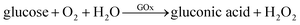Nanocalorimetric platform for accurate thermochemical studies in microliter volumes†
Rima Padovani,
Thomas Lehnert,
Raphaël Trouillon and
Martin A. M. Gijs*
Laboratory of Microsystems, Ecole Polytechnique Fédérale de Lausanne, CH-1015 Lausanne, Switzerland. E-mail: martin.gijs@epfl.ch
First published on 2nd November 2015
Abstract
A straightforward and general way for monitoring chemical reactions is via their thermal signature. Such approach requires however an experimental setup with a high thermal stability that simultaneously allows time-resolved heat detection with high sensitivity. We present a nanocalorimetric platform for accurate thermochemical studies of (bio-)chemical reactions in a miniaturized format (tens of microliter volume), characterized by a fast thermalization time to a preset temperature (<30 minutes), an excellent base temperature stability (±1 mK) and a fast sensing response time (few seconds). The platform is built around a commercial thermopile-based sensor chip, on which an open-well reservoir holding the sample is directly positioned. The sample is, prior to the experiment, pipetted into the reservoir, in which small aliquots of reagents are injected subsequently and sequentially via thermalized microfluidic conducts. The design of the platform is optimized by means of numerical simulations. Via thermoelectric calibration using a resistive heater positioned either on the sensor chip or in the reservoir, we obtain a maximum power sensitivity of 2.7 V W−1 and a heat limit of detection of 70 nW. The excellent functionality of the platform is demonstrated by measuring the reaction enthalpy of 1-propanol in water and the rate constant k and enthalpy change of the oxidation reaction of glucose catalyzed by glucose oxidase, showing good agreement with literature data. Our versatile platform may be applied to many thermochemical studies, including thermodynamic analysis and kinetic reaction analysis, and its ease of use will allow implementation of many different experimental protocols.
Introduction
Calorimetry is a powerful analytical method for thermochemical characterization of physical, chemical or biological processes. Its working principle is based on the transduction of heat into an electrical signal. It is a very versatile method that is suitable for a variety of applications, provided that the chemical reaction process involves an enthalpy variation.1 In particular, in life science research, calorimetry is considered the gold standard for studying biomolecular interactions, as it allows both the full thermodynamic profiling, including the quantification of enthalpy, free energy and entropy2 and the kinetic analysis of bioprocesses.3–5 Furthermore, this technique has the unique capability of measuring directly the metabolic heat produced by living organisms,6,7 enabling applications in disease diagnosis, drug development, toxicology, and environmental testing.8,9 The advantages of calorimetry are many: (i) it is a label-free technique, which does not require specific prior sample preparation, (ii) it is non-invasive, as it does not alter the nature of the sample allowing further analyses, and (iii) it is not limited by sample opacity, in contrary to the use of optical characterization methods.1,10 However, the omnipresence of thermo-chemical phenomena in nature qualifies heat as a non-specific signal, which may become a disadvantage if experiments are not carefully designed. Indeed, unwanted reactions, nonspecific molecular binding events, and evaporation may cause misleading interpretation of the experimental data.1,7,11 The main drawbacks of classical calorimetry are the requirement for relatively large sample volumes (at least a few ml) to achieve a large heat signal that allows detection with sufficient sensitivity, equipment cost and inflexibility in terms of its application to living organism studies and in terms of high-throughput implementation.12The miniaturized version of calorimetry, often named nanocalorimetry, uses volumes in the nl–μl range, resulting in proportionally smaller heat signals, but benefiting from the existence of extremely sensitive miniaturized sensors, enabling detection in the nW range or below.13 The versatility of nanocalorimetry has now been demonstrated by a wide range of applications that are documented in literature.14 The great majority of nanocalorimeters relies on the use of a thermopile sensor, which is microfabricated on a thin membrane with low heat capacity that is suspended in a thicker frame. This configuration guarantees a high thermal insulation and enables the detection of heat produced by small sample volumes.12,14 Thermopile-based sensors for liquid applications are commercially available,15 but they have been also custom-built,16–20 driven by particular experimental designs. Also, thermistor-based nanocalorimeters have been developed.5,21–23
