Open Access Article
Open Access ArticleAcetone alkylation with ethanol over multifunctional catalysts by a borrowing hydrogen strategy
Gy.
Onyestyák
 *,
Gy.
Novodárszki
,
R.
Barthos
,
Sz.
Klébert
,
Á. Farkas
Wellisch
and
A.
Pilbáth
*,
Gy.
Novodárszki
,
R.
Barthos
,
Sz.
Klébert
,
Á. Farkas
Wellisch
and
A.
Pilbáth
Institute of Materials and Environmental Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, H-1519 Budapest, P. O. Box 286, Hungary. E-mail: onyestyak.gyorgy@ttk.mta.hu
First published on 20th November 2015
Abstract
Step by step alkylation of acetone (A) with ethanol (E) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was investigated. A fixed bed flow-through reactor system was used at a total pressure of 21 bar and in the temperature range of 150–350 °C in inert He or a reducing H2 medium. Following the hydrogen borrowing methodology, two types of catalysts were prepared; using neutral activated carbon (AC) and alkaline hydrotalcite (HT) supports, namely 5 wt% Pd/AC in the presence of alkaline additives (10, 20 and 30 wt% KOH or 20% K3PO4); 9 wt% Cu/HT and 5 wt% Pd/HT. The catalysts were activated in a H2 flow at 350 °C. Different yields of mono- or dialkylated ketones were observed. In a hydrogen medium over the same catalyst systems the ketone products could be reduced to alcohols. In this study the Pd/HT catalyst seems to be the most promising for fuel production based on biomass fermentation.
2 was investigated. A fixed bed flow-through reactor system was used at a total pressure of 21 bar and in the temperature range of 150–350 °C in inert He or a reducing H2 medium. Following the hydrogen borrowing methodology, two types of catalysts were prepared; using neutral activated carbon (AC) and alkaline hydrotalcite (HT) supports, namely 5 wt% Pd/AC in the presence of alkaline additives (10, 20 and 30 wt% KOH or 20% K3PO4); 9 wt% Cu/HT and 5 wt% Pd/HT. The catalysts were activated in a H2 flow at 350 °C. Different yields of mono- or dialkylated ketones were observed. In a hydrogen medium over the same catalyst systems the ketone products could be reduced to alcohols. In this study the Pd/HT catalyst seems to be the most promising for fuel production based on biomass fermentation.
Introduction
The depletion of fossil fuels has prompted interest in producing alternatives that are compatible with petroleum liquids. Technologies on various biomass platforms involve combinations of mechanical, thermal, chemical and biochemical processes.1–3 Instead of the less selective thermochemical method, favourable microbiological destruction processes are preferred.4 Commercial production of ethanol as a fuel blend has already been well recognized worldwide. Compared to ethanol, butanol has about 33% higher energy content per mass and is more compatible with the existing petroleum infrastructure. Nowadays, the anaerobe bacteria of genus Clostridium attract again great interest to produce acetone, butanol and ethanol (ABE mixture) in a 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.7
3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 molar ratio from sugars, carbohydrates, lignocelluloses, etc. for use as renewable alternative transportation fuels.5 Although co-production of acetone lowers the yield of alcohol biofuels, but its presence give an important potential to obtain longer carbon chains via alkylation with alcohol content of the mixture. Nucleophilic α-carbons of acetone can form C–C bond with electrophilic α-carbon of the alcohols, resulting in longer chain length organic compounds than the original fermentation products from two-carbon, three carbon and four-carbon precursors.
1.0 molar ratio from sugars, carbohydrates, lignocelluloses, etc. for use as renewable alternative transportation fuels.5 Although co-production of acetone lowers the yield of alcohol biofuels, but its presence give an important potential to obtain longer carbon chains via alkylation with alcohol content of the mixture. Nucleophilic α-carbons of acetone can form C–C bond with electrophilic α-carbon of the alcohols, resulting in longer chain length organic compounds than the original fermentation products from two-carbon, three carbon and four-carbon precursors.
First Anbarasan and co-workers5 proposed the alkylation route to convert ABE fermentation product into fuel. Using stirred pressurized tubes, small batch systems at 110 °C in toluene C5–C11 or longer-chain ketones were formed with different yields depending on composition of the reaction mixture and the applied catalysts. Ketones may be deoxygenated to paraffins, the components of fuel (in this cited work only partial reduction to alcohols was presented over a Pt-catalyst). Carbon supported palladium using in various forms was found to be outstanding compared with other metals (Ir, Ru, Rh, Pt, Ni). Different bases were added in some molar equivalent to the reaction mixture. K3PO4 as alkaline additive seemed to be the most efficient.5 Although the well-reviewed borrowing hydrogen methodology6,7 is suggested applying metal catalysts, type and amount of the used bases catalysing aldol reaction step are also important factors. In the shown experiments high, at least equivalent amount of bases are used.
