A fluorescent biosensor of lysozyme-stabilized copper nanoclusters for the selective detection of glucose†
Chan Wanga,
Shili Shuc,
Yagang Yao*b and
Qijun Song*a
aKey Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical & Material Engineering, Jiangnan University, Wuxi 214122, China. E-mail: qsong@jiangnan.edu.cn; Tel: +86-510-85917763
bSuzhou Institute of Nano-tech and Nano-bionics, Chinese Academy of Sciences, Suzhou 215123, China. E-mail: ygyao2013@sinano.ac.cn; Tel: +86-512-62872829
cDepartment of Chemistry, Tangshan Normal University, Tangshan 063000, China
First published on 18th November 2015
Abstract
Glucose biosensors have attracted increased attention, as the rapid and sensitive detection of glucose is highly desirable for diabetes diagnosis. In this article, we designed a type of lysozyme functionalized fluorescence copper nanoclusters (Lys-CuNCs) to detect glucose levels in blood samples. Fluorescence measurements were carried out to optimize the synthesis conditions (e.g. mass ratio, pH and reaction time) for the biosensor. Under optimum conditions, the obtained Lys-CuNCs with an average diameter of 2 nm exhibited bright orangey-red fluorescence with high quantum yields (up to 5.6%). The fluorescence signal of Lys-CuNCs was quenched upon the addition of glucose, presumably due to the reduction of Cu(I) on the NCs surface by glucose. Thus the Lys-CuNCs can be served as a biosensor for glucose detection and two linear response ranges respectively in 0.03–10 μM and 0.5–10 mM of glucose were observed with a detection limit of 1.9 nM. Furthermore, this biosensor showed superior selectivity for various interferences, including light radiation, metal ions, carbohydrates and amino acids. In view of these properties, the Lys-CuNCs biosensor was applied in the determination of glucose in blood samples, and the results agreed well with that obtained from a currently used clinical method. Finally the visualized fluorescence variation of Lys-CuNCs may further enable the rapid and simple detection of glucose level in blood.
Introduction
Glucose is one of the most important metabolic carbohydrate energy sources, which is crucial to maintaining health. The blood glucose concentration beyond the normal range of 3.1–6.6 mM may cause hyperglycemia (above 6.6) or hypoglycemia (under 3.1).1,2 Abnormal glucose levels can result in diabetes mellitus, which causes serious health problems including blindness, heart disease, kidney failure and hypertension.3 Therefore, it is of great significance to monitor glucose levels for diagnosis and treatment of diabetes in a simple, sensitive and efficient way. Since the appearance of the first glucose biosensor in 1962, a lot of effort has been devoted to developing glucose biosensors.4 Based on the detection method, these biosensors may be classified into colormetry,5 electrochemistry,6,7 electrochemiluminescence,8 and fluorescence method.9 Among them, fluorescence biosensors became a hotspot in recent years, owing to their operational simplicity, excellent sensitivity, low cost and the availability of multiple signals.Metal nanoclusters (NCs) have gained great interest because of their unique physicochemical, optical, and electrical properties,10,11 and thus are widely used in biological imaging,12,13 catalysis,14 and chemical sensors.15–17 In the past decades, metal NCs are one of the most popular fluorescence materials for glucose biosensors. Especially gold nanoclusters (AuNCs) have attracted much attention due to their ultrasmall sizes, bright fluorescence, good biocompatibility and high stability.18,19 For instance, Xia et al. reported a glucose oxidase (GOD)-functionalized AuNCs as probes for glucose.20 Radhakumary et al. developed a colorimetric probe for glucose using a glucose oxidase/gold nanoparticles bioconjugate.21 The biomolecule-stabilized AuNCs were also used as a novel fluorescence probe for sensitive and selective detection of glucose.22
Compared with Au, the non-precious Cu is earth-abundant and significantly cheaper. CuNCs have also been proved to possess unique photoluminescence (PL) properties, low toxicity and excellent biocompatibility.10,23 In present work, we developed CuNCs as a fluorescent biosensor for glucose detection. Lysozyme was chosen as template for the preparation of CuNCs, and the fluorescence signals of as-prepared Lys-CuNCs bioconjugates exhibited smart response to the presence of glucose in aqueous solution. This attractive feature enables the CuNCs to serve as probe for glucose-responsive functional materials. Hence, we explore the utility of the Lys-CuNCs for glucose detection in blood.
