Effect of microwave irradiation on reduction of graphene oxide films
H. J. Han,
Y. N. Chen and
Z. J. Wang*
Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, 72 Wenhua Road, Shenyang 110016, China. E-mail: wangzj@imr.ac.cn
First published on 23rd October 2015
Abstract
In this study, the effect of microwave irradiation on reduction of graphene oxide films was investigated. The few-layer graphene (FLG) sheets were prepared by the electrochemical exfoliated method. The reduction process was completed with a microwave power as low as 42 W below a temperature of 250 °C in air. The Raman and XPS spectra demonstrated that a larger amount of oxygen functional groups were removed by microwave irradiation, which is much more effective than the usual method of mild-thermal treatment at 250 °C for 30 min. The FLG films treated by microwave irradiation showed a low value of sheet resistance around 6 × 103 Ω □−1. The results demonstrate that the use of microwave irradiation provides a simple and effective method for eliminating oxygen functional groups to produce highly conductive graphene films.
Introduction
Graphene, as a single-atom-thick layer of graphite, has been extensively studied for its many functional properties, such as excellent optical transparency, flexibility and electrical conductivity. Monolayer graphene is almost optically transparent, and it absorbs only 2.3% of the visible light.1 Also, it exhibits considerably high electron mobility, which exceeds 2 × 105 cm2 V−1 s−1 at a carrier concentration of n = 1012 cm−2.2 These unique properties make graphene films appropriate material for transparent conductive films applying in transparent conductors,3,4 solar cells5,6 and optoelectronic devices.7So far, large efforts have been made to develop simple methods for the production of graphene with a large scale and a high quality. Several methods such as micromechanical exfoliation,8 epitaxial growth on SiC,9 chemical vapor deposition (CVD),10 chemical exfoliation11 and electrochemical exfoliation12,13 have been widely studied. As a novel one-step method for obtaining high-quality graphene, electrochemical exfoliation of graphite has attracted great interest due to its potential advantages of low-cost and solution-processed fabrication. This method uses a chemical aqueous or non-aqueous solution as electrolyte, and an electrical current is applied to drive structural expansion and exfoliation of graphite for producing graphene sheets. Exfoliation in non-aqueous (such as ionic liquid) solution usually leads to functionalization of graphene with ionic liquid.14,15 Also, large consumption of ionic liquid makes this method expensive. Whereas, using aqueous acid solution as electrolyte is an appealing method for producing graphene in high quality and large lateral size. However, the application of sulphuric acid and positive potentials to the graphite unavoidably lead to oxidation of graphite, resulting in the formation of graphene with a certain degree of oxidation.12,16–18 Thus, to obtain high conductivity graphene films, a post-reduction process is needed for the electrochemical exfoliated graphene. Generally, the subsequent reduction process for graphene oxide involves thermal or chemical treatments to partially eliminate the oxygen functional groups. In the chemical reduction method, graphene oxide can be reduced via chemical reaction with N2H4,19 NaBH4,20 and HI,21 but these chemical agents are hazardous and usually introduce impurities in reduced graphene oxide. While for the thermal treatment of graphene oxide, high temperature (around 1000 °C) under inert-gas protection is usually needed to improve the high conductivity of graphene,22 which is impossible for glass or plastic substrates. Therefore, an environmental friendly reduction of graphene oxide at a low temperature seems to be very important.
Microwave irradiation, as a convenient and efficient heating method, has been widely used in the field of material synthesis, such as in the synthesis of colloidal inorganic nanocrystals23 and nanoparticles.24 Recently, it has been reported that few-layer graphene with a high quality can be produced from graphite oxide by microwave irradiation.5,25,26 This method includes a reduction and exfoliation of graphite oxide simultaneously. For the microwave-assisted exfoliation method, the main attention has been paid to the improvement of quality and yield for graphene production. However, it should be noticed that microwave irradiation also has a direct reduction effect on graphite oxide. It is well known that the main difference between graphene oxide and graphite oxide is the number of single-atom layers. In the case of graphene oxide, there are less than 5 layers, while for graphite oxide; its thickness is much larger than 5 layers. Therefore, microwave irradiation may be an effective method for the reduction of graphene oxide. In addition, in those previous studies, a high power about 1000 W was used and the local temperature of graphite oxide could exceed 1000 °C, so that the reduction effect on graphite oxide was attributed to such a high temperature. In addition to the effect of temperature, the directed interaction between microwave and graphite oxide was less mentioned. As mentioned above, such high temperatures are not suitable for the fabrication of graphene films on flexible substrates. Therefore, the reduction effect of microwave irradiation with a low power on the graphene oxide is needed to be studied.