Nanocalorimeters may be classified into two different types: batch-mode, also called open-chamber systems, and flow-through-mode, also called closed-chamber systems. In open-chamber nanocalorimeters, the sample is dispensed directly into a microwell that is placed on the sensor chip.17,24–27 For instance, Johannessen et al. demonstrated the feasibility of monitoring heat production by a very small number of mammalian cells17,28 in sample volumes of hundreds of pl. In a closed-chamber device, the sample volume is essentially confined, but there is still a fluidic connection of this volume to the outside world with microchannels allowing sample and reagent delivery as well as drain of the reactants and this in a dynamic fashion. Such systems clearly are well adapted for integration and automation of a measurement protocol.10,18–20,29–31 However, the same fluidic connections may introduce additional heat loss paths and worsen the thermal insulation to the environment, thus affecting the sensitivity.14,18 Lee et al. developed a device based on a parylene-polymer microfluidic calorimeter chip combined with soft-polymer fluidic components.18 On-chip vacuum encapsulation was used to improve thermal insulation, and 4.2 nW resolution was demonstrated for nl sample volumes. The investigation of slow metabolic processes require sample volumes of at least a few μl, as the signal to noise ratio is directly proportional to sample volume. For these applications, the precision of the temperature stabilization is particularly critical for obtaining a good signal resolution.32 A miniaturized flow-through calorimeter was therefore installed in a sophisticated thermostat system, providing a temperature stability of better than 40 μK.10,13,16,33–36 More recently, such system was combined with the so-called segmented flow technology, which consists in repetitive sequential processing of sample segments separated by immiscible inert segments.37,38
In this work, we report the development and characterization of a novel nanocalorimetric platform for the accurate study of the heat that is generated by (bio-)chemical reactions using a small sample reservoir of a few tens of μl. In particular, applications in the field of biochemistry and molecular biology have been addressed, often requiring simultaneously both relatively large sample volumes (of the order of a few tens of μl) and precision temperature stabilization. Our system has the advantages of an open-chamber configuration, as initially the sample is simply pipetted in a reservoir that was priorly mounted on a commercial thermopile sensor chip. Hereafter, the reservoir is confined by embedding it into an isothermal sample holder that also provides fluidic connections to the sample via the sidewalls of the reservoir. This allows profiting from the advantages of a flow-through system, as, after thermal stabilization, additional reagents may be easily sequentially injected. The accessibility of the reservoir prior and during the experiment is crucial for the versatility of the platform. In fact, the modularity of the system allows applying sample preparation steps to the reservoir that are independent from the nanocalorimetric measurements themselves, which will be particularly useful for biological samples. Also, during the calorimetric measurements additional reagents may be injected without perturbing the thermalization of the system. Particular attention was given to the platform design which was guided by computational thermal simulations. This approach allowed optimizing two distinctive thermal time constants for the system, namely (i) a time constant for reaching a stable platform setpoint temperature, thereby avoiding long stabilization times, and (ii) a time constant of the sensing system for a good time-resolved detection of heat generation/consumption processes. The platform had a very high temperature stability (down to ±1 mK over a period of about one hour for a setpoint temperature of 25 °C), and its excellent functionality was demonstrated by a thermodynamic study of the mixing enthalpy of 1-propanol in water and by a kinetic study of the glucose oxidase-catalyzed reaction of glucose.
Experimental
The nanocalorimetric platform
Moreover, it is important that heat generated by the sample is conducted only through the sensing area on the chip. The interface between the different components is critical in order to minimize any heat loss through alternative thermal paths. Finally, the different thermal time constants of the platform have to be considered in the design. On the one hand, the platform temperature has to reach steady state temperature in a reasonable time window (i.e. less than an hour), so its volume and thermal inertia should be kept small. On the other hand, reducing the thermal inertia also reduces damping of ambient temperature fluctuations. The second critical time constant relates to the time response of the sensor with respect to heat generated by the sample: it should be fast enough to detect dynamic events related to (bio-)chemical processes.
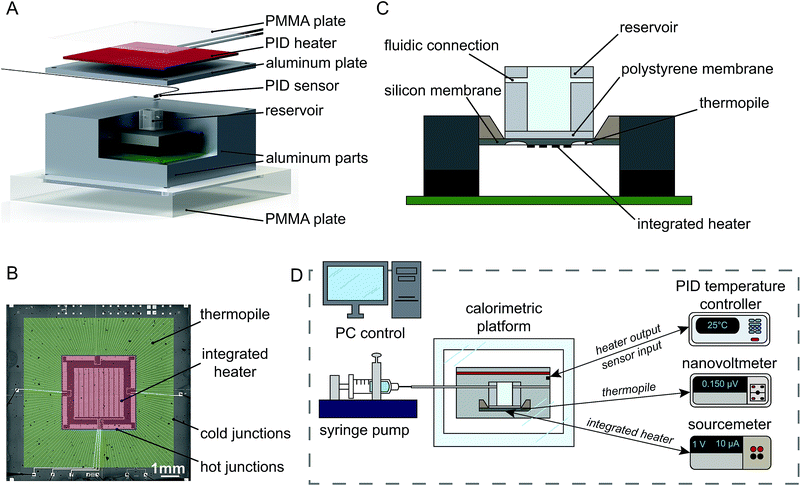
The isothermal holder, developed according to these requirements is sketched in Fig. 1A. It is composed of the following elements: a thermopile sensor (only the outer ceramic frame is visible in the figure), a polycarbonate (PC) sample reservoir, three aluminum parts assembled around the sensor, whose temperature is controlled by a closed loop Proportional-Integral-Derivative (PID) control system and two poly(methyl methacrylate) (PMMA) plates (bottom and top of the holder). An outer PMMA box is also used to provide additional thermal insulation with respect to ambient temperature fluctuations (not included in the sketch).
