Photoelectrocatalytic activity of flexible PEDOT–PSS/silicon carbide nanowire films†
Xin Liaoa,
Zhaoxiang Liua,
Lijuan Dinga,
Jianjun Chen*ab and
Weihua Tang*c
aEngineering Research Center for Eco-Dyeing & Finishing of Textiles, Ministry of Education, Zhejiang Sci-Tech University, Hangzhou 310018, China. E-mail: chen@zstu.edu.cn
bDepartment of Materials Forming and Control Engineering, College of Machinery and Automation, Zhejiang Sci-Tech University, Hangzhou 310018, China
cSchool of Science, Beijing University of Posts and Telecommunications, Beijing 100876, China
First published on 13th November 2015
Abstract
A novel flexible PEDOT/PSS film composite with a laterally bridged SiC nanowire network structure was constructed. Photocatalytic and photoelectrochemical hydrogen evolution activities of SiC–PEDOT/PSS in water were investigated. The fast-response, high sensitivity and continuous stability of the composite film were also demonstrated under simulated sunlight irradiation. The flexible film photoelectrode exhibits improved cathodic current density (1.61 mA cm−2 at 1.0 V vs. Ag/AgCl) under simulated sunlight irradiation, which is higher than that of the pristine SiC nanowire film. It also shows an enhanced hydrogen production rate. The enhanced performance of the SiC–PEDOT/PSS can be ascribed to efficient charge separation resulting from the high hole-mobility of PEDOT/PSS. The novel materials, with a flexible semiconductor nanowire network as the framework support of the organic semiconductor films, would provide a promising composite structure for the new organic–inorganic flexible photoelectrocatalytic films.
Introduction
Photocatalytic and photoelectrochemical hydrogen evolution using semiconductor-based materials have become promising approaches to address clean fuel issues.1–6 As a well-known wide bandgap semiconductor, silicon carbide (SiC) has attracted considerable attention in solar light water splitting due to its high chemical stability, high electron mobility and appropriate band gap (2.4 eV for 3C–SiC).6–8 However, a nonnegligible factor impeding the photocatalytic and photoelectrochemical efficiency is the poor control over the recombination of photo-generated charge carriers, which mainly happens during the excitons diffusion in the bulk as well as on the photocatalyst surface.9 A great number of solutions were investigated to suppress the charge recombination. For example, Ma et al.10 found the p-SiC film on p-Si substrate can generate a cathodic photocurrent, which corresponds to hydrogen production. Wang et al.11 reported that the H2 evolution rate of platinum nanoparticle-decorated SiC nanowire photocatalyst is higher than that of pristine SiC. Zhou and co-workers prepared metal-free multi-walled carbon nanotube–SiC nanowire heterostructures with high photocatalytic capacity.12 Recently, conjugated polymer materials have been recognized to be promising photocatalysts.13–15 Difference from above solutions, a new alternative way is to integrate organic and inorganic materials to constitute photocatalysts. For instance, Liao and co-workers prepared a photonic crystal coupled TiO2/P3HT photocatalyst.16 Fu et al.17 reported a novel C3N4–CdS organic–inorganic heterojunction. Nanda et al.18 developed an organic–inorganic thin film photocatalyst by coupling P3HT on CdS.Poly(3,4-ethylenedioxythiophene) poly(styrene sulfonate) (PEDOT/PSS) conjugated polymer is interesting as a new candidate for applications in solar cells, fuel cells and photocatalysis materials, owing to its advantages of excellent electrical and optical properties, high hole mobility and processability.19–23 In our previous study, a novel flexible SiC nanowire paper was synthesized by a simple suction filtration approach.24 Herein, we report a novel flexible photoelectrocatalytic film produced by the netlike SiC nanowire framework and PEDOT/PSS film. The composite of SiC with PEDOT/PSS may give rise to an interface that may lead to the enhancement of the exciton dissociation and charge collection processes. The photocatalytic and photoelectrochemical activities of the SiC–PEDOT/PSS show improved hydrogen production ability under simulated solar light irradiation.
