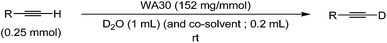Mild deuteration method of terminal alkynes in heavy water using reusable basic resin†
Tsuyoshi Yamada,
Kwihwan Park,
Yasunari Monguchi,
Yoshinari Sawama* and
Hironao Sajiki*
Laboratory of Organic Chemistry, Gifu Pharmaceutical University, 1-25-4 Daigaku-nishi, Gifu 501-1196, Japan. E-mail: sawama@gifu-pu.ac.jp; sajiki@gifu-pu.ac.jp; Fax: +81-58-230-8109; Tel: +81-58-230-8109
First published on 23rd October 2015
Abstract
The mild and efficient deuteration of terminal alkynes (mono-substituted alkynes) proceeded in the presence of a basic anion exchange resin, WA30, which is a polystyrene polymer bearing a tertiary amine residue on the aromatic nuclei, in heavy water (D2O) at room temperature. WA30 could be easily removed by a simple filtration and repeatedly reused.
The utility of deuterium-labeled compounds is widely recognized in various scientific fields, such as human metabolic, reaction mechanistic, analytic and material studies.1,2 Among them, deuterated terminal alkynes were also utilized for mechanistic reaction studies3 and as useful building blocks to construct deuterium labeled materials (e.g., deuterated alkenes3a,b,h,4 by hydrogenation, 1,2,3-triazole5 by Huisgen cycloaddition with azido compounds, and aromatics3f,g by Lewis acid-catalyzed intra or intermolecular annulation and aromatization, etc.). The deuterated terminal alkynes are traditionally prepared via an acetylide, generated by the stoichiometric use of organolithium,3a,b,c,g Grignard reagents3f or Na metal3h in a non-protic solvent, and subsequent quench using deuterium sources, such as D2O and CD3OD, in association with the production of a large amount of metal sludge. Additionally, the deuteration using the silver salt/CD3CO2D combination6 or triazabicyclodecene (TBD)7 and N-heterocyclic carbene8 in CDCl3 has also been reported. Bew et al. recently developed a mild deuteration method of terminal alkynes under basic reaction conditions in the presence of K2CO3 in a D2O/CH3CN mixed-solvent.5 We have also reported a deuteration method using Et3N as an organic base in a D2O/THF mixed-solvent at nearly the same time.4 However, the development of the deuterium-labeled method using a reusable catalyst in D2O as a neutral and cheapest deuterium source without an organic co-solvent is still challenging from the viewpoint of green sustainable chemistry. We now demonstrate a clean and mild deuteration method of terminal alkynes using a reusable solid organic base in D2O at room temperature.
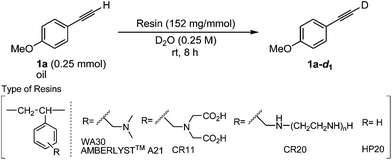
Various polystyrene polymer resins bearing an amine residue within the basic skeleton are readily available, and we have previously utilized basic and neutral resins (WA30, CR11, CR20 and HP20 shown in Table 1) as supports of a heterogeneous transition metal-catalyst for coupling reactions,9 chemoselective reductions10 and oxidations.11 We first investigated the effect of the substituent connected to the polystyrene polymer backbone for the direct deuteration of 4-ethynylanisole (1a) as a terminal alkyne (Table 1). The deuteration of 1a (0.25 mmol: oil) using 115 weight% (wt%) of the polystyrene WA30 resin bearing the tertiary amine residue purchased from the Mitsubishi Chemical Corporation in D2O smoothly proceeded to give the desired deuterated terminal alkyne (1a-d1) with an excellent deuterium content (99% D) and yield (93%) for 8 h (Entry 1). While AMBERLYST™ A21 possessing a structure similar to WA30 (ref. 12) and CR11 bearing the iminodiacetic acid residue as a kind of tertiary amine were also effective (Entries 2 and 3), CR20 possessing secondary and primary amine moieties within the molecule was inefficient as a deuteration catalyst (Entry 4). Meanwhile, the use of the polystyrene resin without amine moieties (HP20) never facilitated the desired deuteration of 1a (Entry 5). WA30 possessing a physically durable structure was chosen as the optimal basic solid catalyst to achieve the high deuterium content of 1a-d1.