Experimental
Materials and apparatus
Lysozyme (CAS number: 12650-88-3, E. C. number: 235-747-3) from chicken egg white was purchased from MP Biomedicals (Irvine, CA). Carbohydrates (e.g. glucose, lactose, fructose, sucrose, soluble starch) and amino acids (cysteine, glycine, tryptophan, glutamic acid, lysine, leucine, alanine, proline and methionine) were ordered from Sigma-Aldrich (St. Louis, MO, USA). Copper sulfate (CuSO4·5H2O), sodium hydroxide, hydrazine hydrate (N2H4·H2O) and ethanol were acquired from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All the chemicals were of analytical grade and used as received without further purification. Deionized water was used in all of the experiments.UV-Vis absorption spectra were recorded with a Lambda 800 spectrophotometer (PerkinElmer, USA). PL experiments were performed with a Shimadzu RF-5301 PC spectrofluorimeter (Shimadzu, Japan) with excitation at 400 nm. X-ray photoelectron spectroscopy (XPS) analysis was performed on a VG ESCALAB MKII spectrometer (Thermo Fisher Scientific, USA) with Mg Kα excitation (1253.6 eV); the binding energy was calibrated with the C 1s band at 284.6 eV. Fourier transform infrared spectroscopy (FT-IR) spectra were recorded in the range of 4000–500 cm−1 with a Nicolet Avatar 360 FT-IR spectrophotometer (Thermo Fisher Scientific, USA). To study the morphology and estimate the mean diameter of the resultant Lys-CuNCs, transmission electron microscopy (TEM) analyses were conducted on a TECNAI F20 (FEI, USA) operating at an accelerating voltage of 200 kV. Glucose detection was conducted on the blood samples by the standard clinical instrument (Hitachi 7080, Japan) as compared.
Lys-CuNCs synthesis and optimization
The lysozyme-stabilized CuNCs were prepared by hydrothermal synthesis. To examine the influence of different experimental parameters, we investigated the content of lysozyme (CuSO4·5H2O/lysozyme, mg mg−1), the amount of N2H4·H2O, the pH values of reaction mixture and the reaction time. Here, the reaction time refers to the time after heating up to the required temperature.In a typical procedure, lysozyme (12.5 mg) and CuSO4·5H2O (6.4 mg) were added to 5 mL of deionized water under vigorous stirring to produce Lys–Cu ion complex. Then 1 mL of N2H4·H2O was introduced to the fully dissolved solution. The mixture was heated to 40 °C under stirring, and the reaction time was 2 h. Subsequently, the NaOH solution (1 M) was added dropwise until a light yellow and transparent solution was obtained, and the corresponding pH was about 12. After cooling to room temperature, the product was collected by precipitating with alcohol and centrifugation at 6000 rpm. The above purification process was repeated three times. The resultant Lys-CuNCs were freeze-dried under vacuum and stored in a refrigerator for subsequent use.
Glucose detection
Results and discussion
Design of Lys-CuNCs biosensor
Owing to the size control ability and the mild reaction conditions,24 proteins have played an important role in the synthesis of metal NCs, involving bovine serum albumin (BSA),10,25 insulin,26 pepsin27 and lysozyme.28,29 Lysozyme is an alkaline protein consisting of a single amino acid chain of 129 residues, which are abundant with free carboxyl and amino groups, as well as four disulfide bonds. Such special groups in lysozyme have high affinities with metal ions and facilitate to the formation and stabilization of metal NCs.30 Thus, we designed a CuNCs biosensor using lysozyme as a template for glucose detection based on the variation in its fluorescence intensity (Scheme 1).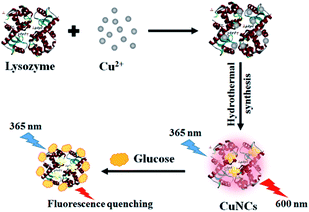 | ||
| Scheme 1 Schematic diagram of the synthesis of fluorescent lysozyme-stabilized CuNCs and the detection of glucose. | ||
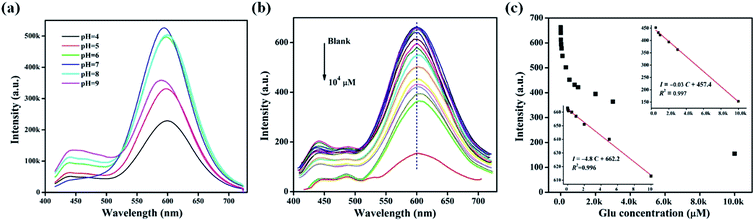
Three important reaction parameters, i.e., the mass ratio of lysozyme and CuSO4·5H2O (Lys/Cu), the pH values of reaction solution and the reaction time were optimized to improve the PL intensity of the Lys-CuNCs. The corresponding PL spectra were recorded, and the results are shown in Fig. 1. With the increase of the lysozyme content, the fluorescence intensity increased, reaching the maximum value at Lys/Cu = 2. Once the ratio is exceeded, the free Lys molecules could adsorb on the CuNCs surfaces, causing a decrease in the fluorescence of the Lys-CuNCs (Fig. 1(a)). As for the effect of the reaction time, the PL intensity increased with the increase of time up to 120 min (Fig. 1(b)). A prolonged reaction time caused rapid decrease in the PL intensity, suggesting the formation of the non-fluorescent copper nanoparticles.31 Therefore, we set the reaction time to 120 min. Moreover, it is necessary to control the end-point by monitoring the pH value of the solution. As shown in Fig. 1(c), the highest fluorescence intensity was achieved at the pH value of 12, presumably due to lysozyme turned into a partially unfolded structure that provided larger internal spaces at this pH.32,33 Such structure helped the formation of more fluorescent CuNCs, resulting in the great increase of PL intensity.