Experimental
Preparation of SiC–PEDOT/PSS flexible films
SiC nanowires (SiC NWs) were synthesized by sol–gel carbothermal reduction approach.25 The SiC NWs were mixed with PEDOT/PSS isopropanol solution containing 1 wt%, 4.5 wt% and 10 wt% of PEDOT/PSS respectively. The solutions were evaporated by heating magnetic stirrer to form slurries. The slurries were then drop-casted separately onto ITO-PET substrates and dried at room temperature.Characterization
X-ray diffraction (XRD) patterns of the catalysts were measured by Bruker diffractometer (D8-Discover, German) using Cu Kα radiation (λ = 0.15405 nm). Scanning electron microscopy (SEM) images and energy dispersive spectrometer (EDS) analysis of the samples were observed by a FE-SEM (S-4800, Hitachi, Japan). Transmission electron microscopy (TEM) analyses were measured by Jeol JEM-2010 HR (Jeol, Tokyo, Japan). X-ray photoelectron spectroscopy (XPS) spectra were carried out using an ESCALAB 250Xi machine (ThermoFisher Scientific, USA). The Fourier transform infrared (FT-IR) spectra (KBr pellets) were recorded on a Bruker Tensor 27 spectrometer. UV-Vis diffuse reflection spectra (DRS) were investigated on a UV-Vis spectrometer (U3900, Hitachi) at room temperature. The photoluminescence (PL) spectra were observed by a fluorescence spectrometer (F-4600, Hitachi) at room temperature by the excitation wavelength of 340 nm.Photoelectrochemical measurements
The photoelectrochemical analyses were accomplished by conducting a three-electrode electrochemical configuration (CHI660 electrochemical workstation, Shanghai Chenhua Instruments, China) at scan rate of 10 mV s−1 in 0.1 mol L−1 Na2S and 0.1 mol L−1 Na2SO3 solution. A 300 W Xe lamp without a UV-light filter served as the light source. The flexible films (ITO-PET substrates, 1.5 cm × 1.2 cm) were used as the working electrodes. Ag/AgCl electrode and platinum foil were employed as the reference electrode and counter electrode, respectively. The incident photo to current conversion efficiency (IPCE) spectra was measured with interval wavelength of 20 nm.Photocatalytic measurements
The photocatalytic hydrogen evolution reactions of the products were carried out in a photocatalytic hydrogen evolution system with a 300 W Xe lamp (NBeT, HSX-F300, Beijing). The photocatalyst (20 mg) was dispersed in a quartz glass reactor containing 100 mL of 0.1 M Na2S and 0.1 M Na2SO3 solution. The evolved gases were analyzed online with a gas chromatograph (GC-9790, TCD, with N2 as carrier gas). The temperature of the reactor was maintained at room temperature by a circulating water system during the reaction.Results and discussion
The crystal structure of the as-synthesized products was measured by XRD. Fig. S1† shows the XRD patterns and digital photo of the as-prepared samples. No significant change of diffraction peaks was observed in the XRD pattern (bottom, red line) of the SiC–PEDOT/PSS film. It is clear that the addition of PEDOT/PSS has no effect on the crystal structure of SiC.The morphology of the SiC–PEDOT/PSS composite was observed by SEM. Fig. 1A shows the pristine SiC consisting of nanowires with an average diameter of 100 nm. The SiC nanowires have high purity and uniformity, intertwisting together to form a three-dimensional network-like structure. The stacking faults and microtwins can be clearly observed on the surface of SiC nanowires (Fig. S2†). Fig. 1B shows the smooth and flat morphology of PEDOT/PSS. From Fig. 1C and D, it is evident that the PEDOT/PSS are uniformly distributed at SiC NW network. The three-dimensional network of nanowires was laterally bridged by the PEDOT/PSS film. The novel laterally bridged SiC nanowire network and PEDOT/PSS film composite structure was constructed. The inset of Fig. 1D is the EDS of the SiC–PEDOT/PSS composite, which indicates the existence of PEDOT/PSS.
 | ||
| Fig. 1 SEM images of pristine SiC NWs (A), PEDOT/PSS (B) and SiC–4.5 wt%-PEDOT/PSS thin film (C and D); the inset of (D) is the EDS spectrum. | ||
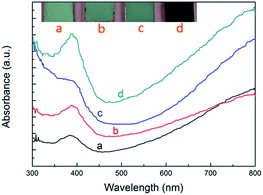
The optical absorption properties of the products were investigated using UV-Vis absorption spectroscopy. Fig. 2 and S3† show the UV-Vis absorption spectra of the pristine SiC, the pristine PEDOT/PSS and all the SiC–PEDOT/PSS samples. As Fig. 2 depicted, all the samples exhibit an obvious absorption in ultraviolet. In addition, the absorption intensity of SiC–PEDOT/PSS composites is also enhanced with the increase of the ratios of PEDOT/PSS, which can be reflected by the gradual colour change of the composites from pale green (pristine SiC) to dark blue (SiC–PEDOT/PSS).