| Entry | Resin | D content (%) | Yielda (%) |
|---|---|---|---|
| a WA30, CR11, CR20 and HP20 were commercially available from Mitsubishi Chemical Corporation. AMBERLYST™ A21 was commercially available from the ORGANO Corporation. All resins were washed with water and methanol, then dried in vacuo before using them. | |||
| 1 | WA30 | 99 | 93 |
| 2 | AMBERLYST™ A21 | 93 | 86 |
| 3 | CR11 | 98 | 93 |
| 4 | CR20 | 34 | 96 |
| 5 | HP20 | 0 | 100 |
We next examined the efficiency based on the WA30 usage (Table 2). The deuterium contents of 1a increased in tandem with the WA30 usage (Entries 1, 3 and 5). The efficient deuterium incorporation of 1a to 1a-d1 was never achieved using 10 or 50 wt% WA30 (Entries 2 and 4). Although 115 wt% of WA30 versus 1a was required to obtain the quantitative deuterium efficiency for the shorter reaction time (Entries 4 vs. 5), it is remarkable that WA30 could be reused at least 5 times without any deactivation and technical loss to accomplish the excellent D contents and isolated yield of 1a-d1 (Table 3).
The present reaction efficiencies were significantly affected by the physical state of the substrates (oil or solid, Table 3 vs. eqn (1) and Table 4). An oily substrate, such as 1a, smoothly and efficiently underwent the deuteration in D2O (Table 3). While 1-ethynyl-4-phenylbenzene (1b) as a solid-state substrate was never deuterated in D2O (eqn (1)), the addition of a small amount of toluene as a co-solvent to dissolve the substrate dramatically improved the deuterium content to give the quantitatively deuterated 1b-d1 (the effect of other organic solvents are described in the ESI†). Furthermore, WA30 used in a D2O/toluene mixed-solvent could also be repeatedly reused (see ESI†).
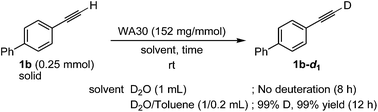 | (1) |
| Entry | Product | Co-solvent | Time (h) | D content (%) [yield (%)] | |
|---|---|---|---|---|---|
| a At 50 °C.b 0.5 mL of the co-solvent was added. | |||||
| Oily substrates | |||||
| 1 |  |
— | 8 | 96 [95] | |
| 2 | 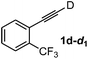 |
— | 8 | 99 [59] | |
| 3 | 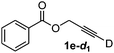 |
— | 8 | 99 [quant.] | |
| 4 | 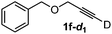 |
— | 8 | 99 [90] | |
| 5 |  |
— | 8 | 95 [93] | |
| 6a | 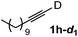 |
— | 16 | 93 [65] | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Solid-state substrates | |||||
| 7 | 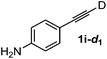 |
— | 8 | 15 [91] | |
| 8 | Toluene | 12 | 97 [97] | ||
| 9 | 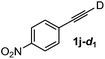 |
— | 8 | 18 [94] | |
| 10 | Tolueneb | 12 | 94 [97] | ||
| 11 | 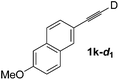 |
— | 8 | 19 [98] | |
| 12 | Toluene | 12 | 96 [quant.] | ||
| 13 | 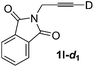 |
— | 8 | 19 [86] | |
| 14a | Toluene | 12 | 35 [89] | ||
| 15 | 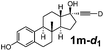 |
— | 8 | 0 [84] | |
| 16a | AcOEtb | 24 | 92 [94] | ||