In this study, few-layer-graphene (FLG) sheets with a low level of oxygen functional groups are obtained by the electrochemical exfoliation of highly oriented pyrolytic graphite (HOPG). The morphology and thickness of the FLG sheets are observed by transmission electron microscopy (TEM) and high-resolution TEM (HRTEM). Statistical distributions for the thickness and lateral size of the FLG sheets are estimated using atomic force microscopy (AFM). Raman spectroscopy and X-ray photoelectron spectroscopy (XPS) are used to investigate the defects and chemical composition of the FLG films before and after microwave irradiation. The results show that a simple and effective method is realized by using microwave irradiation to reduce graphene oxide for producing highly conductive graphene films.
Experimental procedure
Materials and methods
Highly oriented pyrolytic graphite (HOPG; 1.5 cm × 1.5 cm × 0.3 mm) was purchased from Sigma Aldrich and used as received. 98% H2SO4, KOH were supplied by China Medicine and used without further purification. Deionized (DI) water was used as the solvent throughout the experiments. FLG sheets were obtained by electrochemical exfoliation of the HOPG, as shown in Fig. 1. For preparation of FLG films on SiO2/Si substrates, the FLG sheets was dispersed in a dimethylformamide (DMF) solution, and then the DI water was mixed in the solution by volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The FLG thin film self aggregated on the surface of the above solution, and then was transferred on the SiO2/Si substrate by dip coating. For microwave irradiation, we used a single mode microwave (frequency = 2.45 GHz) generator with a waveguide in TE103 mode, which has been described clearly in one of our previous papers.27 The wavelength (λg) of the standing wave formed in this cavity is 12.24 cm. The maximum electric or magnetic field can be established at a specific position by moving a plunger at the end of the waveguide. Each sample was placed at the center of the highest magnetic field for microwave irradiation. During the microwave irradiation procedure, the temperature of the sample was measured by using an infrared thermometer (CTLaser2 M; Optris, Berlin, Germany). This sensor could not measure sample temperatures below 250 °C. The microwave irradiation treatment was carried out with an output power of 42 W for 5 min in air. No temperature over 250 °C could be detected during the microwave irradiation process, indicating that the temperature of the FLG film was below 250 °C. For comparison, the FLG films were also treated at 250 °C for 30 min by conventional annealing (CA) in an electric furnace at the atmosphere. We have done the experiment about the effect of microwave irradiation for many times, and the reproducibility of the experimental results has been confirmed.
5. The FLG thin film self aggregated on the surface of the above solution, and then was transferred on the SiO2/Si substrate by dip coating. For microwave irradiation, we used a single mode microwave (frequency = 2.45 GHz) generator with a waveguide in TE103 mode, which has been described clearly in one of our previous papers.27 The wavelength (λg) of the standing wave formed in this cavity is 12.24 cm. The maximum electric or magnetic field can be established at a specific position by moving a plunger at the end of the waveguide. Each sample was placed at the center of the highest magnetic field for microwave irradiation. During the microwave irradiation procedure, the temperature of the sample was measured by using an infrared thermometer (CTLaser2 M; Optris, Berlin, Germany). This sensor could not measure sample temperatures below 250 °C. The microwave irradiation treatment was carried out with an output power of 42 W for 5 min in air. No temperature over 250 °C could be detected during the microwave irradiation process, indicating that the temperature of the FLG film was below 250 °C. For comparison, the FLG films were also treated at 250 °C for 30 min by conventional annealing (CA) in an electric furnace at the atmosphere. We have done the experiment about the effect of microwave irradiation for many times, and the reproducibility of the experimental results has been confirmed.