Materials used for (bio-)chemical experiments
The validation of this platform is based on the measurement of the heat released/absorbed during different chemical reactions. The first reaction tested is the mixing of 1-propanol (1-propanol anhydrous, 99.7%, Sigma-Aldrich) in deionized water (DIW). The second reaction is the oxidation of glucose (D-glucose ≥ 99.5%, Sigma-Aldrich) catalyzed by glucose oxidase (glucose oxidase from Aspergillus niger 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2 50
000–2 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per g, Sigma-Aldrich). In this second case, phosphate buffered saline (PBS pH 7.4 Gibco, Life Technologies) was used as the buffer solution.
000 units per g, Sigma-Aldrich). In this second case, phosphate buffered saline (PBS pH 7.4 Gibco, Life Technologies) was used as the buffer solution.
Results and discussion
Numerical simulation of the system and its experimental thermal stability
Simulations have been carried out in Comsol Multiphysics, in order to model the heat transfer phenomena in the isothermal holder. Detailed results are reported in the ESI section.† In particular, our studies allowed to optimize the system with (i) a time constant τ′ of 40 min to both reach a stable platform temperature and minimize the thermal gradient over the sensing area, and (ii) a time constant τ′′ of 8 s to obtain a stable thermal gradient between hot and cold junctions of the thermopile in response to heat generated by the sample.Results of experimental tests, aiming at both evaluating the temperature stability of the platform and estimating the background signal, are also reported in the ESI section.† In particular, the thermalization waiting time was reduced to 10–15 minutes to reach the setpoint temperature, and in total to 20–25 minutes to reach the maximum temperature stability. The temperature stability σT is estimated to be ±1 mK, which is the maximum stability reachable for the temperature controller in use, and the corresponding voltage signal stability σV is ±80 nV.
Thermo-electrical calibration and limit of detection
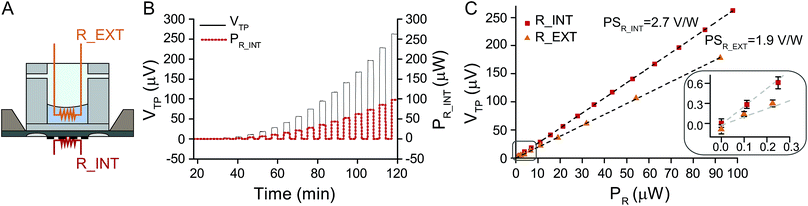
For calibrating the nanocalorimetric platform, the power sensitivity coefficient PS = VTP/PR, i.e. the conversion coefficient that relates the thermopile voltage signal VTP to the applied heat power PR has to be determined for different experimental configurations. Two different configurations, schematically shown in Fig. 2A have been considered. The first one uses the resistive heater integrated in the silicon chip membrane (R_INT, no reservoir), while the second one uses an external resistive heater (R_EXT), which is a miniaturized 100 kΩ thermistor (Micro-BetaCHIP Thermistor Probe, Measurement Specialties) immersed into the reservoir filled with 50 μl DIW. The second calibration procedure mimics more closely the real experimental situation, i.e. heat generation by a liquid sample in the reservoir that is placed on the sensor membrane. In both cases, periodically increasing electrical power was applied to the resistive heaters, which is converted by the Joule effect into heat power (PR_INT and PR_EXT for the integrated and external heater, respectively) and the corresponding thermopile voltage VTP is measured. This sequence is shown for the integrated heater in Fig. 2B. The calibration curves for the thermopile voltage VTP as a function of the heat power generated by the resistive heater PR for both configurations are presented in Fig. 2C. The corresponding power sensitivity coefficients can be determined as PSR_INT = 2.7 V W−1 and PSR_EXT = 1.9 V W−1, for the internal and external heater, respectively. The power sensitivity using the integrated heater is higher, as the heating element and the sensing element are integrated on the same silicon membrane resulting in very efficient heat power to voltage conversion with minimum heat loss. When including a reservoir with an external heater heat losses through alternative thermal paths arise, thus reducing the power sensitivity of the system. The inset in Fig. 2C shows the calibration curves for very small heat power (0–300 nW), from which we determine a limit of detection (LOD) of the platform of 70 nW for the integrated heater, and a LOD of about 170 nW for the external heater (corresponding to ±3σP where σP = PS × σV is the standard deviation of the heat power signal, and σV is the standard deviation of the thermopile voltage VTP). The first value can be considered as the ultimate intrinsic limit of detection of the platform, whereas the second value corresponds better to a real experimental situation.Heat of mixing of 1-propanol in water
As already pointed out by Wadsö et al.,39 calibration based on electrically generated heat may cause systematic errors as the induced thermal gradients may not fully reflect the experimental conditions of heat-generating (bio-)chemical reactions. For this reason, a test reaction having well-known thermochemical properties is required to further validate the calibration and the sensing system.The mixing of 1-propanol in water at 25 °C was chosen here for this purpose, as data for the molar enthalpy of mixing, or molar excess enthalpy HE, of 1-propanol in water are available in literature.39,40 Data reported by Davis et al.40 were taken as a reference and are summarized in Fig. 3A. The differential molar excess enthalpy dHE/dX, plotted on the same figure, was obtained by differentiating HE with respect to X, where X is the mole fraction of 1-propanol in water.