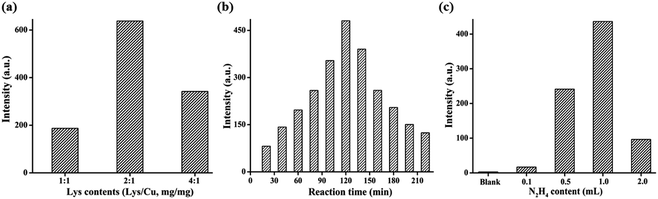 | ||
| Fig. 1 Effect of (a) lysozyme contents, (b) reaction time, and (c) pH values on the PL intensities at λmax = 600 nm of the resultant Lys-CuNCs. | ||
Characterization of the Lys-CuNCs
According to the above experiments, we synthesized the Lys-CuNCs at 40 °C followed by 120 min incubation with a mass ratio of Lys/Cu = 2, maintaining the pH at 12. The UV-Vis and PL spectra of the resultant Lys-CuNCs are shown in Fig. 2. The Lys-CuNCs with good dispersion show a yellow color under ambient light, and a bright orangey-red fluorescence under UV irradiation (see inset of Fig. 2). In addition, the presence of a strong absorption peak below 300 nm and the absence of characteristic surface plasmon resonance of the copper particles both implied the formation of the Lys-CuNCs. Upon excitation at 365 nm, the CuNCs exhibited strong orange emission with a peak centering at approximately 600 nm, whereas a weaker fluorescence in the range of 400–450 nm resulted from the lysozyme. The QY of the Lys-CuNCs was determined to be 5.6%, using rhodamine 6 G (QYs of 95% in ethanol) as the reference.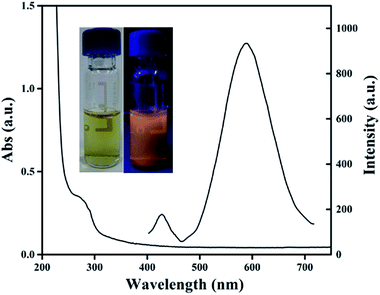 | ||
| Fig. 2 UV-Vis absorption and emission spectra of the resultant Lys-CuNCs. Inset: photographs of the Lys-CuNCs under the irradiation of visible (left) and UV (right) light. | ||
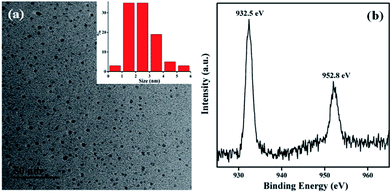
The sizes of the Lys-CuNCs were estimated by transmission electron microscope (TEM), and the results are shown in Fig. 3(a). The Lys-CuNCs are uniformly dispersed with an average size of around 2 nm, without visible large metal nanoparticles or aggregation. Moreover, DLS measurement was used to provide sufficient evidence for particle size, and the result showed the average size was 2.6 nm (Fig. S2(a)†), which was slightly larger than that in TEM images due to the presence of hydrodynamic radius. X-ray photoelectron spectroscopy (XPS) was utilized to determine the oxidation state of Cu in the Lys-CuNCs. As shown in Fig. 3(b), two peaks appear at 932.5 and 952.8 eV, which can be ascribed to the binding energies of the 2p3/2 and 2p1/2 electrons of Cu(0), respectively.34 The absence of the Cu 2p3/2 satellite peak around 942.0 eV confirms that the Cu(II) electrons are not present.35 As the binding energy of Cu(0) is only 0.1 eV away from that of Cu(I),36 it is not possible to exclude the formation of Cu(I), and the valence state of the Cu in CuNCs most likely lies between 0 and +1. The Cu atoms in such tiny clusters were expected to be positively charged, and the presence of Cu(I) could have contributed to the enhancement of both the stability and the PL intensity of the CuNCs, as supported by previous reports.37–39 The XPS spectra of other elements (i.e., S 2p, C 1s, N 1s and O 1s) are shown in Fig. S1 in the ESI.† The formation of CuO/Cu2O could be excluded by the HRTEM and XRD analysis. As shown in Fig. S2(b),† the crystal lattice fringes are 2.05 Å apart which indicates the (111) planes of the metallic Cu, and there was no other crystal lattices in HRTEM image. Moreover, there was no apparent diffraction peaks in the range of 2θ = 30–60° as shown in Fig. S2(c).† As reported by literatures, the NCs are too small to possess all of the characteristic diffraction peaks of bulk nanoparticles.
