Laser-induced synthesis of Ag nanoparticles on the silanized surface of a fiber taper and applications as a SERS probe†
Jie Cao*,
Di Zhao and
Qinghe Mao
Anhui Provincial Key Lab of Photonics Devices and Materials, Anhui Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Hefei 230031, China. E-mail: candj@mail.ustc.edu.cn
First published on 13th November 2015
Abstract
A surface-enhanced Raman scattering (SERS) tapered fiber probe was prepared by silanization of the surface of an optical fiber with 3-aminopropyltrimethoxysilane (APTMS) and subsequent laser-induced synthesis of Ag nanoparticles (AgNPs) on the silanized fiber taper. We revealed that the fiber taper after silanization can anchor AgNPs at far higher particle coverage density and faster anchoring rate in the following laser-induced reaction than the naked fiber taper. The SERS activity of the fiber probe was demonstrated with the detection of 4-aminothiophenol (4-ATP) aqueous solution. The experimental results indicate that the silanized fiber probe with the optimal laser-induced time exhibited very strong SERS activity and good measurement reproducibility.
Surface-enhanced Raman scattering (SERS) has attracted great interest due to its high sensitivity, specificity, and fingerprint effect in the detection and identification of trace chemicals, leading to important applications in the fields of chemistry, biology and environmental science.1–3 As is well known, the SERS effect relies on the enhancement of electromagnetic fields around nanostructured metal surfaces (typically silver or gold) to amplify the weak Raman signal of molecules adsorbed on nano-metal.4 Usually, the SERS spectrum is measured using SERS substrate with noble metal nanoparticles placed on a planar platform such as glass slide,4 copper foil,5 and silicon wafer.6 Compared with the conventional planar substrates, the combination of SERS and optical fiber for sensing and detection takes full advantage of the features of each: ultra-high sensitivity and specificity from the former, and immunity to electromagnetic interference and easy deployment even to remote or hazardous environment from the latter.7 Moreover, optical fibers provide an attractive platform for SERS sensing because they can simplify the optics systems and enhance the SERS excitation and collection efficiencies while being relatively cheap and easily obtainable.8
Generally, fiber SERS probes are applied to molecular detection and identification using a so-called “optrode” configuration,9 in which a single optical fiber transmits both the excitation light to the sample and the backscattered SERS signal to the spectrometer; a SERS-active layer usually consisting of metal nanoparticles or a metal film is placed directly at the end of the excitation fiber. To obtain a high SERS enhancement activity in a fiber SERS probe, the improvements are generally made by optimizing the geometry of the fiber tips and tailoring of the SERS-active layer. To date, fiber SERS probes with different geometries, including flat,10 angled,11 or tapered fiber,12 have been explored in order to improve the SERS detection sensitivity. Among them, the tapered fiber probe has the advantages of high light transmission efficiency and large interaction areas for excitation light and SERS material.13 Meanwhile, various methods have been successfully used for preparing SERS-active layers on the optical fiber tips include noble metal evaporation or sputtering,13,14 assembly of metal colloidal nanoparticles,15 metal integrated onto the fiber by autoclave reaction method,16 etc.
As another option, photo-induced methods have been developed to deposit silver or gold nanoparticles on a glass slide or silica, which can serve as stable and reproducible SERS substrates.17 Bjerneld et al. demonstrated laser-induced growth and deposition of silver nanoparticles at the interface of a glass slide and a silver ion solution at the focus of a laser beam.18 In 2008, Zheng et al. made a fiber SERS probe by photochemical deposition of AgNPs on a fiber tip, and this provides a low-cost and effective route for preparing fiber SERS probes.19 However, none has attempted to apply laser-induced method to the direct nucleation and growth of noble metal nanoparticles on the tapered fiber silanized with APTMS, aiming at SERS application in an “optrode” detection mode.
Herein, we present fiber SERS probes prepared by laser-induced synthesis of AgNPs on the fiber taper silanized with APTMS. Compared with the naked fiber taper, the silanized fiber taper can speed up the anchoring rate of AgNPs and result in far higher particle coverage density. With 4-ATP as the target molecule, the silanized fiber probe with the optimal laser-induced time exhibited very strong SERS activity and good measurement reproducibility.