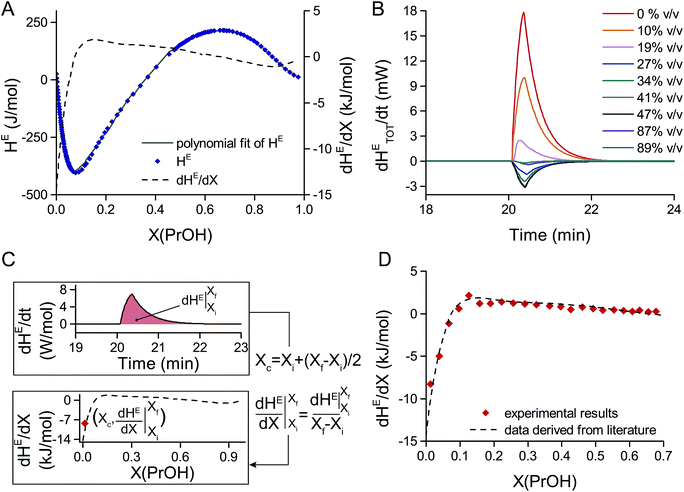 | ||
| Fig. 3 Measurements of the heat of mixing of 1-propanol in water at 25 °C. (A) Literature data for the heat of mixing per mole (or molar excess enthalpy) HE of 1-propanol in water vs. the mole fraction X(PrOH) of 1-propanol in water.40 Additionally, the differential molar excess enthalpy dHE/dX is shown, obtained by differentiating the polynomial fit of the molar excess enthalpy HE with respect to the mole fraction X(PrOH) (dashed line). (B) Differential excess enthalpy with respect to time dHETOT/dt generated by injecting and mixing 5 μl of pure 1-propanol into 45 μl of 1-propanol solutions at different initial concentrations. The differential excess enthalpy dHETOT/dt corresponds to the heat power detected by the thermopile and it was obtained by measuring the thermopile voltage VTP and applying a power sensitivity coefficient of 0.6 V W−1. (C) Procedure used for data analysis: (i) the measured value dHETOT/dt is first normalized (dividing by the number of moles in the mixture) to obtain the differential molar excess enthalpy dHE/dt. (ii) Integrating over time and dividing by the mole fraction difference (Xf − Xi, after and before injection) results in the differential molar excess enthalpy dHE/dX with respect to the mole fraction X. (iii) The central mole fraction Xc is calculated as the average mole fraction between the initial (Xi) and final (Xf) mole fraction and is used to plot the differential molar excess enthalpy data points (Xc, dHE/dX). (D) Comparison of the differential molar excess enthalpy dHE/dX derived from literature40 with the values calculated from our experimental data. | ||
In order to validate the functionality of the nanocalorimetric platform, differential molar excess enthalpy dHE/dX values obtained with this platform were compared with literature data. The experimental procedure consisted in prefilling the reservoir with 45 μl of 1-propanol in water solutions at different initial concentrations. The inlet tubing connecting the reservoir was filled with 5 μl of pure 1-propanol. After allowing a thermalization time of 20 min to reach the setpoint temperature of 25 °C, 5 μl of pure 1-propanol were injected in the reservoir, resulting in a total volume of 50 μl. As the inlet tube passes through the metal block of the platform, the injected volume is well thermalized at 25 °C. As a result, a single experiment may last less than 30 minutes, which is advantageous when planning many sequential and independent experiments. Additionally, an alternative experimental protocol was also tested, and it is described more in detail in the ESI section:† it consisted in applying up to three sequential injections of 5 μl of pure 1-propanol in the same reservoir prefilled with 45 μl of 1-propanol in water solution. This second approach allows to carry on tests at increasing concentrations of reactant in an even more time-efficient manner.
The results of a series of experiments using an initial concentration of 1-propanol in water from 0% to 89% (v/v) is shown in Fig. 3B. The curves show the heat power generated upon mixing corresponding to the total enthalpy change over time dHETOT/dt. In order to compare with literature values, some data processing is required to calculate the corresponding differential molar excess enthalpy dHE/dX, as summarized in Fig. 3C. The following steps were applied: (i) normalizing the total differential excess enthalpy dHETOT/dt by the number of moles in solution in order to obtain the corresponding molar value which is dHE/dt; (ii) integrating with respect to time to obtain the excess molar enthalpy HE, which is the molar enthalpy change measured when the solution concentration varies from the initial mole fraction Xi to the final mole fraction Xf; (iii) dividing by the mole fraction difference (Xf − Xi) to obtain the differential molar excess enthalpy dHE/dX. Being a discrete derivative obtained from the experimental measurements, the central mole fraction Xc = Xi + (Xf − Xi)/2 has been calculated in order to plot pairs of values (Xc, dHE/dX) on the dHE/dX vs. X curve. Fig. 3D shows the comparison between the thus calculated data points and the differential molar excess enthalpy curve derived from literature and clearly demonstrates a good match. A power sensitivity coefficient PSexp of 0.6 V W−1 was used, which is a value that is about 3 times lower than the conversion coefficient PSR_EXT obtained with the external heater. This fact underlines the importance of system calibration using chemical reactions, which generate a heat signal that physically is originating from a distance further away from the sensor membrane than when using a resistive heater.
