Exploration of using thermally responsive polyionic liquid hydrogels as draw agents in forward osmosis†
Abstract
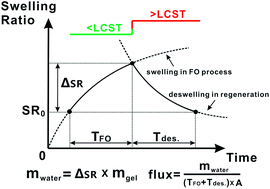
Thermally responsive hydrogels based on ionic liquid monomers of tetrabutylphosphonium p-styrenesulfonate (P4444 SS) and tributylhexylphosphonium p-styrenesulfonate (P4446 SS) were prepared by bulk polymerization in the presence of a crosslinker, and explored as draw agents in forward osmosis for the first time. Unlike traditional copolymer hydrogels from N-isopropylacrylamide (NIPAm) and sodium acrylate (SA) or sodium 2-acrylamido-2-methylpropane sulfonate (AMPS), the combination of thermo-sensitivity and the ionic nature in the hydrogel was achieved by subtle structure design of the ionic liquid monomers. These polyionic liquid hydrogels were proved to be able to generate a reasonable water flux from brackish water, and effectively release the desalinated water at temperatures above their Lower Critical Solution Temperature (LCST). In addition, a set of rational criteria for assessing responsive hydrogel draw agents was proposed, for the first time, to improve the hydrogels' comprehensive performance. Our studies revealed that, both intrinsic factors including hydrogel structure, membrane structure and hydrogel particle–particle contact conditions as well as extrinsic parameters such as the hydrogel's area density on the membrane and FO–regeneration cycle duration, would have a profound impact on the desalination efficacy using hydrogels.

 Please wait while we load your content...
Please wait while we load your content...