Fig. S4† shows the FT-IR spectrum of the as-synthesized samples. Obviously, several new bands can be observed after the addition of PEDOT/PSS. XPS analyses of the thin film samples were also carried out. Fig. S5† depicts the XPS spectra of S 2p of the PEDOT/PSS. The peaks at the binding energy (BE) values of 163.3 and 164.4 eV are due to sulphur atoms in PEDOT, while the BE values of 167.4 eV and 168.5 eV can be assigned to neutral and ionic sulfur in PSS, respectively.26,27 Moreover, the BE values of S in the SiC–4.5 wt%-PEDOT/PSS shift toward higher binding energy than that of the pristine PEDOT/PSS, implying that the surrounding electrons of S are transferred to other atoms.
To investigate the photoelectrochemical properties of the pristine SiC nanowire film and the novel SiC nanowire–PEDOT/PSS composite film photoelectrodes, the linear sweep voltammograms (I–V) were measured by an aqueous electrolyte solution containing 0.1 M Na2S and 0.1 M Na2SO3 in a three electrode system. As described in Fig. 3, it is clear that the SiC–4.5 wt%-PEDOT/PSS photoelectrode exhibits the improved cathodic current density (1.61 mA cm−2 at 1.0 V vs. Ag/AgCl) under the simulated solar light irradiation, which is higher than that of the pristine SiC nanowire film (1.01 mA cm−2 at 1.0 V vs. Ag/AgCl). Moreover, the onset potential of SiC–PEDOT/PSS under simulated light irradiation is also shift to −0.35 V (vs. Ag/AgCl), lower than that of the pristine SiC (−0.45 V vs. Ag/AgCl). Compared with the pristine SiC nanowire film, the novel SiC nanowire–PEDOT/PSS composite film exhibits the enhanced separation ability of photogenerated charges and high performance photoelectrochemical activity. The different concentrations of PEDOT/PSS used for loading on SiC nanowires were also investigated. SiC–4.5 wt%-PEDOT/PSS possesses higher current density under the light irradiation than that of SiC–1 wt%-PEDOT/PSS (1.36 mA cm−2 at 1.0 V vs. Ag/AgCl) and SiC–10 wt%-PEDOT/PSS (1.51 mA cm−2 at 1.0 V vs. Ag/AgCl) (Fig. S6†). Additionally, the I–V curve of bare PEDOT/PSS was also measured by the same method. The pristine SiC shows higher cathodic current density under simulated solar light irradiation than that of the PEDOT/PSS (0.79 mA cm−2 at 1.0 V vs. Ag/AgCl), as the Fig. S6† showed.
 | ||
| Fig. 3 Current density of the pristine SiC (A) and the SiC–4.5 wt%-PEDOT/PSS (B) photoelectrodes under dark and simulated solar light in Na2S/Na2SO3 electrolyte. | ||
To evaluate the photoresponse and stability of the flexible photoelectrodes, current vs. time (I–t) curves were investigated. The SiC–PEDOT/PSS photoelectrode was studied using chopped light at a constant potential at 0 V (vs. Ag/AgCl) in Na2S/Na2SO3 solution. As Fig. 4A showed, the SiC–4.5 wt%-PEDOT/PSS photoelectrode had a high photocurrent with the light illumination, while the photocurrent declined after the light was switched off. Fig. 4B presents the current density of the pristine SiC and SiC–4.5 wt%-PEDOT/PSS photoelectrodes at 0.9 V (vs. Ag/AgCl) under the simulated light illumination. A larger current was observed with the SiC–PEDOT/PSS photoelectrode. After 4000 s of continuous irradiation, the two photoelectrodes still keep the stable current density, revealing the photoelectrodes possess high photoelectrochemical stability. In addition, the IPCE spectra of the pristine SiC and SiC–4.5 wt%-PEDOT/PSS were estimated with interval wavelength of 20 nm (Fig. S7†). As expected, the significant improvement can be observed in the SiC–4.5 wt%-PEDOT/PSS.