The chemical composition of the Lys-CuNCs was further examined by FT-IR measurements (Fig. S3†). The absorption band located between 3330 and 3400 cm−1 is assigned as the absorption peak of the N–H or O–H group, respectively, and the band at 2960 cm−1 is related to C–H stretching. Moreover, lysozyme is a globular protein, and it exhibits a number of characteristic IR absorption bands of protein secondary structures,40,41 i.e., amide I (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, 1645 cm−1), amide II (N–H in-plane bending vibration coupled with C–N stretching vibration, 1527 cm−1) and amide III (1238 cm−1). The peak observed at 2932 cm−1 and 2872 cm−1 can be assigned to the C–H symmetric and antisymmetric modes, respectively. Owing to the weak absorption of disulfide bonds, their characteristic IR bands cannot be seen in Fig. S3.† Moreover, the amide I band showed no discernible shifts, but the peak intensity decreased after assembled to CuNCs, indicating disorder in structure increased and few helical structure were still present.42,43
O stretching vibration, 1645 cm−1), amide II (N–H in-plane bending vibration coupled with C–N stretching vibration, 1527 cm−1) and amide III (1238 cm−1). The peak observed at 2932 cm−1 and 2872 cm−1 can be assigned to the C–H symmetric and antisymmetric modes, respectively. Owing to the weak absorption of disulfide bonds, their characteristic IR bands cannot be seen in Fig. S3.† Moreover, the amide I band showed no discernible shifts, but the peak intensity decreased after assembled to CuNCs, indicating disorder in structure increased and few helical structure were still present.42,43
Sensitivity and selectivity of Lys-CuNCs biosensor
To explore the potential application of the obtained Lys-CuNCs for sensing of glucose in blood, we investigated their performance in the aqueous solution. The PL spectra were measured by adding different concentrations of glucose into Lys-CuNCs solutions. By increasing the concentration of glucose from 0.03 μM to 10 mM, the fluorescence intensity of Lys-CuNCs exhibited a gradual decrease at 600 nm (Fig. 4(b)). Moreover, no discernible changes in the maximum peaks or shape of the fluorescence spectrum accompanied quenching, except a slight red shift at higher glucose concentration, as listed in Fig. 4(b). The two different linear relationships of fluorescence intensity versus the glucose concentration are shown in the inset of Fig. 4(c). For glucose concentration from 0.03 to 10 μM, the calibration equation is I = −4.8C + 662.2 with a correlation efficiency of 0.996, and the detection limit at a signal-to-noise ratio of 3 was estimated to be 1.9 nM.44 Moreover, a second calibration range can be obtained for the glucose concentration from 0.5 to 10 mM with an equation of I = −0.03C + 457.4. This detection range covers the normal level (3.1–6.6 mM) of glucose in blood from healthy persons, suggesting the applicability of our Lys-CuNCs as a blood glucose biosensor.The effect of pH was investigated in order to evaluate the feasibility of fluorescent Lys-CuNCs, and the results were shown in Fig. 4(a). The as-prepared Lys-CuNCs displayed relative high fluorescent intensity in the pH range of 5–9. As shown in Fig. S4(a),† the fluorescence intensity of Lys-CuNCs at pH = 5 did not respond to the addition of glucose until the concentration up to 0.01 M, suggesting the probe at this pH was not suitable for glucose detection. In contrast at pH 9, the fluorescence intensity of Lys-CuNCs exhibited a gradual decrease with the increase of glucose concentration from 0.01 μM to 0.1 mM (Fig. S4(b) and (c)†), but the sensitivity of the sensor was substantially lower than that obtained at the physiological pH 7–8 (Fig. 4(b) and (c)). According to the above analysis, the Lys-CuNCs exhibited good applicability as a blood glucose biosensor at the physiologically relevant pH range. The effect of ionic strength was also considered in present work. As shown in Fig. S5,† the PL intensity and maximum peaks or shape of Lys-CuNCs did not changed up to the addition of 200 mM KCl or NaCl, which corresponds to the concentration of 0.9% saline (wt). Thus it can be concluded that the Lys-CuNCs showed the high stability, suggesting a promising applicability of the probe for serum analysis.