Characterization
The morphology and microstructure of FLG sheets were investigated by transmission electron microscopy (TEM; Tecnai G2 F20, FEI). For TEM investigations, the diluted FLG sheets dispersion was dropped on a carbon-coated copper grid and dried under ambient conditions. The statistical distributions of the thickness and lateral size for the FLG sheets were estimated by atomic force microscopy (AFM; Nanoscope IV, Veeco). The substrate was smooth enough for a reliable analysis of the FLG sheet thickness by AFM. Raman spectra of the FLG films were collected by JY HR800 (laser wave length 632 nm and laser spot size is ∼0.5 μm). Chemical composition of FLG films were determined by X-ray photoelectron spectroscopy (XPS; Thermo ESCALAB 250, Thermal Fisher). Measurements for the electrical conductivity of FLG films were carried out on a TCP-610 using a four-point-probe head with a pin distance of 1 mm.Results and discussion
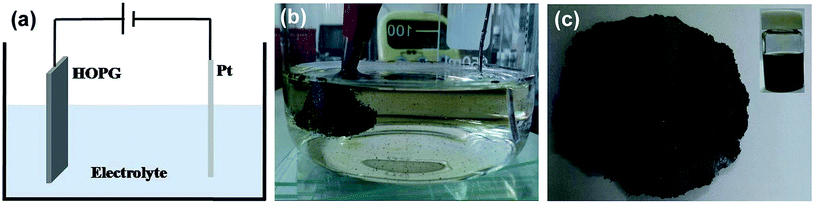
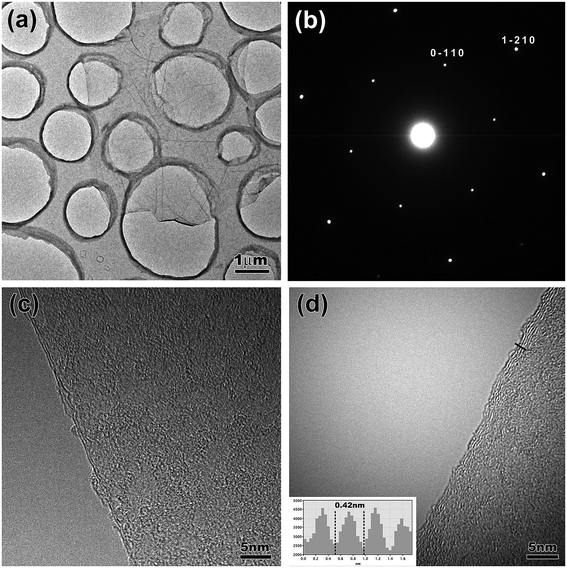
Fig. 1(a) shows a schematic illustration of the experimental setup for the electrochemical exfoliation of the HOPG. Typically, this procedure is carried out in a double electrode device, where the HOPG and Pt wire are used as the working electrode and counter electrode, respectively. The electrolyte was prepared by diluting 98% H2SO4 in 100 ml of deionized water. 30% KOH solution was added for adjusting the pH value to slow down the oxidation rate by H2SO4. Prior to the exfoliation of HOPG, a low bias voltage (2.5 V) was applied on the working electrode for 1 min to make a micro-expansion of the HOPG and probably lead to the intercalation of SO42− ions more efficient. Then an altering high bias voltage (+10/−10 V) was given to the working electrode until desired amounts of exfoliated FLG sheets were obtained. When applying a positive voltage (+10 V), the HOPG begin to expand quickly, then small pieces of graphene sheets dissociated from the HOPG, and dispersed in to the electrolyte surface, as shown in Fig. 1(b). When the bias voltage was switched to −10 V, the oxidation functional groups were eliminated at some extent through the cathodic reduction reaction. Afterward, the exfoliated FLG sheets were collected by vacuum filtration and washed with DI water repeatedly to remove the residual electrolyte. After drying, a piece of “graphene paper” was obtained, as shown in Fig. 1(c). The “graphene paper” was dispersed in the DMF solution. This solution contained a homogeneous dispersion of FLG sheets and large amounts of macroscopic graphite particles, which were also produced in the electrochemical exfoliation process. To remove such unwanted macroscopic particles, the solution was subjected to a centrifugation at 2500 rpm for 30 min. Finally, a dark dispersion was obtained as shown as the inset in Fig. 1(c). This FLG dispersion was used for the preparation of the FLG films.Fig. 2 shows typical TEM and HRTEM images of the exfoliated FLG sheets. As shown in Fig. 2(a), a FLG sheet with a lateral size about 5 μm is observed. Fig. 2(b) shows the selected area electron diffraction (SAED) pattern. The typical 6-fold symmetry in the SAED patterns is consistent with the hexagonal structure, and the stronger diffraction from the (1 ![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 1 0) plane than from the (0
1 0) plane than from the (0 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 0) plane indicates the high crystallinity of a bilayer graphene sheet. As shown in Fig. 2(c) and (d), the HRTEM images show the exfoliated FLG sheets with 2 and 4 layers, respectively. In addition, the interlayer distance of the FLG sheet is estimated to be about 0.42 nm, which is larger than that of graphite (0.335 nm). This may be attributed to the intercalation of oxygen functional groups and anionic SO4− originated from the electrolyte during the exfoliation process.12 In the subsequent X-ray photoelectron spectroscopy (XPS) analysis, a large amount of oxygen and a small amount of sulfur were detected in the as-prepared FLG film, which confirms this conjecture.