Enzymatic study of glucose oxidation, catalyzed by glucose oxidase
The nanocalorimetric platform was also used to study the enzymatic activity of glucose oxidase (GOx) at 25 °C, when catalyzing the oxidation of glucose. Glucose oxidase uses molecular oxygen to catalyze the oxidation of glucose to gluconic acid and the overall reaction can be described as follows:41,42In particular, experiments were performed in presence of dissolved oxygen (DO) under atmospheric conditions, which corresponds to an initial DO concentration of 0.25 mM.43 The experimental procedure for this experiment is very similar to the previously applied protocol, except for the liquid volumes used. The reservoir was filled with 25 μl of glucose oxidase solution (250 U ml−1 in PBS), the tubing was filled with 1.5 μl of glucose solution in PBS, the temperature was set to 25 °C and 30 minutes of thermalization were allowed before injecting the glucose solution. The volume injected was adjusted so that the final glucose concentration ranged from 0 to 40 mmol l−1. Recalibration of the platform using 1-propanol in water as test reaction with the reduced sample volume (∼25 μl instead of ∼50 μl) resulted in an improved power sensitivity PSexp of 1 V W−1. A small sample volume results in heat generation closer to the thermopile sensor, which is more efficiently detected indeed. This power sensitivity coefficient was applied to all glucose oxidation experiments.
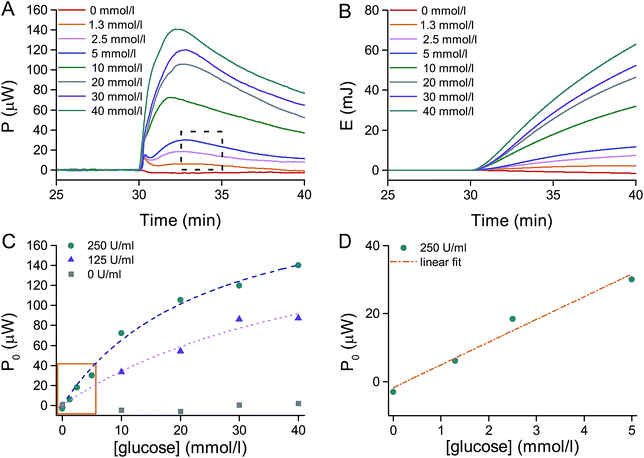
Results from a series of tests are presented in Fig. 4A, showing the heat power P released over time when glucose is injected in the glucose oxidase solution. It is possible to observe that the heat power released P corresponds to the heat energy released per unit time, and it is directly proportional to the number of moles of glucose which are consumed per unit time, that is the reaction rate V in mol (l s)−1:
| P = hV | (1) |
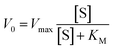 | (2) |
As the heat power P is directly proportional to the reaction rate V, a similar behavior is observed when plotting the initial heat power P0 as a function of the glucose concentration. Experiments were carried out for different GOx concentrations and fitting with the Michaelis–Menten-like equation P0([S]) was carried out as shown in Fig. 4C (dashed lines; adjusted R-squared higher than 0.97) to obtain the two distinctive parameters PMAX and KM. The maximal heat power PMAX estimated is about 210 μW and 230 μW for a GOx concentration of 125 U ml−1 and 250 U ml−1, respectively. More interestingly, the corresponding Michaelis constants KM are about 50 and 25 mmol l−1, which are very close to reported values in literature.47–49 Considering the curve for the GOx concentration of 250 U ml−1, it is possible to observe the linear region of the Michaelis–Menten plot for low glucose concentration ([glucose] ≪ 25 mmol l−1), which is typical of a first order enzyme–substrate reaction, before enzyme saturation becomes important. Fig. 4D shows a magnification in the 0–5 mmol l−1 range of glucose concentrations: in this range of glucose concentrations, the heat power P0 and, thus, the reaction rate V0 is directly proportional to the initial substrate concentration [S0]. A linear fitting (dashed line; adjusted R-squared equal to 0.96) allows the estimation of the slope of the fitted line α, according to the following equation:
| P0 = α[S0] | (3) |
The estimated value of α from the experimental data is 6.68 μW (mmol/l)−1. Furthermore, for this type of reactions, the substrate concentration [S] decays exponentially over time as:
| [S] = [S0]e−kt | (4) |
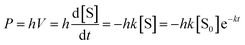 | (5) |
Interestingly it is possible to estimate the rate constant k by fitting the experimental data shown in Fig. 4A, highlighted by the dashed rectangle. In particular, a short duration after the power peak was chosen to guarantee that the system was still in the conditions close to its initial status and that the first order reaction obtained from the simplification of Michaelis–Menten is still a good approximation. From the exponential fitting done over a time window of 2 minutes after the maximal peak, for the glucose concentration range from 1.3 to 5 mmol l−1 ([glucose] ≪ 25 mmol l−1), the estimated average rate constant k is 0.0022 s−1 (adjusted R-squared higher than 0.80). Finally, by applying eqn (5) to the time instant of the maximal peak P0 (considered as the onset of the reaction, once the system has overcome its mass transport limitation) and knowing the proportionality coefficient α from eqn (3), the following relation can be deduced:
| P0 = −hk[S0] = α[S0] | (6) |
As the rate constant k and the proportionality coefficient α have been estimated, it is possible to calculate h which, divided by the sample volume v of 26.5 μl, gives the information on the energy released during the reaction per unit of mole, that is the enthalpy change. The enthalpy change estimated with this procedure is −115 ± 15 kJ mol−1 (for the 250 U ml−1 GOx solution), which is in good agreement with values available from literature.48
