Cellulose-immobilized NHC–Cu(i) complex: an efficient and reusable catalyst for multicomponent synthesis of 1,2,3-triazoles†
Abstract
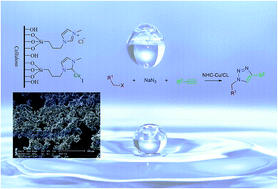
A novel cellulose supported copper NHC complex has been prepared by the reaction of cellulose supported imidazolium salt with copper(I) iodide. The catalyst is active in the synthesis of 1,2,3-triazoles via a one-pot reaction of alkyl/benzyl halides or tosylates and terminal alkynes, with sodium azide in water.

 Please wait while we load your content...
Please wait while we load your content...