Mimicking real aqueous ABE fermentation product G. Xu and co-workers8 showed direct α-alkylation of ketones with alcohols in a small autoclave using water as solvent instead of toluene5 over various Pd/C catalysts. In this study equivalents of different bases (LiOH, NaOH, KOH or K3PO4) to the amount of ketone were also added to the reaction mixture in the batch system. Q. Xu and co-workers9 conceive that transition-metal-catalysed α-alkylation of ketones with alcohols still have drawbacks, consequently they prefer the “catalyst-free” dehydrative α-alkylation studying only various aromatic reactants. However, high amount of bases (NaOH or KOH) are still applied in the studied alkylation reactions.
The significant basicity and intercalated copper ions in Mg, Al hydrotalcite supported copper sample showed multifunctional activity in various catalytic transformations of alcohols.10 Cu–hydrotalcite was found to be as an efficient and cheap catalyst for α-alkylation of various aromatic compounds such as ketones, β-alkylation of alcohols, alkylation of amines and amides.11
The cited literature shows as yet only investigations in small batch reactor systems5,8–11 which have several disadvantages and those tests give information with limited details. Broad spectrums of catalysts were already tested, but the acting catalysts were not at all characterized. A lot of different reaction mixtures were studied in ref. 5, but at presented low yields the comparison of the results are vague. Thus the results of the literature data are hard to be compared significantly with our new results.
Processing of ABE mixture – which can be one important way of biomass utilization in high volume – needs an effective continuous procedure. For this purpose our first aim in this study was to move from batch to flow-through system. Furthermore to avoid application of alkalies as additives, the use of alkaline support (hydrotalcite) instead of the practically inert carbon was aimed.
In this study working in vapour phase through fixed beds α-alkylation using acetone and ethanol, as model reactants was investigated in more details than in the literature shown. In our work it should be taken in consideration that third component of ABE mixture, butanol was not applied as reactant in order to follow and understand better the reaction mechanism. However, it can inevitably form as Guerbet side product in a self-aldol reaction of ethanol. Our findings are more general than processing of ABE mixture, because alkylation of ketones with alcohols has great importance independently on resources of the reactants. The suggested method is using less hydrogen in the production of longer carbon chain hydrocarbons as fuel compounds through longer ketone formation. The formation of one molecule of C7–C11 product is a result of two dehydration steps. Three oxygen atoms per one longer alkane molecule can be removed, using two hydrogen molecules only for the reduction of longer dialkylated ketones.
Experimental
Materials
Ref. 5 and 8 did not give specification about the used “5% palladium on carbon” catalysts. In this study, a commercial pelletized activated carbon (AC) (cylinders with 0.8 mm diameter and 2–4 mm length, Norit ROX 0.8 EXTRA, specific area: 1150 m2 g−1) as inert support was applied in order to compare the results obtained over the newly suggested hydrotalcite support.10,11 The AC first was dried at 110 °C, then impregnated with KOH (Fluka AG) or K3PO4 (Aldrich) solutions using incipient wetness method and dried again at 110 °C. For 1 g support 0.1 g, 0.2 g or 0.3 g bases were added resulting in plus 10, 20 or 30 m% loadings. Finally 5 m% palladium is also loaded using tetraamine–palladium(II)nitrate solution (STREM Chemicals).Preparation and physico-chemical properties of Cu–Mg, Al hydrotalcite (HT) was described in details by Dixit and co-workers.10 Documentation was carefully followed obtaining catalysts with identical features. Incipient wetness impregnation of HT was carried out with aqueous solution of copper acetate (Aldrich). Only discrepancy from the ref. 10 is the higher copper loading used: 9 wt% instead of 3–3.5 wt%. Catalyst containing 5 wt% palladium instead of copper was also prepared for comparison impregnating HT with tetraamine–palladium(II)nitrate solution applying incipient wetness method. These samples were first dried in air slowly heating up to 110 °C and were named 9Cu/HT and 5Pd/HT. The HT samples were calcined at 520 °C for 4 h to obtain Mg–Al mixed oxides (AHT). Usually the metal catalysts are formed in flow of H2 gas before testing the catalytic performance in dehydrogenation/hydrogenation reactions however, in the cited studies10,11 the samples were used without reducing the copper species. In other important cited ref. 5 and 8 information couldn't be found about the state of metallic components. In those studies investigations are carried out in small batch reactor systems in liquid phase below 200 °C. Contrary we used flow-through system in vapour phase at higher reaction temperatures where the applied reactants surely can reduce the precursors of Cu or Pd. Consequently, in general the catalysts were pre-treated equally in situ in hydrogen flow in the reactor at 350 °C and 21 bar for 1 h in order to obtain active metallic surface.