A posteriori analysis of possible limiting effects of dissolved oxygen depletion, which were documented in literature,48,50 has also been carried out. Such limitation was not observed experimentally in our case and the results were indeed in agreement with literature on the Michaelis–Menten model.46,48 Oxygen diffusion from the top surface of the liquid to the bottom of the reservoir may take up to 4 minutes, if we consider the inner size of the reservoir of 5 × 5 mm2, the sample volume of 25 μl, and thus a mean diffusive path of up to 1 mm (a diffusion coefficient D of oxygen in water of 2 × 10−5 cm2 s−1 was taken51 and the mean diffusive time t calculated as x2/(2 × D)). Therefore, re-supply of oxygen over the duration of the experiment is continuously enabled. Moreover, the highest consumption of glucose per minute, which is observed at the maximum reaction rate, may be estimated by dividing the heat power measured (Fig. 4A) by the reaction enthalpy known from literature.48 Considering a glucose concentration of 5 mmol l−1, which is the highest in the linear range of Fig. 4D, the thus estimated highest consumption of glucose would be 14 × 10−9 mol min−1. Consequently, 14 × 10−9 mol min−1 of oxygen are also consumed, which is of the same order of magnitude as the initial DO moles available in solution (7 × 10−9 mol, assuming 0.25 mM as the initial DO concentration). We can conclude that the continuous replenishment of oxygen from air and the relatively fast diffusion of oxygen may explain the ability to test high glucose concentrations without oxygen-limiting effects. This is particularly true in the linear range of the Michaelis–Menten model, as no linearity between heat power signal and glucose concentration would have been observed if oxygen was limiting the reaction. In contrast, we cannot exclude that oxygen depletion may be partially taking place in the saturation regime of the Michaelis–Menten model (glucose concentration higher than 5 mmol l−1), even though the system follows the expected theoretical behaviour for such reaction. Finally, the combination of glucose oxidase with the oxygen-regenerating enzyme catalase may be foreseen in future to create less limiting experimental conditions from the oxygen depletion point-of-view.
The validation of the nanocalorimetric platform with this biochemical reaction demonstrates its applicability to the thermodynamic study of chemical reactions, by measuring the enthalpy change of reaction, but also to the kinetic study of biochemical and, in particular, enzymatic assays. The versatility of the platform makes it an excellent tool for studying unknown biochemical reactions and, more interestingly, the effects of chemical compounds on specific enzymatic reactions: the latest is particularly relevant in the field of drug design for drugs that are targeting enzymes, which are considered one of the major drug targets.52
Conclusions
The development and the validation of a new nanocalorimetric platform has been presented and its applicability to the study of both the thermodynamics and kinetics of (bio-)chemical reactions has been demonstrated. In particular, the design of the platform allowed achieving high temperature stability in the mK range, a thermalization time of less than 30 minutes and a sensing response time of a few seconds. The platform is easy to use, as it does not require any specific sample preparation prior to the experiment and potentially any thermochemical reaction may be investigated. Sample and reagent solutions can either be preloaded in an on-chip reservoir or injected directly into the reservoir via an external thermalized conduct, allowing very versatile experimental protocol definitions. The good performance of the nanocalorimetric platform to measure thermodynamic properties, in particular the heat of mixing of 1-propanol–water test solutions and the study of the enzymatic activity of glucose oxidase, was successfully demonstrated. The following advantages of our platform were identified: (i) a high power sensitivity, as heat power signals as small as a few μW were successfully detected, (ii) small sample volumes in the μl range, which is of benefit for testing chemical compounds which are expensive or limited in quantity, (iii) a short thermalization time, enabling fast turnover between two experiments, (iv) an adequate temporal resolution for the sensor response, enabling studying fast chemical reactions. At the present, the system relies on passive diffusive mixing of the small injected sample volume into the larger on-chip reservoir. Faster mixing is key to further improvement of the platform performance and this may be achieved by integrating a custom-designed microfluidic chip system with the sensor chip in future. The present platform design is already compatible with such an implementation.Finally, the presented work not only shows a new highly performing calorimetric platform per se, but it also represents a significant advance in the field of more user-friendly and versatile technologies for biology. We believe our approach will be in favour of exploitation of a powerful sensing technique like calorimetry in advanced biology studies, and particularly in molecular biology. In particular, such system holds great potential in particularly interesting fields of applications, such as drug design, allowing to easily study the mechanism of action of specific compounds on enzymatic reactions, or in the field of fundamental research in molecular biology, where specific molecular pathways which are regulated by enzymes may be investigated.