Various mono aryl- and alkyl-substituted alkynes could be efficiently deuterated to give the corresponding mono-deuterium labeled alkynes (Table 4). 2-Methoxy (1c) and trifluoromethyl (1d) ethynylbenzene as oily substrates were smoothly deuterated with excellent deuterium efficiencies in D2O (1c-d1 and 1d-d1) (Entries 1 and 2). The oily propargyl alcohol derivatives (1e–g) were also efficiently deuterated in quantitative deuterium contents (1e-d1, 1f-d1, 1g-d1) accompanied without hydrolysis of the ester (1e) or decomposition of the benzyl ether (1f) and sulfide moiety (1g) under the present reaction conditions (Entries 3–5). Dodecyne (1h) as an oily aliphatic terminal alkyne was effectively deuterated in D2O by heating at 50 °C (Entry 6). Although the 4-amino (1i) and nitro (1j) ethynylbenzenes and a naphthalene derivative (1k) as solid-state substrates were inefficiently deuterated in D2O (Entries 7, 9 and 11), the addition of toluene as a co-solvent facilitated the deuteration of 1i, 1j and 1k to give the corresponding deuterium-labeled alkynes, respectively (1i-d1, 1j-d1 and 1k-d1) (Entries 8, 10 and 12). On the other hand, the deuteration of the solid-state N-(propargyloxy)-phthalimide (1l) resulted in the low deuterium incorporation regardless of the addition of toluene as a co-solvent (1l-d1) for some unaccountable reason (Entries 13 and 14). Ethynylestradiol (1m), which is crucial medicinal compound as the solid-state substrate, never underwent the deuteration in D2O. The addition of AcOEt as a co-solvent facilitated the deuteration of 1m (Entries 15 vs. 16), while the addition of toluene was ineffective (see ESI†).
Conclusions
We have developed a mild and efficient deuteration method of terminal alkynes by using a heterogeneous basic polystyrene resin (WA30) in D2O under mild conditions. It is noteworthy that oily substrates smoothly underwent the direct deuteration in D2O, while the deuteration of solid substrates could be facilitated by the addition of a small quantity of a co-solvent, such as toluene and AcOEt. A wide variety of functional groups (e.g., nitro, propargyl ester, sulfide, benzyl ether, etc.) could be tolerant under the present mild reaction conditions. WA30 could be repeatedly used without any loss of catalyst activity. The present clean deuteration method of terminal alkynes is expected to be utilized in not only laboratories, but also industrial fields as an economic and eco-friendly reaction.Notes and references
- Review: (a) J. Atzrodt, V. Derdau, T. Fey and J. Zimmermann, Angew. Chem., Int. Ed., 2007, 46, 7744–7765 CrossRef CAS PubMed; (b) Y. Sawama, Y. Monguchi and H. Sajiki, Synlett, 2012, 23, 959–972 CrossRef CAS; (c) J. M. Herbert, J. Labelled Compd. Radiopharm., 2010, 53, 658–661 CrossRef CAS.
- Our recent H/D exchange reactions using heterogeneous platinum metal on carbon; (a) Y. Sawama, Y. Yabe, H. Iwata, Y. Fujiwara, Y. Monguchi and H. Sajiki, Chem.–Eur. J., 2012, 18, 16436–16442 CrossRef CAS PubMed; (b) Y. Sawama, T. Yamada, Y. Yabe, K. Morita, K. Shibata, M. Shigetsura, Y. Monguchi and H. Sajiki, Adv. Synth. Catal., 2013, 355, 1529–1534 CrossRef CAS; (c) T. Yamada, Y. Sawama, K. Shibata, K. Morita, Y. Monguchi and H. Sajiki, RSC Adv., 2015, 5, 13727–13732 RSC.