As we know, the fluorescence quenching follow different mechanisms, (a) exited state reactions, (b) energy transfer, (c) dynamic quenching and (d) static quenching. The last two mechanisms were mainly considered, and the static quenching is usually resulted from the formation of a stable complex between the protein and quencher, while, the dynamic quenching is usually resulted from collisional encounters between the protein and quencher.45,46 The dependence of PL intensity on the quencher concentration can be used to predict the quenching mechanism.47–49 This was done by fitting our experimental data to the Stern–Volmer eqn (1).
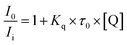 | (1) |
According to the data from Fig. S6,† the average lifetime of Lys-CuNCs in the absence of glucose was measured by a picosecond-resolved time correlated single-photon counting (TCSPC) technique. The numerical fitting of the luminescence collected at 495 nm reveals time constants of 2.03 ns (22%) and 8.21 ns (78%), and the average lifetime of Lys-CuNCs is 6.85 ns. After putting it into the eqn (1), the Kq is calculated to be 2.2 × 1011 L mol−1 s−1, which is much larger than the constant of collision–diffusion between quencher and fluorescence molecules (2 × 1010 L mol−1 s−1),47 and thus the mechanism can be ascribed to static quenching, i.e., a ground-state non-fluorescent complex is formed between Lys-CuNCs and glucose. Two factors may be responsible for fluorescence quenching in our hypothesis. First, the oxidized-state Cu(I) on the surface of CuNCs could be reduced to Cu(0) by glucose, of which the mechanism is similar to the silver mirror reaction. The presence of Cu(I) greatly increases the PL intensity of Lys-CuNCs, hence its reduction could cause the quenching fluorescence. In order to further investigate the fluorescence provider in our hypothesis, the fluorescence quenching of pristine lysozyme (without copper) in the presence of glucose was considered, and the result was shown in Fig. S7.† Apparently, lysozyme had no response to glucose, which confirmed that the fluorescence signal was belonged to CuNCs provider.
According to the experiments of pH effect, the fluorescence intensity of Lys-CuNCs were highly sensitive to glucose detection in the weakly alkaline or neutral aqueous solution as shown in Fig. S4.† As reported by references,50,51 the alkaline condition was helpful for promoting the oxidization of glucose to gluconic acid. Therefore, these results could confirm the detection of glucose followed the redox mechanism. Another possibility could be ascribed to the coordination reaction between the carboxyl groups on the produced gluconic acid (GluC) and Cu(I) in Lys-CuNCs, leading to the subsequent formation of the non-fluorescent GluC@CuNCs.52 Meanwhile, this complex could have prevented CuNCs from absorption with each other and hence no aggregation occurred. This hypothesis was confirmed by the results shown in Fig. S8.† When the 1 mM glucose was added, the color of Lys-CuNCs became lighter than that in inset of Fig. 2. In addition, the solution was still well dispersed with no precipitation, and the size of Lys-CuNCs increased to 4–10 nm after adding glucose, which may prove the formation of the GluC@CuNCs complex.
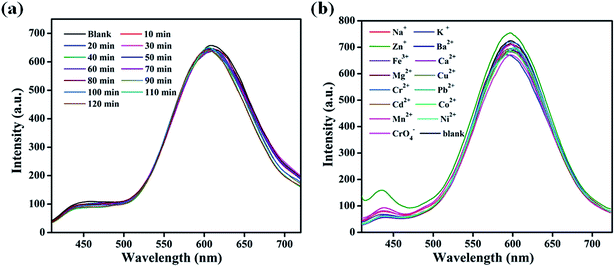
To investigate the ability of the Lys-CuNCs as sensors for the selective detection of glucose, the interferences of light radiation and metal ions were examined, and the results are shown in Fig. 5. The Lys-CuNCs solution was transferred into a 1 mL quartz cuvette, which was put in the cell holder of spectrofluorimeter with 450 nm Xenon lamp. Fig. 5(a) shows the time evolution of PL intensity of CuNCs during a 120 min light radiation period. Within the investigated duration, Moreover, a variety of metal ions (Na+, K+, Zn2+, Fe3+, Ca2+, Mg2+, Cu2+, Cr2+, Pb2+, Cd2+, Co2+, Mn2+, Ni2+ and CrO42−) were respectively introduced into the Lys-CuNCs solution with pH 7.4 PBS buffer, ensuring the ion concentration at 10 μM. As could be seen in Fig. 5(b), these foreign ions do not lead to any significant fluorescence changes at all.