Acknowledgements
This work was supported by the EPFL and funding was provided by the EU Ideas program (ERC-2012-AdG-320404). We also thank members of the EPFL workshops for printed circuit board fabrication and for micromechanics for the technical assistance.Notes and references
- W. Lee, J. Lee and J. Koh, Journal of Nanobiosensors in Disease Diagnosis, 2012, 2012, 17–29 CrossRef.
- J. B. Chaires, Annu. Rev. Biophys., 2008, 37, 135–151 CrossRef CAS PubMed.
- A. E. Beezer, Thermochim. Acta, 2001, 380, 205–208 CrossRef CAS.
- M. L. Bianconi, Biophys. Chem., 2007, 126, 59–64 CrossRef CAS PubMed.
- M. I. Recht, F. E. Torres, D. D. Bruyker, A. G. Bell, M. Klumpp and R. H. Bruce, Anal. Biochem., 2009, 388, 204–212 CrossRef CAS PubMed.
- A. E. Beezer, Biological microcalorimetry, Academic Press Inc. Ltd, London, 1980 Search PubMed.
- I. Wadsö, Trends Biotechnol., 1986, 4, 45–51 CrossRef.
- O. Braissant, D. Wirz, B. Göpfert and A. U. Daniels, FEMS Microbiol. Lett., 2010, 303, 1–8 CrossRef CAS PubMed.
- O. Braissant, D. Wirz, B. Göpfert and A. U. Daniels, Sensors, 2010, 10, 9369–9383 CrossRef CAS PubMed.
- L. M. Ahmad, B. Towe, A. Wolf, F. Mertens and J. Lerchner, Sens. Actuators, B, 2010, 145, 239–245 CrossRef CAS.
- I. Wadsö and L. Wadsö, J. Therm. Anal. Calorim., 2005, 82, 553–558 CrossRef.
- T. Maskow, T. Schubert, A. Wolf, F. Buchholz, L. Regestein, J. Buechs, F. Mertens, H. Harms and J. Lerchner, Appl. Microbiol. Biotechnol., 2011, 92, 55–66 CrossRef CAS PubMed.
- J. Lerchner, A. Wolf, H.-J. Schneider, F. Mertens, E. Kessler, V. Baier, A. Funfak, M. Nietzsch and M. Krügel, Thermochim. Acta, 2008, 477, 48–53 CrossRef CAS.
- F. Yi and D. A. La van, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2012, 4, 31–41 CrossRef CAS PubMed.
- A. W. van Herwaarden, Thermochim. Acta, 2005, 432, 192–201 CrossRef CAS.
- V. Baier, R. Födisch, A. Ihring, E. Kessler, J. Lerchner, G. Wolf, J. M. Köhler, M. Nietzsch and M. Krügel, Sens. Actuators, A, 2005, 123–124, 354–359 CrossRef CAS.
- E. A. Johannessen, J. M. R. Weaver, L. Bourova, P. Svoboda, P. H. Cobbold and J. M. Cooper, Anal. Chem., 2002, 74, 2190–2197 CrossRef CAS PubMed.
- W. Lee, W. Fon, B. W. Axelrod and M. L. Roukes, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15225–15230 CrossRef CAS PubMed.
- S.-K. Nam, J.-K. Kim, S.-C. Cho and S.-K. Lee, Sensors, 2010, 10, 6594–6611 CrossRef CAS PubMed.
- L. Wang, D. M. Sipe, Y. Xu and Q. Lin, J. Microelectromech. Syst., 2008, 17, 318–327 CrossRef CAS.
- K. Ramanathan and B. Danielsson, Biosens. Bioelectron., 2001, 16, 417–423 CrossRef CAS PubMed.
- M. I. Recht, D. D. Bruyker, A. G. Bell, M. V. Wolkin, E. Peeters, G. B. Anderson, A. R. Kolatkar, M. W. Bern, P. Kuhn, R. H. Bruce and F. E. Torres, Anal. Biochem., 2008, 377, 33–39 CrossRef CAS PubMed.
- F. E. Torres, P. Kuhn, D. D. Bruyker, A. G. Bell, M. V. Wolkin, E. Peeters, J. R. Williamson, G. B. Anderson, G. P. Schmitz, M. I. Recht, S. Schweizer, L. G. Scott, J. H. Ho, S. A. Elrod, P. G. Schultz, R. A. Lerner and R. H. Bruce, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 9517–9522 CrossRef CAS PubMed.
- B. Lubbers and F. Baudenbacher, Anal. Chem., 2011, 83, 7955–7961 CrossRef CAS PubMed.
- K. Verhaegen, K. Baert, J. Simaels and W. van Driessche, Sens. Actuators, A, 2000, 82, 186–190 CrossRef CAS.