1 0) plane indicates the high crystallinity of a bilayer graphene sheet. As shown in Fig. 2(c) and (d), the HRTEM images show the exfoliated FLG sheets with 2 and 4 layers, respectively. In addition, the interlayer distance of the FLG sheet is estimated to be about 0.42 nm, which is larger than that of graphite (0.335 nm). This may be attributed to the intercalation of oxygen functional groups and anionic SO4− originated from the electrolyte during the exfoliation process.12 In the subsequent X-ray photoelectron spectroscopy (XPS) analysis, a large amount of oxygen and a small amount of sulfur were detected in the as-prepared FLG film, which confirms this conjecture.
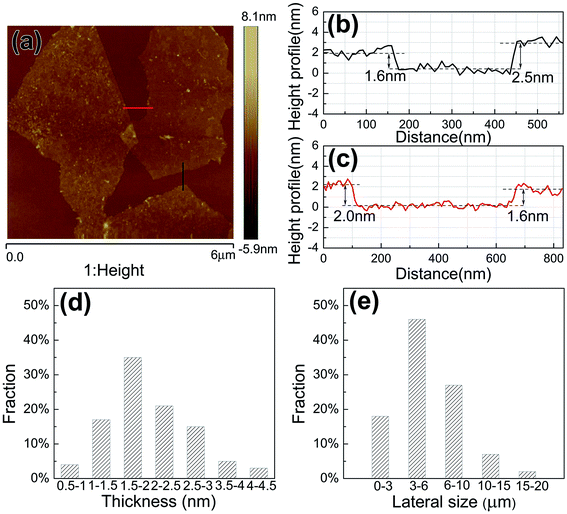
To confirm the average size and thickness of the electrochemically exfoliated FLG sheets, the atomic force microscopy (AFM) was used for further estimation. Fig. 3(a) shows the typical AFM image of the FLG sheets transferred to a SiO2/Si substrate, in which two small pieces of FLG sheets with a lateral size about 3 μm can be seen. The AFM image also shows that the FLG sheets are connected by jointing the edges of them. As shown in Fig. 3(b) and (c), the thickness for the three FLG sheets is about 1.6 nm, 2.0 nm and 2.5 nm. According to the interlayer distance of the FLG sheet obtained by HRTEM, the sheets with thickness of 1.6 nm, 2.0 nm and 2.5 nm correspond to 4, 5 and 6 layers of graphene, respectively. The statistical distributions of thickness and lateral size for the FLG sheets were analyzed by AFM on about 60 FLG sheets which were randomly selected, and the analysis results are shown in Fig. 3(d) and (e). It can be seen that over 50% of the FLG sheets have 2–5 layers. The monolayer graphene absorbs only 2.3% of the visible light,1 so that a large amount of FLG sheets within 5 layers can be estimated to be more than 80% light transmittance. Most of the lateral size of the FLG sheets ranges from 3 to 10 μm. It has been reported that sheet resistance of graphene films will decrease with increasing lateral size of graphene sheets because of the reduction in the number of intersections between graphene sheets which always introduce large interflake resistance.28 Therefore, it is necessary to further increase the average lateral size of the FLG sheets to decrease the sheet resistance of the FLG films.