Methods
Nitrogen physisorption measurements were carried out at −196 °C using Quantachrome Autosorb 1C instrument. Before the adsorption analysis samples were outgassed under high vacuum at 200 °C for 3 h.The catalytic alkylation of acetone (A) (99.5%, Reanal) with ethanol (E) (99.7%, Reanal) mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio was studied in a high-pressure fixed bed flow-through reactor12 at 21 bar total pressure in the temperature range of 150–350 °C using inert helium or reducing hydrogen streams. The reaction was allowed to run one hour at each condition to attain steady state. The effluent during the second hour was collected, depressurized and cooled to room temperature. The liquid product mixture at ambient conditions was analysed by gas chromatograph using a GC-MS (Shimadzu QP2010 SE) capable to identify products formed in low concentration, equipped with a ZB-WAX plus capillary column. The gaseous reactor effluent was analysed for detection of CO2, CO, CH4 and light hydrocarbons using an on-line gas chromatograph (HP 5890) with thermal conductivity detector (TCD) on Carboxen 1006 PLOT capillary column.
2 molar ratio was studied in a high-pressure fixed bed flow-through reactor12 at 21 bar total pressure in the temperature range of 150–350 °C using inert helium or reducing hydrogen streams. The reaction was allowed to run one hour at each condition to attain steady state. The effluent during the second hour was collected, depressurized and cooled to room temperature. The liquid product mixture at ambient conditions was analysed by gas chromatograph using a GC-MS (Shimadzu QP2010 SE) capable to identify products formed in low concentration, equipped with a ZB-WAX plus capillary column. The gaseous reactor effluent was analysed for detection of CO2, CO, CH4 and light hydrocarbons using an on-line gas chromatograph (HP 5890) with thermal conductivity detector (TCD) on Carboxen 1006 PLOT capillary column.
The conversion of the two component reaction mixture cannot be well defined, due to the complex reaction network shown later. Both of the reactants are transformed to such a kind of by-products, which can take part further in the main alkylation reaction network. The main alkylated products (ketones and alcohols) yields are used for characterizing the activity and selectivity of the applied catalysts.
Results and discussion
Adsorption properties of supports and supported catalysts
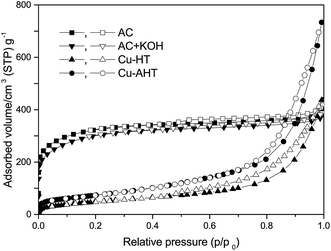
Since the structure of support can strongly affect the formation, location and accessibility of the catalytically active components, checking of the overall catalyst texture is essential – including both the support and the active species, changes in the course of the different preparation steps. For instance the diffusion resistance of the material may change after each step and the consequently altered mass transport may result in significantly altered yields and selectivities. In Fig. 1 adsorption properties of activated carbon (AC) and Mg–Al hydrotalcite (HT) supported catalysts are compared. Different textural features of the applied supports are reflected by the nitrogen adsorption and desorption isotherms.AC support, which is practically inert under conditions of the investigated reactions, has a well-defined pore structure. Shape of isotherms (typical type I indicating a microporous adsorbent with slight mesoporosity) reflects highly microporous materials with high specific surface area (>1000 m2 g−1) which is characteristic for activated carbons containing less slit-like pores than 1 nm between carbon sheets. Appearance of hysteresis loop indicates mesopores (mean pore diameter (BJH) is 4 nm) of low diffusional resistances evolved in the course of pellet formation process. Presence of impregnated bases and/or palladium on AC only hardly decrease the adsorbed amount of nitrogen related to AC content (in Fig. 1 only the parent carbon and the 30KOH/AC samples are shown). This observation means that alkaline salts (and loaded palladium metal also) can form larger crystals than the entrance of micropores in the mesopores. However, the mesoporous volumes, area of hysteresis loops are decreased significantly. Since active components, bases and the metal are located in the larger pores consequently the catalytic reactions take place in the mesopores.
Hydrotalcite (HT) isotherms are of the form well known in the literature as type IV with H2 hysteresis loop. These are practically overlapping applying different loadings (Cu or Pd and precursors of them) however different calcination temperatures (200 °C – Cu/HT and 520 °C – Cu/AHT) influencing the HT structure, result in significantly different isotherms and consequently different BET surface areas, 178 and 274 m2 g−1, respectively. Decomposition bellow 250 °C was due to removal of physisorbed water and interlayer water molecules. In the range of 270–500 °C structure change was attributed to loss of hydroxyls of brucite layer and interlayer anions. The mean mesopore diameter in all the samples is approx. the same (16 nm). Presence of metal, copper or palladium particles do not influence the texture similarly to the impregnated bases and/or palladium on AC. Since only slight decrease can be observed of the adsorbed amount of nitrogen for different loadings thus catalytic influence of textural changes can be also negligible. Differences in texture for different supports (AC or HT) are evident however in our case these hardly influence the pathway of alkylation reaction or the product distribution as it can be seen hereunder. The role of catalytically active species, quantity and quality of alkaline and metallic active site centres seems to be dominant compared to the support texture.