- J. Xu, R. Reiserer, J. Tellinghuisen, J. P. Wikswo and F. J. Baudenbacher, Anal. Chem., 2008, 80, 2728–2733 CrossRef CAS PubMed.
- E. A. Johannessen, J. M. R. Weaver, P. H. Cobbold and J. M. Cooper, IEEE Transactions on NanoBioscience, 2002, 1, 29–36 CrossRef PubMed.
- E. A. Johannessen, J. M. R. Weaver, P. H. Cobbold and J. M. Cooper, Appl. Phys. Lett., 2002, 80, 2029–2031 CrossRef CAS.
- B. S. Kwak, B. S. Kim, H. H. Cho, J. S. Park and H. I. Jung, Microfluid. Nanofluid., 2008, 5, 255–262 CrossRef CAS.
- B. Wang and Q. Lin, Sens. Actuators, B, 2013, 180, 60–65 CrossRef CAS.
- Y. Zhang and S. Tadigadapa, Biosens. Bioelectron., 2004, 19, 1733–1743 CrossRef CAS PubMed.
- J. Lerchner, A. Wolf, G. Wolf and I. Fernandez, Thermochim. Acta, 2006, 446, 168–175 CrossRef CAS.
- J. Lerchner, A. Wolf, G. Wolf, V. Baier, E. Kessler, M. Nietzsch and M. Krügel, Thermochim. Acta, 2006, 445, 144–150 CrossRef CAS.
- J. Lerchner, A. Wolf, F. Buchholz, F. Mertens, T. R. Neu, H. Harms and T. Maskow, J. Microbiol. Methods, 2008, 74, 74–81 CrossRef CAS PubMed.
- J. Lerchner, D. Mueller-Hagen, H. Roehr, A. Wolf, F. Mertens, R. Mueller, W. Witte and I. Klare, J. Therm. Anal. Calorim., 2011, 104, 31–36 CrossRef CAS.
- T. Maskow, J. Lerchner, M. Peitzsch, H. Harms and G. Wolf, J. Biotechnol., 2006, 122, 431–442 CrossRef CAS PubMed.
- T. Hartmann, N. Barros, A. Wolf, C. Siewert, P. L. O. Volpe, J. Schemberg, A. Grodrian, E. Kessler, F. Hänschke, F. Mertens and J. Lerchner, Sens. Actuators, B, 2014, 201, 460–468 CrossRef CAS.
- A. Wolf, T. Hartmann, M. Bertolini, J. Schemberg, A. Grodrian, K. Lemke, T. Förster, E. Kessler, F. Hänschke, F. Mertens, R. Paus and J. Lerchner, Thermochim. Acta, 2015, 603, 172–183 CrossRef CAS.
- I. Wadsö and R. N. Goldberg, Pure Appl. Chem., 2001, 73, 1625–1639 CrossRef.
- M. I. Davis and E. S. Ham, Thermochim. Acta, 1991, 190, 251–258 CrossRef CAS.
- M. F. Chaplin and C. Bucke, Enzyme Technology, CUP Archive, Cambridge, 1990 Search PubMed.
- A. Crueger and W. Crueger, in Microbial Enzymes and Biotechnology, ed. W. M. Fogarty and C. T. Kelly, Elsevier Science Publishers, Essex, 2nd edn, 1990, pp. 177–226 Search PubMed.
- D. Kondepudi and I. Prigogine, in Modern Thermodynamics, John Wiley & Sons, Ltd, 2014, pp. 207–229 Search PubMed.
- J. M. Berg, J. L. Tymoczko and L. Stryer, Biochemistry: International Edition, W. H. Freeman & Co Ltd, New York, International, of 6th revised, 2006 Search PubMed.
- K. A. Johnson and R. S. Goody, Biochemistry, 2011, 50, 8264–8269 CrossRef CAS PubMed.
- L. Michaelis and M. L. Menten, Biochem. Z., 1913, 49, 333–369 CAS.
- S. B. Bankar, M. V. Bule, R. S. Singhal and L. Ananthanarayan, Biotechnol. Adv., 2009, 27, 489–501 CrossRef CAS PubMed.
- R. Hüttl, K. Bohmhammel, K. Pritzkat and G. Wolf, Thermochim. Acta, 1993, 229, 205–213 CrossRef.
- R. Wilson and A. P. F. Turner, Biosens. Bioelectron., 1992, 7, 165–185 CrossRef CAS.
- B. Danielsson, K. Gadd, B. Mattiasson and K. Mosbach, Clin. Chim. Acta, 1977, 81, 163–175 CrossRef CAS.
- P. Han and D. M. Bartels, J. Phys. Chem., 1996, 100, 5597–5602 CrossRef CAS.
- J. G. Robertson, Biochemistry, 2005, 44, 5561–5571 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Numerical simulation of characteristic thermal time constants, experimental evaluation of platform stability and sensitivity. Experimental protocol for sequential chemical testing. See DOI: 10.1039/c5ra22248f |
| This journal is © The Royal Society of Chemistry 2015 |