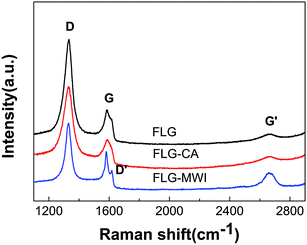
Raman spectroscopy is a non-destructive and essential technique to investigate the structure of carbon materials, especially for determining the concentration and type of defects in graphene sheets. Fig. 4 shows the Raman spectra for the as-prepared graphene films (FLG), the graphene films that after conventional annealing (FLG–CA) and microwave irradiation (FLG–MWI), respectively. In the Raman spectrum, there are three characteristic peaks which correspond to the D band appears at 1350 cm−1, the G band appears at 1574 cm−1, and the G′ band appears at 2684 cm−1. Sometimes, the G band peak appears a shoulder which is usually denoted as the D′ peak. The G band originates from the in-plane vibration of sp2 hybrid carbon atoms. Both the D band (corresponding to disorder sp3 structure in graphene) and D′ band (related to disordered edge carbons) are associated with the appearance of structural disorder in graphene. Usually, the ID/IG ratio is employed to evaluate the defect concentration in a sp2 network structure of graphene, and the ID/ID′ ratio is used to identify the type of defects. The intensity of the G′ band qualitatively represents the thickness of the FLG sheets. In the Raman spectra of the FLG film, an obvious D band peak appears which is probably due to the presence of sp3 defects formed by unavoidable oxidation in the electrochemical exfoliation process. The ID/IG ratio for FLG, FLG–CA and FLG–MWI is 2.89, 2.34 and 1.56 respectively. The value for the ID/IG ratio is simply proportional to the amount of oxygen functional groups in the three kinds of films with the same size. As mentioned above the ID/IG ratio indicated the defect density in the sp2-bonded structure, so the highest value of ID/IG ratio for the FLG film means the largest number of oxygen functional groups in it. After conventional annealing or microwave irradiation, the decrease in the ID/IG ratio indicates that the oxygen functional groups were removed partly. Moreover, microwave irradiation is more effective than conventional annealing. In the Raman spectra of FLG–MWI, the D′ band is observed clearly, and the ID/ID′ is about 2.5, which is close to the value of 3.5 (related to boundary-like defects in graphene). In addition, the increased peak of the G′ band is observed in the Raman spectra of FLG–MWI. These results suggested that a small extent of exfoliation for FLG sheets happened in the microwave irradiation process, leading to that some thick graphene sheets were exfoliated into small and thin pieces, which may also contribute to the high quality of the FLG films. The effect of microwave irradiation can also be confirmed by the following X-ray photoelectron spectroscopy (XPS) results.
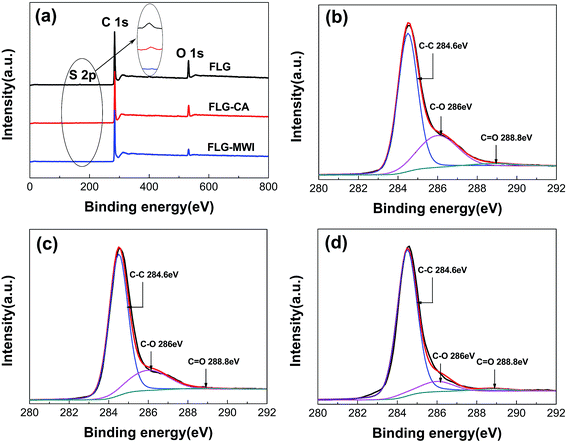
Fig. 5(a) shows the full-scale XPS spectra of the three films. Two peaks corresponding to C 1s at 284.6 eV and O 1s at 532.6 eV appeared in all spectra. The presence of the O peak in the as-prepared graphene film indicates that the FLG sheets were still somewhat oxidized during the electrochemical exfoliation process. After conventional annealing or microwave irradiation, the C/O ratio is increased from 7.8 to 9.3 and 17.5, respectively, indicating that both treatment methods can remove oxygen functional groups. Moreover, microwave irradiation shows a better reduction effect than conventional annealing. Such a high value of 17.5 for C/O ratio is almost comparable to that of reduced graphene oxide (RGO) obtained by chemical or thermal reduction method19–22 and much higher than that of multi-layer graphene prepared by microwave-assisted exfoliation of graphite oxide.29,30 In these reports, graphite oxide with a C/O ratio around 1 was reduced and exfoliated to multi-layer graphene with a C/O ratio around 3 by microwave irradiation with a high power. The peak of S 2p is detected in the as-prepared FLG film, as shown in the inset of Fig. 5(a). But it disappeared after microwave irradiation, indicating that the purity of the FLG film was improved by microwave irradiation. On the other hand, a further characterization of C 1s spectrum in Fig. 5(b) also indicate a minor extent of oxidation with oxygen functional groups assigned to C–O bonds (such as hydroxyl or epoxide groups) at around 286 eV and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds relating to carboxylic acid groups at around 288.8 eV. As shown in Fig. 5(c) and (d), after treated by conventional annealing or microwave irradiation, the intensity of the peaks for C–O and C
O bonds relating to carboxylic acid groups at around 288.8 eV. As shown in Fig. 5(c) and (d), after treated by conventional annealing or microwave irradiation, the intensity of the peaks for C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O greatly reduced. Moreover, in the case of microwave irradiation, this phenomenon is more obvious. These results also indicate that most of oxygen functional groups can be eliminated by microwave irradiation.