Characteristic transformations in inert atmosphere over 5Pd/AC
Tables 1–3 are designed in identical structure to demonstrate significant differences in formation of main products over various catalysts and using inert (helium) or reactive (hydrogen) carrier gases, respectively. In helium the mono-alkylated (2-pentanone, 2-heptanone) and the bi-alkylated-ketones (4-heptanone, 4-nonanone) are the main products formed with desired good selectivity over AC supported Pd catalysts. Table 1 shown the results obtained on catalyst loaded with different alkaline materials (KOH and K3PO4) and with KOH applied in various concentrations. The influence of reaction temperature is also reflected in Table 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)
| Catalyst | 5Pd,10KOH/AC | 5Pd,20KOH/AC | 5Pd,30KOH/AC | 5Pd,30KOH/AC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reac. temp. °C | 200 | 250 | 3000 | 200 | 250 | 300 | 200 | 250 | 300 | 200 | 250 | 300 |
| Isopropyl alc. | 1.3 | 2.2 | 2.4 | 1.2 | 1.6 | 1.8 | 1.4 | 1.3 | 1.4 | — | 0.9 | 2.0 |
| Butanol | — | — | — | — | — | — | — | — | — | — | — | — |
| Ethyl acetate | — | — | — | — | — | — | — | — | — | — | — | — |
| 2-Pentanone | 16.1 | 22.0 | 24.0 | 16.3 | 21.9 | 24.4 | 16.4 | 21.8 | 24.6 | 0.7 | 18.3 | 24.9 |
| 2-Pentanol | — | — | — | — | — | — | — | — | — | — | — | — |
| 4-Heptanone | 3.5 | 17.9 | 26.3 | 5.1 | 17.5 | 25.9 | 7.9 | 17.1 | 25.6 | 0.8 | 4.6 | 14.3 |
| 2-Heptanone | — | 1.8 | 2.2 | 0.2 | 2.1 | 2.9 | 0.5 | 2.7 | 4.0 | — | 1.1 | 1.7 |
| 4-Heptanol | — | — | — | — | — | — | — | — | — | — | — | — |
| 4-Nonanone | 0.4 | 1.5 | 9.9 | 0.4 | 3.6 | 9.1 | 0.3 | 6.4 | 9.9 | 0.2 | 0.6 | 1.8 |
| CO | — | 2.0 | 2.2 | — | 0.9 | 1.1 | — | 0.5 | 0.7 | 0.3 | 1.8 | 4.1 |
| CH4 | — | 3.4 | 3.3 | — | 1.9 | 2.2 | — | 0.9 | 1.2 | — | 1.7 | 4.2 |
| CO2 | — | 3.8 | 3.4 | — | 2.1 | 2.3 | — | 1.5 | 1.9 | — | 0.3 | 1.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)
| Catalyst | 5Pd,20KOH/AC | 5Pd,20K3PO4/AC | ||
|---|---|---|---|---|
| Reac. temp. °C | 300 | 350 | 300 | 350 |
| Isopropyl alc. | 11.3 | 5.4 | 15.0 | 9.1 |
| Butanol | 3.2 | 2.1 | 3.1 | 1.2 |
| Ethyl acetate | — | — | — | — |
| 2-Pentanone | 11.6 | 17.9 | 10.6 | 19.5 |
| 2-Pentanol | 10.6 | 9.9 | 9.4 | 5.5 |
| 4-Heptanone | 12.8 | 18.9 | 7.6 | 18.4 |
| 2-Heptanone | 1.2 | 2.6 | 0.5 | 2.0 |
| 4-Heptanol | 1.2 | 3.9 | 2.7 | 2.7 |
| 4-Nonanone | 1.4 | 9.1 | 1.1 | 3.6 |
| CO | 1.3 | 3.0 | 1.8 | 6.7 |
| CH4 | 1.5 | 3.1 | 2.6 | 5.6 |
| CO2 | 0.6 | 1.6 | 2.8 | 2.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)
| Catalyst | 9Cu/AHT + He | 9Cu/AHT + H2 | 5Pd/AHT + He | 5Pd/AHT + H2 | ||||
|---|---|---|---|---|---|---|---|---|
| Reac. temp. °C | 300 | 350 | 300 | 350 | 250 | 300 | 250 | 300 |
| Isopropyl alcohol | 6.3 | 5.6 | 20.5 | 14.3 | 2.9 | 5.3 | 12.5 | 8.5 |
| Butanol | 4.3 | 2.5 | 3.4 | 2.9 | — | — | — | — |
| Ethyl acetate | 11.6 | 3.3 | 3.2 | 1.1 | — | — | — | — |
| 2-Pentanone | 10.6 | 20.5 | 5.9 | 7.0 | 15.6 | 20.7 | 5.7 | 8.3 |
| 2-Pentanol | — | — | 11.6 | 18.4 | — | — | 8.6 | 11.4 |
| 4-Heptanone | 1.3 | 4.5 | — | — | 3.9 | 7.6 | 6.9 | 16.3 |
| 2-Heptanone | 1.1 | 3.2 | 0.3 | 0.3 | 0.4 | 0.6 | — | — |
| 4-Heptanol | — | — | 1.3 | 3.2 | — | — | 5.2 | 11.8 |
| 4-Nonanone | — | — | — | — | — | — | — | 0.9 |
| CO | 0.2 | 1.3 | 0.7 | 2.4 | 2.2 | 9.0 | 0.2 | 2.8 |
| CH4 | 0.1 | 0.3 | 0.8 | 2.9 | 1.3 | 5.1 | 0.1 | 2.0 |
| CO2 | 3.3 | 8.1 | 2.2 | 5.1 | — | — | — | 0.3 |
Without palladium loading very low catalyst activities can be observed (not shown in Table 1). The “catalyst-free” (in presence only of alkaline material, i.e. over alkaline monofunctional catalyst) dehydrative α-alkylation9 cannot be suggested in flow-through system. Without bases (K3PO4 or KOH) over monofunctional Pd/C catalyst only low alkylation activity (also not shown in Table 1) can be observed similarly to one component Pd-free catalyst, but the Pd/C catalyst is very active in producing of fragmented molecules. In absence of hydrogen high concentration of the undesired carbon dioxide, methane and carbon monoxide gases can be produced by splitting the C–C bonds of the reactants.