The fiber used in the experiment is the common multimode silica optical fiber with a core diameter of 200 μm and a cladding diameter of 220 μm. The naked fiber tapers were prepared using a dynamic hydrofluoric acid etching method.20 After etching, the tapered fiber was cleaned with deionized water. To active the surface of the optical fiber, the naked fiber tapers were immersed in piranha solution (1 volume of 30% H2O2 and 3 volumes of concentrated H2SO4) for 5 h and washed thoroughly with deionized water. The piranha solution here could remove the retained plastic cladding and supply hydroxyl groups to the surface of the fiber tapers, as shown in Fig. 1 (left). Then, we introduce amino groups onto the hydroxylated optical fiber by silanization of the fiber tapers in a solution (30% v/v of APTMS (97%), 40% v/v of methanol and 30% v/v of deionized water) for 16 h under inert N2 purging. Due to the affinity of metal toward amino groups of APTMS, the fiber silanized with APTMS can capture the metal nuclei in the following laser-induced reaction.
The experimental setup for the laser-induced synthesis of AgNPs on the silanized fiber taper is displayed in Fig. 1. A 785 nm semiconductor laser is used as the laser source for laser-induced reaction. The output laser power was fixed at 100 mW and coupled into one cleaved end of the fiber. Then, the other end (silanized fiber taper) was immersed in a reaction solution (mixing 0.005 M AgNO3 and 0.005 M trisodium citrate aqueous solution in a volume ratio of 1 : 1) with different laser-induced time. After that, the fiber SERS probe was created and cleaned with water before being used. The general morphology of the probe was characterized using field-emitting scanning electron microscope (FESEM, Quanta-200, FEI, USA). Optical micrographs were taken using digital optical microscope (model XHC-MV2). The surface plasmon resonance (SPR) spectrum of the AgNPs scraped off from the silanized fiber probe was measured by the UV-Vis-NIR spectrophotometer (LAMBDA 950).
To test the performances of the fiber SERS probes, we took 4-ATP (10−7 M) as a target molecule and detected their SERS spectra in an “optrode” detection mode (Fig. 1, right). The SERS excitation source used in the experiment was the same laser source as the laser-induced reaction mentioned above. The excitation light was coupled into the tapered fiber probe from its cleaved endface. The tapered portion of the probe has SERS active structures and was immersed in the analyte solution (4-ATP aqueous solution). The tapered SERS probes were back excited by the laser beam and the resulting SERS signal was coupled into the fiber, traveled backwards and detected using a commercial Raman spectrometer (B&W TEK Opto-electronics, MiniRam). All spectra were collected by using the same accumulation time of 2 s, and using the excitation laser power of 20 mW. In our experiments, the background Raman spectrum introduced by the naked fiber taper is shown in Fig. S1† (curve 1), and the silica fiber Raman appears because of guiding of the light in the fiber core. The Raman spectrum of the silanized fiber probe with the citrate ions and APTMS molecules hung on is shown in Fig. S1† (curve 2). Apart from the Raman background of the silica fiber, no remarkable peaks derived from the citrate ions and APTMS molecules can be observed in the entire spectrum. In order to exclude interference, before the 4-ATP molecules testing, such signals were recorded as the background noise. All the SERS spectra were baseline corrected to remove the background noise.
Fig. 2(a) shows optical micrograph of naked fiber taper produced by hydrofluoric acid etching method. From the photograph, one can clearly see that the fiber diameter is decreasing gradually with etching rate gradient, generating the conical shape of the tip. The optical micrograph of the silanized SERS probe prepared by silanization optical fiber with APTMS and subsequent laser-induced reaction for 20 min is shown in Fig. 2(b). As can be seen, the obvious contrast between the silanized SERS probe [Fig. 2(b)] and the naked tapered optical fiber [Fig. 2(a)] confirms that AgNPs can be anchored onto the entire surface of the fiber taper. The low magnification SEM image [Fig. 2(c)] gives an overview of the silanized SERS probe and represents that it keeps the perfect tapered structure after the whole preparation procedure. From a closer observation [Fig. 2(d)], it can be seen clearly that the surface of the tapered fiber is covered by the AgNPs. They are evenly distributed on the taper surface with average size of 100 nm. The UV-Vis-NIR spectrum of the AgNPs is given in Fig. S2,† which indicates that the main absorption peak is around 460 nm, corresponding to the SPR of the AgNPs.
