising way for energy harvesting using CNTs-based TECs prepared via the EPD technique.

 Please wait while we load your content...
Please wait while we load your content...