The significant production of 2-heptanone and 4-nonanone is interesting which testify that butanol can form as a Gourbet by-product, although it cannot be directly detected using helium. Consequently numerous variations of potential ketones can be detected in very different concentration alike as real ABE mixture has been studied.
Characteristic transformations in reducing atmosphere on 5Pd/AC
Alcohols didn't appear applying helium carrier gas. Those were significantly formed only in hydrogen (see in Table 2). Over Pd catalysts ketones can be reduced only to alcohols. Alkanes preferable for fuels can be detected only under severe reaction conditions, at high temperatures where numerous useless by-products can be also formed. The alkaline loaded Pd/C catalyst is highly efficient in the desired alkylation reactions however total deoxygenation could not be reached similarly to the results of P. Anbarasan and co-workers.5 In order to increase the hydrogenation reaction rate higher than atmospheric pressure was applied. Any alkenes or unsaturated ketones could not be detected, thus the used carbon support seems to be inert. It is interesting that the reactant acetone in hydrogen flow can be fully reduced to isopropyl alcohol already below 200 °C over the tested Pd-catalysts. Also the alkylation is materialized despite of the significant consumption of the acetone in the isopropyl alcohol formation.The ABE mixture can become a compatible fuel after less or deeper transformations. The simplest method is if the reactive acetone content is eliminated. A present study was aimed to develop an improved Clostridum acetobutylicum strain with enhanced alcohol fuel production capability.13 Contrary, our finding is more efficient that in hydrogen atmosphere, over Pd catalysts acetone content of ABE mixture can be completely reduced to isopropyl-alcohol below 200 °C, which method seems to be simpler than the efforts shown in ref. 13.
Using hydrogen, significant butanol formation can be already observed. In situ formed butanol results in mimicking of ABE mixture. Gaseous, cracked by-products are formed in less quantity by applying hydrogen stream over the applied “multifunctional” catalysts (dehydrogenation + aldol reaction + dehydration + hydrogenation), which is the primal advantage of H2 use together with alcohol production from ketones. Necessarily elevated total pressure also decreases production of fragmented compounds which can result in more molecules in the reactor space. Finally, the use of inert carrier gas can be preferred to attain higher yields of alkylates since there is no significant loss of reactive acetone in H2 medium due to reduction to isopropyl alcohol. A second reactor (hydrogenating) can be suggested for production of alkanes from ketones obtained in the first stage.
The yield of alkylated products is increasing at higher temperature, but gaseous by-products are lowered at lower temperatures.
The efficiencies of the studied Pd/C catalysts (5Pd,20K3PO4/AC; 5Pd,10KOH/AC; 5Pd,20KOH/AC; 5Pd,30KOH/AC) applied in different medium (He and H2) is compared in Tables 1 and 2. The nature of different bases (e.g. KOH or K3PO4) seems to be important in line with the batch results.5,8 However under conditions of this continuous process K3PO4 as alkaline additive was proven to be less efficient than KOH similarly than in ref. 8. Using KOH more dialkylated products can be obtained reflecting its higher activity in the aldol reaction. The concentration of alkaline component (KOH) on the catalyst surface seems to be less important regarding to main product yields, however significantly less yields of undesirable gaseous by-products can be observed with increasing KOH content.
The alkaline loaded catalysts seem to be stable during the long experimental time and sustain a constant activity. Thus vapour phase alcohol reactant or water product cannot remove alkaline salts from the support.
Applying reductive carrier gas in the reactor (i.e. hydrogen flow), lower gaseous by-product yields can be obtained (see Table 2). However it seems to be not too characteristics in line with the lower yields of desired products.
In this work the reaction temperature is much higher than in batch studies and gas/solid interactions are studied resulting in higher reaction rates. In our flow-through reactor system the productivity seems to be higher at least with more than one magnitude of order.