- (a) J. M. Brown and G. C. Lloyd-Jones, J. Am. Chem. Soc., 1994, 116, 866–878 CrossRef CAS; (b) J. E. Baldwin and R. C. Burrell, J. Org. Chem., 1999, 64, 3567–3571 CrossRef CAS PubMed; (c) J.-C. Cintrat, F. Pillon and B. Rousseau, Tetrahedron Lett., 2001, 42, 5001–5003 CrossRef CAS; (d) M.-Y. Chou, A. B. Mandal and M.-k. Leung, J. Org. Chem., 2002, 67, 1501–1505 CrossRef CAS PubMed; (e) C. H. Oh, H. H. Jung, K. S. Kim and N. Kim, Angew. Chem., Int. Ed., 2003, 42, 805–808 CrossRef CAS PubMed; (f) A. S. K. Hashmi, M. Rudolph, H.-U. Siehl, M. Tanaka, J. W. Bats and W. Frey, Chem.–Eur. J., 2008, 14, 3703–3708 CrossRef CAS PubMed; (g) T. Tsuchimoto, H. Matsubayashi, M. Kaneko, Y. Nagase, T. Miyamure and E. Shirakawa, J. Am. Chem. Soc., 2008, 130, 15823–15835 CrossRef CAS PubMed; (h) G. Zhang, L. Cui, Y. Wang and L. Zhang, J. Am. Chem. Soc., 2010, 132, 1474–1475 CrossRef CAS PubMed.
- Y. Yabe, Y. Sawama, Y. Monguchi and H. Sajiki, Chem.–Eur. J., 2013, 19, 484–488 CrossRef CAS PubMed.
- S. P. Bew, G. D. Hiatt-Gipson, J. A. Lovell and C. Poullain, Org. Lett., 2012, 14, 456–459 CrossRef CAS PubMed.
- G. S. Lewandos, J. W. Maki and J. P. Ginnebaugh, Organometallics, 1982, 1, 1700–1705 CrossRef CAS.
- C. Sabot, K. A. Kumar, C. Antheaume and C. Mioskowski, J. Org. Chem., 2007, 72, 5001–5004 CrossRef CAS PubMed.
- F. Perez, Y. Ren, T. Boddaert, J. Rodriguez and Y. Coquerel, J. Org. Chem., 2015, 80, 1092–1097 CrossRef CAS PubMed.
- (a) Y. Kitamura, K. Taniguchi, T. Maegawa, Y. Monguchi, Y. Kitade and H. Sajiki, Heterocycles, 2009, 77, 521–532 CrossRef CAS; (b) Y. Monguchi, K. Sakai, K. Endo, Y. Fujita, M. Niimura, M. Yoshimura, T. Mizusaki, Y. Sawama and H. Sajiki, ChemCatChem, 2012, 4, 546–558 CrossRef CAS; (c) Y. Monguchi, K. Nozaki, T. Maejima, Y. Shimoda, Y. Sawama, Y. Kitamura, Y. Kitade and H. Sajiki, Green Chem., 2013, 15, 490–495 RSC; (d) Y. Monguchi, Y. Sawama and H. Sajiki, Heterocycles, 2015, 91, 239–264 CrossRef; (e) Y. Monguchi, T. Ichikawa, M. Netsu, T. Hattori, T. Mizusaki, Y. Sawama and H. Sajiki, Synlett, 2015, 26, 2014–2018 CrossRef CAS.
- (a) Y. Monguchi, Y. Fujita, K. Endo, S. Takao, M. Yoshimura, Y. Takagi, T. Maegawa and H. Sajiki, Chem.–Eur. J., 2009, 15, 834–837 CrossRef CAS PubMed; (b) Y. Monguchi, T. Ichikawa, K. Nozaki, K. Kihara, Y. Yamada, Y. Miyake, Y. Sawama and H. Sajiki, Tetrahedron, 2015, 71, 6499–6505 CrossRef CAS.
- Y. Monguchi, F. Wakayama, H. Takada, Y. Sawama and H. Sajiki, Synlett, 2015, 26, 700–704 CrossRef CAS.
- Although WA30 and AMBERLYST™ A21 both partially possess tert-amino functionalities on the polystyrene backbone, the apparent density and total ion-exchange capacity should be different. Nevertheless, both catalysts possess similar high catalyst activities.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the general procedure for deuteration, and NMR spectral data of the products. See DOI: 10.1039/c5ra18921g |
| This journal is © The Royal Society of Chemistry 2015 |