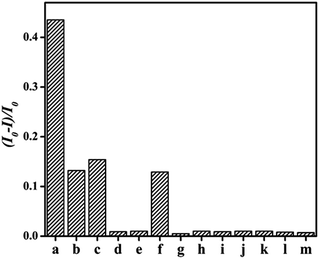
Subsequently, the as-prepared Lys-CuNCs were treated respectively with other carbohydrates (e.g. lactose, fructose, sucrose, starch soluble) and amino acids (e.g. cysteine, glycine, tryptophan, glutamic acid, lysine, leucine alanine, proline and methionine), and the fluorescence intensity rations were recorded. As shown in Fig. 6, the highest value of (I0 − I)/I0 was observed in the case of glucose treatment. The addition of lactose, fructose and cysteine also showed some influence on the fluorescence intensity, whereas the remaining analytes had a negligible effect under identical conditions. These results further illustrated that non-reducing analytes cannot cause the quenching effect on the fluorescence, suggesting the Lys-CuNCs sensors have good selectivity for glucose detection.
Glucose sensing blood samples
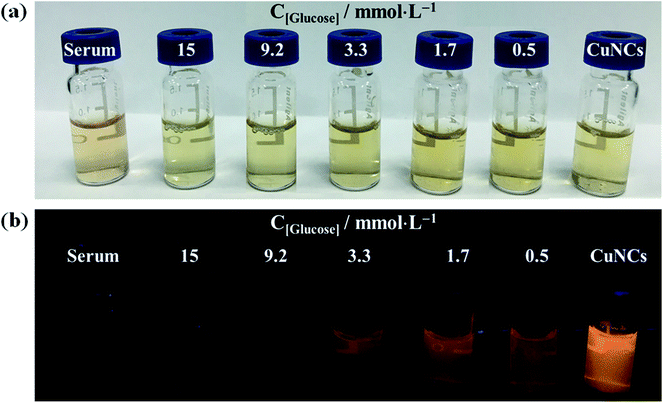
To evaluate the feasibility of our proposed method, the Lys-CuNCs sensor was employed to detect glucose in blood samples. A series of blood samples from healthy persons were collected, and the serums were separated from the coagulated blood. The spiked samples were respectively added to the Lys-CuNCs and were incubated for 15 min. The photographs of serum/glucose@Lys-CuNCs complexes are presented in Fig. 7, and the corresponding PL spectra are shown in Fig. S9.† As can be seen, the color of all samples look very similar by naked eyes. Under UV irradiation, the pure serum and Lys-CuNCs exhibit blue and bright orangey-red fluorescence, respectively. After adding glucose, the color changed from light orange to pale blue, therefore, it is particularly convenient to detect blood glucose by colorimetric assay, without any complicated instrumentation or operation procedure. Fig. S4† shows that the fluorescence intensity at about 600 nm decreases with the increase of glucose concentration, and these data are similar to that calculated from the second calibration equation as shown in the inset of Fig. 4(b).According to the above investigations, the good correlation was obtained between the PL intensity and the glucose concentrations, suggesting the feasibility of the use of our method for the quantitative measurement of glucose in blood. To validate the new method, glucose detection was further conducted on the blood samples with unknown concentration levels, and the results were compared with that obtained from the standard clinical method (STM). The glucose levels were quantified using the calibration plot (I = −0.03C + 457.4), and the results are shown in Table 1. The data obtained by our method are close to that by STM. Hence it is feasible to determination of glucose level in blood by the lysozyme-stabilized CuNCs sensors.
Conclusions
We present a convenient method to prepare fluorescence copper nanoclusters using lysozyme as a template. The resultant Lys-CuNCs show a number of remarkable features, including water solubility, bright orangey-red fluorescence, and high quantum yields. Significantly, the PL intensity of Lys-CuNCs is found to be selectively quenched by glucose, and two linear ranges from 0.03 to 10 μM and 0.5 to 10 mM with high sensitivity were obtained for glucose quantification. Meanwhile, the visualization fluorescence variation of Lys-CuNCs may further enable the rapid and simple detection of blood glucose level. Given the attractive fluorescence features, Lys-CuNCs biosensor are quite promising for applications in sensing blood glucose level.Acknowledgements
This work was supported by Natural Science Foundation of Jiangsu Province, China (No. BK20140392), Natural National Science Foundation of China (No. 51502115, No. 51372265 and No. 21175060), the Postdoctoral Research Foundation of Jiangsu Province, China (No. 1401058B), China Postdoctoral Science Foundation (No. 2015M581719), and the Open Foundation of State Key Laboratory of Materials-Oriented Chemical Engineering of Nanjing University of Technology (2014, KL14-02).Notes and references
- S. Canivell and R. Gomis, Autoimmun. Rev., 2014, 13, 403–407 CrossRef CAS PubMed
.