Qualitatively the product distributions demonstrate good analogy with cited references, reflecting identical reaction mechanism in the reacting zones, in the course of solid–liquid or solid–gas catalytic surface chemistry. However the quantitative comparison is very complicated because of significant difference of the studied catalytic systems and mainly of poor data shown in the cited papers.5,8
Reaction network of acetone alkylation with ethanol
Based on products shown in Tables 1 and 2 and further components detectable by GC-MS a complex reaction scheme can be recognized. The main variation of the alkylation reactions are presented in Scheme 1, but several products are formed in low concentration as well. The alcohols, the ethanol reactant and the in situ formed butanol are dehydrogenated by the palladium catalyst, generating the reactive aldehyde and hydrogen. At this stage the aldehydes undergo either a self-aldol reaction to form Guerbet product precursor, or an aldol reaction with acetone. Under the conditions employed here, condensation with acetone seems to be favoured because acetone is present at much higher concentrations than the transient product aldehydes. By changing inert carrier gas to hydrogen flow the initial acetone–alcohol ratio significantly decreases due to the transformation of a great part of acetone to isopropyl alcohol, which results in more significant production of butanol as by-product. The longer, mono-alkylated ketones produced in increased concentration have concentration-proportionated chance to react instead of acetone resulting in dialkylated products. | ||
| Scheme 1 A basic approach to the transition-metal catalysed reaction network for alkylation of acetone with alcohols on the base of detected products in significant concentration. | ||
Subsequent dehydration of aldol product yields α,β-unsaturated ketones which undergo metal-catalysed hydrogenation with the hydrogen generated in the first step. Completing the same cycle with monoalkylated products attains the expected double alkylation. The appearance of metal-adsorbed hydrogen after the ethanol dehydrogenating step (even in inert atmosphere) isopropyl alcohol can necessarily form from acetone resulting in branched products also (e.g. 4-methyl-2-pentanone, 2-methyl-4-heptanone, etc.). Naturally longer ketones formed with butanol or isopropyl alcohol can be also reduced to alcohols in significant, but lower concentrations. The visualization of these compounds in the scheme would make the scheme very diffuse.
Summarizing, the studied alkylation reactions involves a complex sequence of many reactions. This route includes four different types of reactions: I dehydrogenation, II aldolization, III dehydration and IV hydrogenation. The most commonly accepted path involves the aldolization reaction as the C–C forming step.
Alkalinity of the multifunctional catalyst given by the support
One important aspect of this catalytic process is the use of additional alkalis in high amount to efficiently stimulate the aldol reaction step. Availability of base particles in mixed batch systems containing liquid phase contacted with solid or in flow-through reactors working with vapour/solid interactions are severely undefined. For example only a small part of the impregnated KOH in the inert activated carbon support can work because practically equal product distributions were obtained on 5Pd,10KOH/AC, 5Pd,20KOH/AC and 5Pd,30KOH/AC catalyst samples (see Table 1). The use of an originally basic support such as MgO or its mixed oxides instead of a neutral or slightly acidic carbon support can be supposed to be imposing. Different hydrotalcites and their derived mixed oxides have received a great attention in recent years as an appropriate support for copper being highly active for catalytic transformations of alcohols due to synergistic effect between the basicity of the support and the fast spill over of Cu nanoparticles.10 The scope of Cu–hydrotalcite was shown as an efficient catalyst for α-alkylation of ketones, β-alkylation of alcohols, alkylation of amines and amides.11 Catalytic activity of such supported copper catalysts in alcohol activation by borrowing hydrogen was shown in numerous examples but it was investigated only with aromatic alcohols as reactants.11The main aim of this work was to avoid use of alkalies as catalyst additives using an originally basic support instead of the practically inert carbon. We applied well defined and thoroughly characterized Mg–Al hydrotalcite and Mg–Al mixed oxide supported copper catalysts which seemed to be efficient in other reactions demonstrated by M. Dixit et al.10,11
Table 3 demonstrates evidently applicability of hydrotalcite based catalysts. However under the same reaction conditions these samples show significantly lower activities than studied KOH loaded carbon supported forms (compared with Tables 1 and 2). As it turned out, differences can be found using different alkalines (see in Table 1 or ref. 5 and 8). Mg–Al hydrotalcite has also different basicity as well as different accessibility of basic sites. Along with lower conversion the product distributions contrast also strikingly with former samples which is not too surprising having significant structural and chemical differences.
Dehydrogenating/hydrogenating steps can be rate-determining in the reaction mechanism of alkylation. As is well-known copper has drastically lower activity than palladium. This difference is strikingly reflected in Table 3 showing different catalytic properties of the metals. Beyond that the different basicity which can be a crucial effect, differences can be observed better at the less active Cu-catalysts.