For comparison, we fabricated non-silanized SERS probes A and B (as shown in Fig. 3(a) and (b)), which were obtained in the reaction solution using the laser-induced method to direct deposition of AgNPs on the naked fiber tapers with different deposition times of 20 min and 60 min respectively. It should be pointed out that the laser power spread out of the fiber taper and the reaction solution concentration were both kept the same as those used in the preparation of the silanized fiber probe. Compared with the silanized SERS probe [Fig. 2(b)], only the distal end of the probe A is covered by AgNPs at the same laser-induced time of 20 min. Although the AgNPs can deposit onto the entire surface of the non-silanized fiber taper when the laser-induced time is extended to 60 min (probe B), the density of the AgNPs is less than the silanized fiber taper with the laser-induced time of 20 min [Fig. 2(b)]. We used them for further SERS detection. The peaks observed in the SERS spectra of the 4-ATP molecule [Fig. 3(c)] are in good agreement with the literature.21,22 The intensity of the SERS characteristic peak at 1076 cm−1 obtained from the silanized SERS probe (curve 3) is 5.3 and 3.2 times stronger than those detected by the probe A (curve 1) and probe B (curve 2), respectively. All of the above reveals that the silanized fiber surface can accelerate the growth process of AgNPs and anchor vast AgNPs, which allows for strong SERS enhancement effect and exhibits higher detection sensitivity.
The high particle coverage density and fast anchoring rate of AgNPs on the silanized fiber probe can be attributed to the principle of electrostatic attraction. The AgNPs are usually prepared by chemical reduction of Ag(I) salts and can be stabilized by coating with various ligands, which determine the surface charges (either negative or positive) of the AgNPs.23,24 As proved in previous studies, the AgNPs are synthesized in the presence of trisodium citrate, which present the negative-charged surface due to the citrate anions as a stabilizing agent.25,26 On the other hand, APTMS with bifunctional groups can be readily modified onto the silica fiber surface by silanol groups, and provide positively charged surface due to the protonated amino groups, similar to the protonation process described previously.27 As the laser-induced reaction proceeds, citrate-coated AgNPs can be anchored on the APTMS-silanized fiber surface because of the strong electrostatic attraction between the citrate-coated AgNPs with negative charges and APTMS with positive charges. As a result, it is found that AgNPs can be anchored over the entire fiber taper and they are closely packed on the taper surface (see Fig. 2).
Fig. 4 shows the optical micrograph of silanized fiber SERS probes prepared at an inducing laser power of 100 mW in a reaction solution [1 : 1 (v/v) silver nitrate/trisodium citrate, 0.005 M] after different laser-induced times of 10, 20, 30 and 60 min, respectively. As can be seen, when the reaction time is 10 min, only the distal end of the tapered fiber is covered by AgNPs. With the increase of the reaction time, the coverage area of AgNPs spreads from the tip of the taper toward the base of the taper. When the time is 20 min, the AgNPs are already uniformly distributed over the whole fiber taper surface. As the laser-induced time was lengthened from 20 to 30 and 60 min, the tapered portion where the excitation light can leakage from the silica core to interact with the sample were also completely covered by a layer of nanoparticles, and only the thickness of AgNPs anchored on the fiber tapers increased. Based on above analysis, we can know that the densely packed AgNPs uniformly anchored and completely covered the taper surface of the fiber when the laser-induced time reached 20 min. SEM images of AgNPs on the silanized fiber probes (Fig. S3†) show average particle sizes of 55, 160, and 230 nm for laser-induced times of 10, 30, and 60 min, respectively. As discussed above, the silanized fiber probe with laser-induced time of 20 min corresponds to AgNPs about 100 nm in size [Fig. 2(d)]. All these observations indicate that the average size of the AgNPs on the fiber SERS probes can be readily tuned from 55 to 230 nm by varying the laser-induced growth time.