O greatly reduced. Moreover, in the case of microwave irradiation, this phenomenon is more obvious. These results also indicate that most of oxygen functional groups can be eliminated by microwave irradiation.
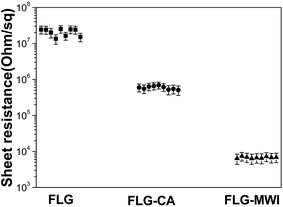
Fig. 6 shows the sheet resistance values of the FLG, FLG–CA and FLG–MWI films measured by a four-point-probe system. The sheet resistance for the as-prepared FLG film is about 107 Ω □−1, and largely reduced to ∼6 × 103 Ω □−1 by microwave irradiation, which can be attributed to the removal of oxygen functional groups caused by microwave irradiation. It has been reported that graphene oxide shows a poor electrical conductivity due to disruption of π-conjugated structures by oxygen functional groups,31 while the electrical conductivity can be restored by removing these functional groups through reduction of graphene oxide. The enhanced conductivity of the FLG films treated by microwave irradiation proved that microwave has a direct reduction effect on the low level oxidized FLG sheets. The FLG films with a low-resistance may be expected to replace currently used high-cost ITO electrodes in the future.
The effect of microwave irradiation on the deoxygenation of low-level oxidized FLG sheets can be considered as follows. Li32 and Chen33 have investigated the change of weight versus temperature for the graphene oxide powders by thermogravimetric analysis (TGA), and reported that there was weight loss in the graphene oxide powders during increasing temperature. In the first step, weight loss about 10% happened around 100 °C, resulting from the elimination of absorbed water. In the next step, weight loss with 30–50% appeared around 200 °C, which due to the decomposition of the thermally labile oxygen functional groups. That is why we choose 250 °C as the reduction temperature. In this study, by using conventional annealing at 250 °C, the oxygen functional groups in the FLG film was removed in some extent. Although the temperature of the FLG film treated by microwave irradiation was below 250 °C during the whole process, Raman and XPS analysis results showed the high level of reduction in the FLG film. Namely, microwave irradiation can induce a larger number of oxygen functional groups to be removed than the conventional annealing treatment, despite a lower temperature and less time used in microwave irradiation. As far as the reason is concerned, the local-thermal effect induced by microwave irradiation plays an important role in the reduction of oxidized FLG sheets. According the results reported in by Li32 and Tang,34 graphite oxide or reduced graphene oxide can strongly absorb microwave through the interaction between microwave and π-conjugated structures in these graphene-based carbon materials, thus the supplied microwave energy could be transformed to Joule heat. For the FLG sheets, there are enough amounts of π-conjugated structures in them, so a rapid increase of the local temperature can be induced by the efficient Joule heating. The transformed microwave energy led to the heating of FLG sheets, resulting in decomposition of some oxygen functional groups. Compared with the conventional annealing at a similar temperature, microwave irradiation showed the high efficiency of reduction for the oxide FLG sheets. Therefore, except for the thermal effect of microwave irradiation, there might be another reason for the reduction of the oxidized FLG sheets by microwave irradiation. It has been reported that microwave has a directly coupling with polar substances in microwave-assisted organic synthesis reactions.35 These oxygen functional groups containing in low-level oxidized FLG sheets are polarized, so the supplied microwave may have a direct interaction with these groups, facilitating the decomposition of the oxygen functional groups. Thus, although the microwave irradiation process happened below 250 °C, the Joule heating produced by π-conjugated structures and the direct interaction of microwave and polar functional groups render a higher efficiency of reduction than the conventional thermal annealing process.