Most eye-catching effect is appearance of a new product, ethyl acetate in high concentration parallel with detectable acetaldehyde intermedier in measurable amount (not shown in Table 3). Although high basicity of Mg–Al hydrotalcite supported copper samples was stated in ref. 10, in fact concentrations of acidic and basic sites were shown to be commensurable. Less basicity can result in lower alkylation rate along aldol reaction of ketones. Under mild reaction conditions the low alkylation rate results in higher acetaldehyde coverage on the basic surface giving more chance for rival ethyl acetate formation in the Tishchenko reaction by coupling of two acetaldehyde molecules. Over similar catalytic systems ethyl acetate formation has been often found to be significant at various reaction conditions.14 The open literature provides a wide variety of possible explanations without conclusive evidence.15
Also the presence of more acetaldehyde can be correlated with higher butanol production, which needs also acetaldehyde precursor. The rate of alcohol coupling is proportional to the aldehyde concentration. What is more, the third pathway of by-product formation (beside butanol or ethyl-acetate production) is also influenced by support change for hydrotalcite base: isopropyl alcohol formation was already significantly increased in absence of reductive atmosphere, in helium stream (compare Tables 1 and 3), too. Hydrogen borrowing metals, copper or palladium, producing hydrogen in the reaction step of alcohol transformation to acetaldehyde, at lower concentration of unsaturated aldol reaction intermediers has more chance to reduce the reactant acetone to isopropyl alcohol in significant extent.
Copper and palladium above highly different efficiency in dehydrogenating/hydrogenating reactions has a very important dissimilarity considering affinities to hydrogen. In the case of catalysts that include copper, dihydrogen can be released from the surface after the dehydrogenation reaction, and the phase dihydrogen and aldehyde can be in equilibrium with alcohol. Contrary palladium can absorb in its crystal structure high amount of hydrogen. This otherness can play important role in the reaction mechanism resulting in different product distribution as reflected in Table 3 and Fig. 2 and 3.
 | ||
| Fig. 2 Yield of significant products obtained over hydrotalcite supported catalysts in helium as a function of space time. The reaction was carried out at 21 bar total pressure and 300 °C. | ||
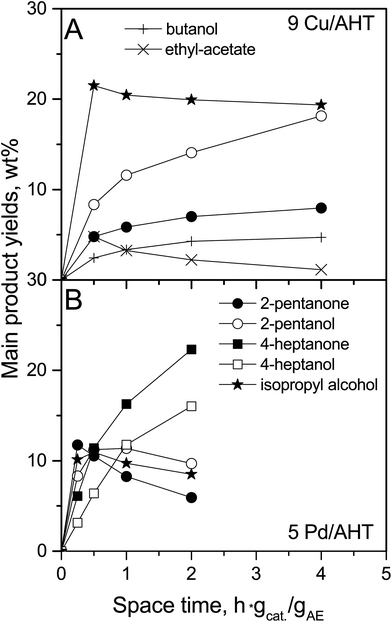 | ||
| Fig. 3 Yield of significant products obtained over hydrotalcite supported catalysts in hydrogen as a function of space time. The reaction was carried out at 21 bar total pressure and 300 °C. | ||
The mentioned three undesired side reaction pathway can be restrict under more severe reaction conditions, as such effect of work at higher reaction temperature can be observed in Table 3. Beyond the reaction temperature control, variation of the space time results in change of conversion and selectivity in a wide range (see Fig. 2 and 3). These results reflect the complexity of the reaction network, especially the progression of consecutive reaction chains. Determination of optimal reaction conditions for the requested products needs compromise. Enhancement of the reaction temperature results in higher yields of the desired alkylates but with higher increase of gaseous by-products. Using longer catalyst bed or feeding less reactant the yield for desired products are increasing with slower increase of gaseous by-products resulting in better selectivity.
Fig. 2 demonstrates that over hydrotalcite catalysts in helium stream the yields of side products (isopropyl alcohol, butanol or ethyl acetate) slowly decreases or hardly increases with enhanced space time in a wide range resulting in significantly improving selectivity for desired products, alkylates.
However Fig. 2 and Table 3 show that, in contrast to ref. 11, hydrotalcite supported copper catalyst is not advantageous for industrial processing of the ABE mixture. In their paper11 Dixit et al. suggests that this copper catalyst has great potential for the catalytic C- and N-alkylation of aromatic compounds.
Combination of basic hydrotalcite support with palladium metal (which was found to be most active and selective on carbon support5) gives a promising catalyst for the selective alkylation of acetone, a key process for converting the ABE mixture into fuels. Beside good alkylation selectivity, the initial reaction rates are also much higher over palladium than on copper, as Fig. 2A and B demonstrate.
It is well-known that palladium is an effective dehydrogenation/hydrogenation catalyst. This property is reflected in the different yields in Fig. 2 and 3, which show them when the alkylation was carried out in helium and hydrogen flow, respectively.
The observed differences demonstrate the competition of coexistent four reaction routes (three side- and one main reaction path) resulting in the consecutive alkylation reaction chain illustrated in Scheme 1.