The set of SERS spectra shown in Fig. 5 was obtained by the detection of 4-ATP (10−7 M) with the fiber probes silanized with APTMS and then laser-induced synthesis of AgNPs on the silanized surface of the fiber taper at different laser-induced times, that is, 10, 20, 30, and 60 min. As can be seen, the SERS signals of 4-ATP obtain by these fiber SERS probes were greatly enhanced. The inset in Fig. 5 shows the intensity at 1076 cm−1 changed as a function of laser-induced time. We can conclude that the fiber SERS probe possesses optimum SERS activity at a laser-induced time of 20 min. As expected, low surface coverage area and overly thick Ag layers are both negative to the high SERS signal. The small coverage area causes fewer AgNPs and a small contribution to SERS enhancement. An overly thick Ag layer would lead to a large light loss, blocking the excited light from arriving in the target molecular and also forbidding SERS signal collection. Therefore, the optimal laser-induced time for the silanized fiber probe is 20 min when other experimental conditions were kept unchanged.
One of the major concerns on the development of fiber SERS probes for practical applications is the reproducibility of the SERS signal. Fig. 6 presents the SERS spectra of 4-ATP detected by eight silanized fiber probes with laser-induced time of 20 min. As can be seen, the intensities of the SERS spectra are dispersed in a relatively narrow range. For the strongest peak at 1076 cm−1, the relative standard deviation (RSD) value of signal intensities is calculated to be approximately 8.2%, which is better than the previous reports.28,29
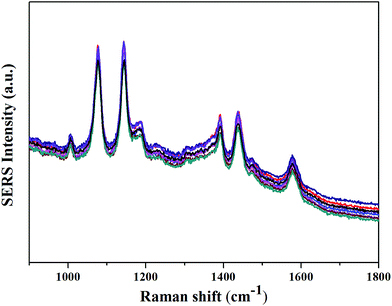 | ||
| Fig. 6 SERS spectra of 4-ATP (10−7 M) detected by eight silanized fiber probes with laser-induced time of 20 min. | ||
The enhancement factor (EF) of 4-ATP on the fiber SERS probe was estimated using the following expression:30,31
| EF = (ISERS/Nads)/(Ibulk/Nbulk) |
It is generally accepted that many factors simultaneously contribute to the SERS enhancement effect. In our case, the significant SERS enhancement of the tapered fiber probes can be explained as follows. First, the good quality SERS spectra were obtained when the SPR peak (460 nm, as shown in Fig. S2†) is deviated from the excitation wavelength (785 nm). The similar results were also observed in the previous report,34 where AgNPs on planar substrate characterized by a strong SPR between 400 and 600 nm yields extremely good SERS spectra at the excitation of 785 nm. They believed that the natural explanation of this fact is the formation of randomly distributed hot spots.34 Although the SPR of these hot spots cannot be easily determined as their proportion in the sample is low, hot spots are very optically active and can yield enhancement effect over five orders of magnitude as compared with that of regular particles.35 In our study, the AgNPs anchored on the silanized fiber probes are closely packed to form narrow interparticle gaps, which are very critical to generate hot spots with strong SERS enhancement.36,37 Second, a portion of the enhancement may be derived from the chemical effects between the 4-ATP molecules and the metal surface.33,37 Third, when transmitted into the taper region of the fiber probe, the excitation light exists partially in the form of evanescent wave, which could interact with the AgNPs on the fiber and improve the SERS excitation efficiencies.12 As a result, the strength of the SERS signal could be further enhanced by using evanescent-wave coupling technique.38 In addition, the laser energy is gradually released to the SERS-active layer as the diameter of the taper's cross section decreases, which can effectively reduce the sample damage caused by the high-power laser. Moreover, multiple reflections of the laser in the fiber taper may lead to a summation of Raman signals from the fiber-metal region and maximize SERS benefit.13
Due to the structural similarity of silane couple agents, this preparation scheme can be easily extended to fabricate more types of silanized fiber probes. Herein, 3-mercaptopropyltriethoxysilane (MPTES), and 3-aminopropyltriethoxysilane (APTES) were selected as examples, further demonstrating the interesting idea. Among them, the MPTES molecules contain thiol groups that have strong affinity to AgNPs surface through Ag–S bonds, whereas APTES can link to AgNPs surface through the similar principle of electrostatic attraction to the APTMS-silanized probe. When other experimental conditions were kept unchanged, the two types of fiber SERS probes silanized with MPTES and APTES were prepared according to the same procedure as the APTMS-silanized probe. The optical micrographs of the MPTES and APTES-silanized fiber probes are shown in Fig. 8(a) and (c), and the corresponding SEM images of AgNPs on the probes are shown in Fig. 8(b) and (d), respectively. The experiment results indicate that the growth habits of AgNPs on MPTES and APTES-silanized fiber tapers are similar to that performed by APTMS. The SERS activities of these two types of fiber probes were also investigated under the same measurement conditions, and their spectra compared with the APTMS-silanized probe are displayed in Fig. 8(e). It can be seen that all these fiber probes exhibit very strong SERS activities, but the SERS strengths of the MPTES and APTES-silanized fiber probes are slightly lower than that of the APTMS-silanized probe. Taken together, our preparation scheme employing laser-induced synthesis of AgNPs on the silanized fiber taper greatly improves the growth habit of AgNPs on the optical fiber as well as the SERS activity of the fiber SERS probe.