- J. Wang, Chem. Rev., 2008, 108, 814–825 CrossRef CAS PubMed
.
- N. A. Calcutt, M. E. Cooper, T. S. Kern and A. M. Schmidt, Nat. Rev. Drug Discovery, 2009, 8, 417–429 CrossRef CAS PubMed
.
- L. C. Clark and C. Lyons, Electrode systems for continuous monitoring in cardiovascular surgery, Ann. N. Y. Acad. Sci., 1962, 102, 29–45 CrossRef CAS PubMed
.
- S. Zhu, X. Zhao, J. You, G. Xu and H. Wang, Analyst, 2015, 140, 6398–6403 RSC
.
- F. Kong, S. Gu, W. Li, T. Chen, Q. Xu and W. Wang, Biosens. Bioelectron., 2014, 56, 77–82 CrossRef CAS PubMed
.
- X. Feng, H. Cheng, Y. Pan and H. Zheng, Biosens. Bioelectron., 2015, 70, 411–417 CrossRef CAS PubMed
.
- X. Tian, S. Lian, L. Zhao, X. Chen, Z. Huang and X. Chen, J. Solid State Electrochem., 2014, 18, 2375–2382 CrossRef CAS
.
- R. Gifford, ChemPhysChem, 2013, 14, 2032–2044 CrossRef CAS PubMed
.
- C. Wang, C. X. Wang, L. Xu, H. Cheng, Q. Lin and C. Zhang, Nanoscale, 2014, 6, 1775–1781 RSC
.
- H. Pei, X. L. Zuo, D. Zhu, Q. Huang and C. H. Fan, Acc. Chem. Res., 2014, 47, 550–559 CrossRef CAS PubMed
.
- Y. Tao, Z. H. Li, E. G. Ju, J. S. Ren and X. G. Qu, Nanoscale, 2013, 5, 6154–6160 RSC
.
- C. L. Liu, H. T. Wu, Y. H. Hsiao, C. W. Lai, C. W. Shih, Y. K. Peng, K. C. Tang, H. W. Chang, Y. C. Chien, J. K. Hsiao, J. T. Cheng and P. T. Chou, Angew. Chem., Int. Ed., 2011, 50, 7056–7060 CrossRef CAS PubMed
.
- M. Liang, L. B. Wang, X. Liu, W. Qi, R. X. Su, R. L. Huang, Y. J. Yu and Z. M. He, Nanotechnology, 2013, 24, 245601–245608 CrossRef CAS PubMed
.
- Y. Yue, T. Y. Liu, H. W. Li, Z. Y. Liu and Y. Q. Wu, Nanoscale, 2012, 4, 2251–2254 RSC
.
- R. Ghosh, A. K. Sahoo, S. S. Ghosh, A. Paul and A. Chattopadhyay, ACS Appl. Mater. Interfaces, 2014, 6, 3822–3828 CAS
.
- Y. Y. Zhao, Y. X. Ma, H. Li and L. Y. Wang, Anal. Chem., 2012, 84, 386–395 CrossRef CAS PubMed
.
- C. Wang, Y. G. Yao and Q. Song, J. Mater. Chem. C, 2015, 3, 5910–5917 RSC
.
- J. B. Liu, M. X. Yu, C. Zhou, S. Y. Yang, X. H. Ning and J. Zheng, J. Am. Chem. Soc., 2013, 135, 4978–4981 CrossRef CAS PubMed
.
- X. D. Xia, Y. F. Long and J. X. Wang, Anal. Chim. Acta, 2013, 772, 81–86 CrossRef CAS PubMed
.
- C. Radhakumary and K. Sreenivasan, Anal. Chem., 2011, 83, 2829–2833 CrossRef CAS PubMed
.
- L. Jin, L. Shang, S. Guo, Y. Fang, D. Wen, L. Wang, J. Yin and S. Dong, Biosens. Bioelectron., 2011, 26, 1965–1969 CrossRef CAS PubMed
.
- C. Wang and Y. Huang, Nano, 2013, 8, 1350054–1350063 CrossRef
.
- Y. L. Xu, J. Sherwood, Y. Qin, D. Crowley, M. Bonizzoni and Y. P. Bao, Nanoscale, 2014, 6, 1515–1524 RSC
.
- J. P. Xie, Y. G. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed
.
- Y. H. Lin and W. L. Tseng, Anal. Chem., 2010, 82, 9194–9200 CrossRef CAS PubMed
.