On the Cu/AHT catalyst every side reactions (ethyl acetate, butanol and isopropyl alcohol formations) are significant compared to the aimed alkylation process. For example, the slope from 0 h gcat gAE−1 to the first measured yield values in Fig. 2 and 3 is much steeper on the Pd catalyst than that on the Cu catalyst, all but one, indicating that the initial rate of ethyl acetate formation through the acetaldehyde intermediate is faster than that through the direct acetone alkylation. High consumption of reactant ethanol for two different and competing side reactions hinders the formation of desired alkylates. In hydrogen flow the consumption of acetone in the third side reaction (reduction to isopropyl alcohol) is dominant (Fig. 3A), which might also slow down the alkylation. In contrast, Pd/AHT is more selective for the aimed alkylation reaction.
Since the hydrotalcite supported Cu- and Pd-catalysts contain the self-same alkaline support the different activity and the salient diverse selectivity can be evidently connected with different properties of the applied metals. However the reaction network is too complicated (see a simplified summary in Scheme 1) future studies are needed to trace significant points determining the selectivity. The importance of the well-known extraordinary hydrogen absorbing property of palladium can be significant in the hydrogenation of unsaturated ketone intermediates formed after the aldolization and dehydration reaction steps. This highly concentrated hydrogen is directly accessible on the palladium surface for the hydrogenation process.
Although over the less active copper catalyst formation of ethyl acetate by-product is significantly restricted working in hydrogen medium however dialkylation is remained negligible. Contrarily, palladium/hydrotalcite catalyst is highly efficient alkylating composite equally in helium or hydrogen. It is important that activity and selectivity is highly sensitive for increasing of space time giving a great potential for the processing of ABE mixture.
Conclusions
In a complicated reaction network of mono- or bi-alkylation of alcohol–acetone mixtures the reacting compounds, intermediates, and products form a complex liquid. Some of these components only form valuable fuel ingredients when partially or totally hydrogenated. We tested metal catalysts with great potential for C-alkylation of acetone with alcohols. We show that the selectivity and overall yield on palladium catalysts is superior compared to a novel copper catalyst. To avoid complications with alkaline additives, which have been previously employed, we used a basic catalyst support, hydrotalcite.We show that various mono- and dialkylated ketones formed with high yields when helium carrier gas was applied. These products were reduced out in hydrogen flow over our Pd/hydrotalcite catalyst. These results promise a new, efficient, continuous, catalytic processing option for ABE mixtures, obtained via biomass degradation, into desirable fuel components.
Acknowledgements
Thanks are due to the National Development Agency (Grant No. KTIA_AIK_12-1-2012-0014) for supporting this research work.Notes and references
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106(9), 4044–4098 CrossRef CAS PubMed.
- D. Kubicka, Collect. Czech. Chem. Commun., 2008, 73, 1015–1044 CrossRef CAS.
- S. N. Naik, V. V. Goud, P. K. Rout and A. K. Dalai, Renewable Sustainable Energy Rev., 2010, 14, 578–597 CrossRef CAS.
- H. N. Chang, N. J. Kim, J. Kang and C. M. Jeong, Biotechnol. Bioprocess Eng., 2010, 15(1), 1–10 CrossRef CAS.
- P. Anbarasan, Z. C. Baer, S. Sreekumar, E. Gross, J. B. Binder, H. W. Blanch, D. S. Clark and F. D. Toste, Nature, 2012, 491, 235–239 CrossRef CAS PubMed.
- Y. Obora, ACS Catal., 2014, 4, 3972–3981 CrossRef CAS.
- K. Shimizu, Catal. Sci. Technol., 2015, 5, 1412–1427 CAS.
- G. Xu, Q. Li, J. Feng, Q. Liu, Z. Zhang, X. Wang, X. Zhang and X. Mu, ChemSusChem, 2014, 7, 105–109 CrossRef CAS PubMed.
- Q. Xu, J. Chen, H. Tian, X. Yuan, S. Li, C. Zhou and J. Liu, Angew. Chem., Int. Ed., 2014, 53, 225–229 CrossRef CAS PubMed.
- M. Dixit, M. Mishra, P. A. Joshi and D. O. Shah, J. Ind. Eng. Chem., 2013, 19, 458–468 CrossRef CAS.
- M. Dixit, M. Mishra, P. A. Joshi and D. O. Shah, Catal. Commun., 2013, 33, 80–83 CrossRef CAS.
- Gy. Onyestyák, Sz. Harnos, Sz. Klébert, M. Stolcova, A. Kaszonyi and D. Kalló, Appl. Catal., A, 2013, 464–465, 313–321 CrossRef.
- S. B. Bankar, G. Jurgens, S. A. Survase, H. Ojamo and T. Granström, Fuel, 2014, 136, 226–232 CrossRef CAS.
- T. Riittonen, K. Eranen, P. Maki-Arvela, A. Shchukared, A. R. Rautio, K. Kordas, N. Kumar, T. Salmi and J. P. Mikkola, Renewable Energy, 2015, 74, 369–378 CrossRef CAS.
- D. Gabriels, W. Y. Hernandez, B. Sels, P. van der Voort and A. Verberckmoes, Catal.: Sci. Technol., 2015, 5, 3876–3902 CAS.
| This journal is © The Royal Society of Chemistry 2015 |