Fig. 5 shows the photocatalytic hydrogen production yield of the pristine SiC NWs and SiC–4.5 wt%-PEDOT/PSS under simulated solar light. Obviously, the SiC–4.5 wt%-PEDOT/PSS shows a higher hydrogen generation rate (100.7 μmol g−1 h−1), which is roughly 1.5 times than that of the pristine SiC (67.5 μmol g−1 h−1). The photocatalytic test of the pristine PEDOT/PSS showed no hydrogen production can be detected. It has no ability of photocatalytic activity. The stability of the SiC–PEDOT/PSS as hydrogen generation photocatalysts was evaluated by the cyclic test for 15 h (Fig. S7†), and there is no apparent decrease of H2 evolution for the SiC–PEDOT/PSS after three cyclic tests. It demonstrated that the SiC–PEDOT/PSS exhibits high stability during photocatalytic H2 evolution.
 | ||
| Fig. 5 H2 evolution (A) and average H2 production rates (B) of the pristine SiC and the SiC–4.5 wt%-PEDOT/PSS photocatalysts. | ||
To investigate the decrease of electron–hole pair recombination, the PL spectra were measured (Fig. S8†). There is a better quenching of fluorescence in the SiC–PEDOT/PSS photocatalysts. The PL result indicated the decreased recombination of photoexcited charge carriers in the case of SiC–PEDOT/PSS. A proposed mechanism of the photocatalytic hydrogen production under simulated solar light in a photocatalyst suspension hydrogen generation system is shown in Fig. S9.† When the photocatalyst is exposed to the simulated solar light, the photogenerated electrons get drafted toward SiC leaving holes in PEDOT/PSS due to hole conductor capacity of the organic, which results in hindrance of photogenerated electron–hole pairs recombination.23 Accordingly, the photo-excited electrons transfer to the reduction of H+ to H2.
Conclusion
In summary, SiC nanowires–PEDOT/PSS organic–inorganic composite films have been designed. Compared with the pristine SiC nanowire film, the novel composite thin films exhibit enhanced photocurrent density and improved hydrogen production rate. The excellent performance of the composite films in the photoelectrochemical and photocatalytic activities may be ascribed to the efficient carriers separation brought by the important function of the PEDOT/PSS as a hole-transporting polymer. The novel materials, with the flexible semiconductor nanowire network as the framework support of the organic semiconductor films, would open a promising approach for new organic–inorganic flexible materials design.Acknowledgements
This work is supported by the National Nature Science Foundation of China (No. 51572243), the Zhejiang Provincial Natural Science Foundation (No. LY15E020012), 521 Talent Training Plan of Zhejiang Sci-Tech University, the Young Researchers Foundation of Zhejiang Provincial Top Key Academic Discipline of Chemical Engineering and Technology, Zhejiang Sci-Tech University (ZYG2015006) and Cultivation Fund Program for Excellent Dissertation, Zhejiang Sci-Tech University (2015YSPY05).Notes and references
- Q. Xiang, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575–6578 CrossRef CAS PubMed.
- S. Wang, Z. Zheng, B. Huang, Z. Wang, Y. Liu, X. Qin, X. Zhang and Y. Dai, RSC Adv., 2013, 3, 5156–5161 RSC.
- C. X. Zhao, H. Luo, F. Chen, P. Zhang, L. H. Yi and K. Y. You, Energy Environ. Sci., 2014, 7, 1700–1707 CAS.
- L. C. Pop, I. Tantis and P. Lianos, Int. J. Hydrogen Energy, 2015, 40, 8304–8310 CrossRef CAS.
- R. Peng, D. Zhao, J. Baltrusaitis, C. M. Wu and R. T. Koodali, RSC Adv., 2012, 2, 5754–5767 RSC.
- Y. Li, Z. M. Yu, J. L. Meng and Y. D. Li, Int. J. Hydrogen Energy, 2013, 38, 3898–3904 CrossRef CAS.