Conclusions
In summary, the silanized fiber SERS probe was prepared by laser-induced synthesis of AgNPs on the silanized surfaces of the fiber tapers. Compared with the fiber probes by direct deposition of AgNPs on the naked fiber tapers, this silanized fiber surface can speed up the anchoring rate of AgNPs and result in far higher particle coverage density, which allows for strong SERS enhancement effect. When tested using a 4-ATP solution, the silanized fiber probe with the optimal laser-induced time exhibited very strong SERS activity and good measurement reproducibility. Furthermore, the combination of the high sensitivity and operating in an attractive “optrode” configuration permits remote SERS sampling to be conducted, which has potential in environmental monitoring and in vivo analysis.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51301166), the National Key Basic Research Program of China (Grant No. 2013CB934304), and the Foundation of President of Hefei Institutes of Physical Science, Chinese Academy of Sciences (Grant No. YZJJ201305). We also appreciated partial support from the NSFC (Grant No. 61377044).Notes and references
- Y. Fang, N. Seong and D. D. Dlott, Science, 2008, 321, 388 CrossRef CAS PubMed.
- W. J. Cho, Y. Kim and J. K. Kim, ACS Nano, 2012, 6, 249 CrossRef CAS PubMed.
- J. Cao, D. Zhao, X. Lei, Y. Liu and Q. H. Mao, Appl. Phys. Lett., 2014, 104, 201906 CrossRef.
- H. B. Zhou, D. T. Yang, N. P. Ivleva, N. E. Mircescu, R. Niessner and C. Haisch, Anal. Chem., 2014, 86, 1525 CrossRef CAS PubMed.
- X. Chen, C. H. Cui, Z. Guo, J. H. Liu, X. J. Huang and S. H. Yu, Small, 2011, 7, 858 CrossRef CAS PubMed.
- M. Yilmaz, E. Senlik, E. Biskin, M. S. Yavuz, U. Tamer and G. Demirel, Phys. Chem. Chem. Phys., 2014, 16, 5563 RSC.
- E. Polwart, R. Keir, C. Davidson, W. E. Smith and D. A. Sadler, Appl. Spectrosc., 2000, 54, 522 CrossRef CAS.
- D. J. White and P. R. Stoddart, Opt. Lett., 2005, 30, 598 CrossRef PubMed.
- Z. Y. Chen, Z. M. Dai, N. Chen, S. P. Liu, F. F. Pang, B. Lu and T. Y. Wang, IEEE Photonics Technol. Lett., 2014, 26, 777 CrossRef CAS.
- X. W. Lan, Y. K. Han, T. Wei, Y. Zhang, L. Jiang, H. Tsai and H. Xiao, Opt. Lett., 2009, 34, 2285 CrossRef CAS PubMed.
- C. Viets and W. Hill, J. Mol. Struct., 2001, 565, 515 CrossRef.
- D. Jin, Y. X. Bai, H. G. Chen, S. P. Liu, N. Chen, J. Huang, S. J. Huang and Z. Y. Chen, Anal. Methods, 2015, 7, 1307 RSC.
- C. Viets and W. Hill, J. Mol. Struct., 2001, 563, 163 CrossRef.