- H. Kawasaki, K. Hamaguchi, I. Osaka and R. Arakawa, Adv. Funct. Mater., 2011, 21, 3508–3515 CrossRef CAS
.
- J. Das and S. O. Kelley, Anal. Chem., 2013, 85, 7333–7338 CrossRef CAS PubMed
.
- W. Y. Chen, J. Y. Lin, W. J. Chen, L. Y. Luo, E. W. G. Diau and Y. C. Chen, Nanomedicine, 2010, 5, 755–764 CrossRef CAS PubMed
.
- L. N. Johnson and D. C. Phillips, Nature, 1965, 206, 761–763 CrossRef CAS PubMed
.
- C. X. Wang, L. Xu, Y. Wang, D. Zhang, X. D. Shi, F. X. Dong, K. Yu, Q. Lin and B. Yang, Chem.–Asian J., 2012, 7, 1652–1656 CrossRef CAS PubMed
.
- T. H. Chen and W. L. Tseng, Small, 2012, 8, 1912–1919 CrossRef CAS PubMed
.
- S. Nonose, K. Yamashita, T. Okamura, S. Fukase, M. Kawashima, A. Sudo and H. Isono, J. Phys. Chem. B, 2014, 118, 9651–9661 CrossRef CAS PubMed
.
- J. J. Brege, C. E. Hamilton, C. A. Crouse and A. R. Barron, Nano Lett., 2009, 9, 2239–2242 CrossRef CAS PubMed
.
- N. Goswami, A. Giri, M. S. Bootharaju, P. L. Xavier, T. Pradeep and S. K. Pal, Anal. Chem., 2011, 83, 9676–9680 CrossRef CAS PubMed
.
- W. T. Wei, Y. Z. Lu, W. Chen and S. W. Chen, J. Am. Chem. Soc., 2011, 133, 2060–2063 CrossRef CAS PubMed
.
- Z. T. Luo, X. Yuan, Y. Yu, Q. B. Zhang, D. T. Leong, J. Y. Lee and J. P. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed
.
- Z. Luo, V. Nachammai, B. Zhang, N. Yan, D. T. Leong, D. Jiang and J. Xie, J. Am. Chem. Soc., 2014, 136, 10577–10580 CrossRef CAS PubMed
.
- C. X. Wang, Y. Wang, L. Xu, X. D. Shi, X. W. Li, X. W. Xu, H. C. Sun, B. Yang and Q. Lin, Small, 2013, 9, 413–420 CrossRef CAS PubMed
.
- G. Thakur and R. M. Leblanc, Langmuir, 2009, 25, 2842–2849 CrossRef CAS PubMed
.
- A. Barth, Biochim. Biophys. Acta, Bioenerg., 2007, 1767, 1011–1073 CrossRef PubMed
.
- S. K. Das, C. Dickinson, F. Lafir, D. F. Brougham and E. Marsili, Green Chem., 2012, 14, 1322–1334 RSC
.
- D. T. Lu, L. L. Liu, F. X. Li, S. M. Shuang, Y. F. Li, M. M. F. Choi and C. Dong, Spectrochim. Acta, Part A, 2014, 121, 77–80 CrossRef CAS PubMed
.
- Analytical Methods Committee, Analyst, 1987, 112, 199–204 RSC
.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed
.
- J. M. Costa-Fernández, R. Pereiro and A. Sanz-Medel, TrAC, Trends Anal. Chem., 2006, 25, 207–218 CrossRef
.
- M. R. Eftink and C. A. Ghiron, Anal. Biochem., 1981, 114, 199–227 CrossRef CAS PubMed
.
- J. R. Lakowicz and G. Weber, Biochemistry, 1973, 12, 4171–4179 CrossRef CAS PubMed
.
- F. Mehranfar, A. K. Bordbar and H. Parastar, J. Photochem. Photobiol., B, 2013, 127, 100–107 CrossRef CAS PubMed
.
- C. C. Chen, C. L. Lin and L. C. Chen, Electrochim. Acta, 2015, 152, 408–416 CrossRef CAS
.
- N. Fujiwara, S. I. Yamazaki, Z. Siroma, T. Ioroi, H. Senoh and K. Yasuda, Electrochem. Commun., 2009, 11, 390–392 CrossRef CAS
.
- J. S. Maier, S. A. Walker, S. Fantini, M. A. Franceschini and E. Gratton, Opt. Lett., 1994, 19, 2062–2064 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: XPS spectra, FT-IR spectra, photographs and TEM micrograph of the Lys-CuNCs adding with glucose, and fluorescence spectra of serum@Lys-CuNCs. See DOI: 10.1039/c5ra19421k |
| This journal is © The Royal Society of Chemistry 2015 |