- C. He, X. Wu, J. Shen and P. K. Chu, Nano Lett., 2012, 12, 1545–1548 CrossRef CAS PubMed.
- J. J. Chen, J. D. Zhang, M. M. Wang, L. Gao and Y. Li, J. Alloys Compd., 2014, 605, 168–172 CrossRef CAS.
- F. E. Osterloh, Chem. Mater., 2007, 20, 35–54 CrossRef.
- Q. B. Ma, B. Kaiser and W. Jaegermann, J. Power Sources, 2014, 253, 41–47 CrossRef CAS.
- M. M. Wang, J. J. Chen, X. Liao, Z. X. Liu, J. D. Zhang, L. Gao and Y. Li, Int. J. Hydrogen Energy, 2014, 39, 14581–14587 CrossRef CAS.
- X. F. Zhou, X. Li, Q. Z. Gao, J. L. Yuan, J. Q. Wen, Y. P. Fang, W. Liu, S. S. Zhang and Y. J. Liu, Catal. Sci. Technol., 2015, 5, 2798–2806 CAS.
- S. Ghosh, N. A. Kouamé, L. Ramos, S. Remita, A. Dazzi, A. Deniset-Besseau, P. Beaunier, F. Goubard, P. H. Aubert and H. Remita, Nat. Mater., 2015, 14, 505–511 CrossRef CAS PubMed.
- Q. Z. Luo, L. L. Bao, D. S. Wang, X. Y. Li and J. An, J. Phys. Chem. C, 2012, 116, 25806–25815 CAS.
- B. Muktha, G. Madras, T. N. Guru Row, U. Scherf and S. Patil, J. Phys. Chem. B, 2007, 111, 7994–7998 CrossRef CAS.
- G. Z. Liao, S. Chen, X. Quan, H. Chen and Y. B. Zhang, Environ. Sci. Technol., 2010, 44, 3481–3485 CrossRef CAS PubMed.
- J. Fu, B. B. Chang, Y. L. Tian, F. N. Xi and X. P. Dong, J. Mater. Chem. A, 2013, 1, 3083–3090 CAS.
- K. K. Nanda, S. Swain, B. Satpati, L. Besra, B. Mishra and Y. S. Chaudhary, ACS Appl. Mater. Interfaces, 2015, 7, 7970–7978 CAS.
- D. Antiohos, G. Folkes, P. Sherrell, S. Ashraf, G. G. Wallace, P. Aitchison, A. T. Harris, J. Chen and A. I. Minett, J. Mater. Chem., 2011, 21, 15987–15994 RSC.
- X. Crispin, F. L. E. Jakobsson, A. Crispin, P. C. M. Grim, P. Andersson, A. Volodin, C. van Haesendonck, M. van der Auweraer, W. R. Salaneck and M. Berggren, Chem. Mater., 2006, 18, 4354–4360 CrossRef CAS.
- C. Teng, X. Lu, Y. Zhu, M. Wan and L. Jiang, RSC Adv., 2013, 3, 7219–7223 RSC.
- A. J. Mozer, D. K. Panda, S. Gambhir, B. Winther-Jensen and G. G. Wallace, J. Am. Chem. Soc., 2010, 132, 9543–9545 CrossRef CAS PubMed.
- Z. Xing, Z. G. Chen, X. Zong and L. Z. Wang, Chem. Commun., 2014, 50, 6762–6764 RSC.
- J. J. Chen, X. Liao, M. M. Wang, Z. X. Liu, L. J. Ding, J. D. Zhang, L. Gao and Y. Li, Nanoscale, 2015, 7, 6374–6379 RSC.
- L. P. Xin, Q. Shi, J. J. Chen, W. Tang, N. Wang, Y. Liu and Y. Lin, Mater. Charact., 2012, 65, 55–61 CrossRef CAS.
- J. Y. Kim, J. H. Jung, D. E. Lee and J. Joo, Synth. Met., 2002, 126, 311–316 CrossRef CAS.
- G. Greczynski, T. Kugler, M. Keil, W. Osikowicz, M. Fahlman and W. R. Salaneck, J. Electron Spectrosc. Relat. Phenom., 2001, 121, 1–17 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19193a |
| This journal is © The Royal Society of Chemistry 2015 |