- V. Guieu, D. Talaga, L. Servant, N. Sojic and F. Lagugné-Labarthet, J. Phys. Chem. C, 2009, 113, 874 CAS.
- S. P. Liu, J. Huang, Z. Y. Chen, N. Chen, F. F. Pang, T. Y. Wang and L. S. Hu, J. Raman Spectrosc., 2015, 46, 197 CrossRef CAS.
- J. Cao and J. Z. Wang, New J. Chem., 2015, 39, 2421 RSC.
- M. Muniz-Miranda, J. Raman Spectrosc., 2004, 35, 839 CrossRef CAS.
- E. J. Bjerneld, F. Svedberg and M. Käll, Nano Lett., 2003, 3, 593 CrossRef CAS.
- X. L. Zheng, D. W. Guo, Y. L. Shao, S. J. Jia, S. P. Xu, B. Zhao, W. Q. Xu, C. Corredor and J. R. Lombardi, Langmuir, 2008, 24, 4394 CrossRef CAS PubMed.
- A. Foti, C. D'Andrea, F. Bonaccorso, M. Lanza, G. Calogero, E. Messina, O. M. Maragò, B. Fazio and P. G. Gucciardi, Plasmonics, 2013, 8, 13 CrossRef CAS.
- G. S. Hong, C. Li and L. M. Qi, Adv. Funct. Mater., 2010, 20, 3774 CrossRef CAS.
- Y. W. Zhang, S. Liu, L. Wang, X. Y. Qin, J. Q. Tian, W. B. Lu, G. H. Chang and X. P. Sun, RSC Adv., 2012, 2, 538 RSC.
- A. Ivask, A. Elbadawy and C. Kaweeteerawat, et al., ACS Nano, 2014, 8, 374 CrossRef PubMed.
- A. Stewart, S. Murray and S. E. J. Bell, Analyst, 2015, 140, 2988 RSC.
- G. Gicheva and G. Yordanov, Colloids Surf., A, 2013, 431, 51 CrossRef CAS.
- O. S. Ivanova and F. P. Zamborini, J. Am. Chem. Soc., 2010, 132, 70 CrossRef CAS PubMed.
- R. A. Masitas, I. V. Khachian, B. L. Bill and F. P. Zamborini, Langmuir, 2014, 30, 13075 CrossRef CAS PubMed.
- C. Viets and W. Hill, Sens. Actuators, B, 1998, 51, 92 CrossRef CAS.
- G. Andrade, M. Fan and A. G. Brolo, Biosens. Bioelectron., 2010, 25, 2270 CrossRef CAS PubMed.
- L. Wang, H. L. Li, J. Q. Tian and X. P. Sun, ACS Appl. Mater. Interfaces, 2010, 2, 2987 CAS.
- G. Q. Liu, W. P. Cai, L. C. Kong, G. T. Duan and F. J. Lü, J. Mater. Chem., 2010, 20, 767 RSC.
- C. J. Orendorff, A. Gole, T. K. Sau and C. J. Murphy, Anal. Chem., 2005, 77, 3261 CrossRef CAS PubMed.
- K. Kim and J. K. Yoon, J. Phys. Chem. B, 2005, 109, 20731 CrossRef CAS PubMed.
- R. A. Álvarez-Puebla, J. Phys. Chem. Lett., 2012, 3, 857 CrossRef PubMed.
- R. Alvarez-Puebla, L. M. Liz-Marzán and F. Abajo, J. Phys. Chem. Lett., 2010, 1, 2428 CrossRef CAS.
- X. F. Gu, S. Tian, Q. Zhou, J. Adkins, Z. M. Gu, X. W. Li and J. W. Zheng, RSC Adv., 2013, 3, 25989 RSC.
- Z. Yi, X. B. Xu, X. Q. Wu, C. H. Chen, X. B. Li, B. C. Luo, J. S. Luo, X. D. Jiang, W. D. Wu, Y. G. Yi and Y. J. Tang, Appl. Phys. A: Mater. Sci. Process., 2013, 110, 335 CrossRef CAS.
- L. Su, T. H. Lee and S. R. Elliott, Opt. Lett., 2009, 34, 2685 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18504a |
| This journal is © The Royal Society of Chemistry 2015 |