On the other hand, the amount of such local energy depended on the output power of microwave irradiation and the amount of π-conjugated structures. For low-level oxidized FLG sheets, a large number of π-conjugated structures are included, so that a low power of 42 W can meet the requirement of energy for the reduction reaction. As far as the high-level oxidized graphene oxide prepared by Hummer's method, a small amount of π-conjugated structures are contained in them, so that a high power may be needed for the reduction of graphene oxide. In addition, to produce graphene sheets by microwave-assisted exfoliation of graphite oxide method, a power as high as 1000 W was reported.5,25,26 In their process, except for the reduction of graphite oxide, exfoliation of reduced graphite oxide to produce pieces of few layer graphene also happened simultaneously. Such a higher power was need to make the thermal explosion and large amounts of gases for the exfoliation of reduced graphite oxide. Although the microwave-assisted exfoliation of graphite oxide is a simple and practicable method for preparation of multi-layer graphene sheets, the average thickness of the product is still large. Therefore, it is not suitable for preparation of high conductivity graphene films. The preparation of low level oxidized FLG sheets by electrochemical exfoliation of graphite excludes utilization of KMnO4 and concentrated H2SO4, avoiding severe damage of π-conjugated structures. Moreover, the microwave irradiation method developed in this work can effectively remove the oxygen functional groups in the FLG sheets, improving their electrical conductivity. The microwave-reduced FLG films with a low sheet resistance can be expected to be used as flexible transparent electrodes for flexible touch screens or thin-film photovoltaic devices.36
Conclusions
In this study, we developed a simple reduction method to remove oxygen functional groups contained in electrochemical exfoliated graphene. By using microwave irradiation, the C/O ratio of the FLG film was obviously increased from 7.8 to 17.5 and the large sheet resistance of 107 Ω □−1 for the as-prepared FLG films were remarkably decreased to ∼6 × 103 Ω □−1, indicating that most of oxygen functional groups were successfully eliminated. This study offers a simple and green method for obtaining highly conductive graphene films.Acknowledgements
This research was supported by the Hundred Talents Program of the Chinese Academy of Sciences and the National Natural Science Foundation of China (No. 51172238). We sincerely thank Dr Xiaotian Zhao for his assistance in the assembly of the electrolytic equipment.References
- R. R. Nair, P. Blake, A. N. Grigorenko, K. S. Novoselov, T. J. Booth, T. Stauber, N. M. R. Peres and A. K. Geim, Science, 2008, 320, 1308 CrossRef CAS PubMed
.
- A. S. Mayorov, R. V. Gorbachev, S. V. Morozov, L. Britnell, R. Jalil, L. A. Ponomarenko, P. Blake, K. S. Novoselov, K. Watanabe, T. Taniguchi and A. K. Geim, Nano Lett., 2011, 11, 2396–2399 CrossRef CAS PubMed
.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270–274 CrossRef CAS PubMed
.
- J. Wang, M. Liang, Y. Fang, T. Qiu, J. Zhang and L. Zhi, Adv. Mater., 2012, 24, 2874–2878 CrossRef CAS PubMed
.
- K. Saranya, N. Sivasankar and A. Subramania, RSC Adv., 2014, 4, 36226–36233 RSC
.
- L. J. Larsen, C. J. Shearer, A. V. Ellis and J. G. Shapter, RSC Adv., 2015, 5, 38851–38858 RSC
.
- T.-H. Han, Y. Lee, M.-R. Choi, S.-H. Woo, S.-H. Bae, B. H. Hong, J.-H. Ahn and T.-W. Lee, Nat. Photonics, 2012, 6, 105–110 CrossRef CAS
.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed
.
- P. W. Sutter, J.-I. Flege and E. A. Sutter, Nat. Mater., 2008, 7, 406–411 CrossRef CAS PubMed
.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed
.
- G. Eda, Y.-Y. Lin, S. Miller, C.-W. Chen, W.-F. Su and M. Chhowalla, Appl. Phys. Lett., 2008, 92, 233305 CrossRef
.
- C.-Y. Su, A.-Y. Lu, Y. P. Xu, F.-R. Chen, A. N. Khlobystov and L.-J. Li, ACS Nano, 2011, 5, 2332–2339 CrossRef CAS PubMed
.
- D. Sun, L. Jin, Y. Chen, J. R. Zhang and J. J. Zhu, ChemPlusChem, 2013, 78, 227–234 CrossRef CAS
.
- Z. Y. Xia, G. Giambastiani, C. Christodoulou, M. V. Nardi, N. Koch, E. Treossi, V. Bellani, S. Pezzini, F. Corticelli, V. Morandi, A. Zanelli and V. Palermo, ChemPlusChem, 2014, 79, 439–446 CrossRef CAS
.
- K. S. Rao, J. Sentilnathan, H.-W. Cho, J.-J. Wu and M. Yoshimura, Adv. Funct. Mater., 2015, 25, 298–305 CrossRef CAS
.
- K. S. Rao, J. Senthilnathan, Y. F. Liu and M. Yoshimura, Sci. Rep., 2014, 4, 4237 Search PubMed
.
- Y. Zhang, L. Chen, W. Yang, J. Ou, B. Zheng, H. Yuan, Y. Guo and D. Xiao, RSC Adv., 2013, 3, 12758–12764 RSC
.
- P. Mahanandia, F. Simon, G. Heinrich and K. K. Nanda, Chem. Commun., 2014, 50, 4613–4615 RSC
.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS
.
- W. Gao, L. B. Alemany, L. Ci and P. M. Ajayan, Nat. Chem., 2009, 1, 403–408 CrossRef CAS PubMed
.
- I. K. Moon, J. Lee, R. S. Ruoff and H. Lee, Nat. Commun., 2010, 1, 73 Search PubMed
.
- Y. Liang, J. Frisch, L. Zhi, H. Norouzi-Arasi, X. Feng, J. P. Rabe, N. Koch and K. Mullen, Nanotechnology, 2009, 20, 434007 CrossRef PubMed
.
- M. Baghbanzadeh, L. Carbone, P. D. Cozzoli and C. O. Kappe, Angew. Chem., Int. Ed., 2011, 50, 11312–11359 CrossRef CAS PubMed
.
- D. H. Youn, J.-W. Jang, J. Y. Kim, J. S. Jang, S. H. Choi and J. S. Lee, Sci. Rep., 2014, 4, 5492 CAS
.
- X. Liu, J. Liu, D. Zhan, J. Yan, J. Wang, D. Chao, L. Lai, M. Chen, J. Yin and Z. Shen, RSC Adv., 2013, 3, 11601–11606 RSC
.
- Y. M. Shulga, S. A. Baskakov, E. I. Knerelman, G. I. Davidova, E. R. Badamshina, N. Y. Shulga, E. A. Skryleva, A. L. Agapov, D. N. Voylov, A. P. Sokolov and V. M. Martynenko, RSC Adv., 2014, 4, 587–592 RSC
.
- Y. N. Chen, Z. J. Wang, T. Yang and Z. D. Zhang, Acta Mater., 2014, 71, 1–10 CrossRef
.
- P. N. Nirmalraj, T. Lutz, S. Kumar, G. S. Duesberg and J. J. Boland, Nano Lett., 2011, 11, 16–22 CrossRef CAS PubMed
.
- B. Yang, Y. Guo, S. Zhang, T. Wen and C. Zhao, RSC Adv., 2014, 4, 64771–64780 RSC
.
- X. Liu, D. Zhan, D. Chao, B. Cao, J. Yin, J. Zhao, Y. Li, J. Lin and Z. Shen, J. Mater. Chem. A, 2014, 2, 12166–12170 CAS
.
- T. Kuila, A. K. Mishra, P. Khanra, N. H. Kim and J. H. Lee, Nanoscale, 2013, 5, 52–71 RSC
.
- Z. Li, Y. Yao, Z. Lin, K.-S. Moon, W. Lin and C. Wong, J. Mater. Chem., 2010, 20, 4781–4783 RSC
.
- D. Chen, L. Li and L. Guo, Nanotechnology, 2011, 22, 325601 CrossRef PubMed
.
- P. Tang, G. Hu, Y. Gao, W. Li, S. Yao, Z. Liu and D. Ma, Sci. Rep., 2014, 4, 5901 CAS
.
- A. de la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164–178 RSC
.
- J. Wang, M. Liang, Y. Fang, T. Qiu, J. Zhang and L. Zhi, Adv. Mater., 2012, 24, 2874–2878 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |