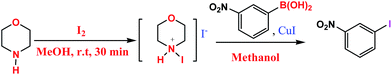
A practical and general ipso iodination of arylboronic acids using N-iodomorpholinium iodide (NIMI) as a novel iodinating agent: mild and regioselective synthesis of aryliodides†
R. H. Tale*,
G. K. Toradmal and
V. B. Gopula
School of Chemical Sciences, S.R.T.M. University, Nanded-431606, India. E-mail: rkht_2008@rediffmail.com
First published on 29th September 2015
Abstract
A mild and efficient protocol for the ipso-iodination of aryl boronic acids using N-iodomorpholinium iodide (NIMI) generated in situ from morpholine and molecular iodine as a novel iodinating agent has been developed. The addition of a catalytic amount of copper iodide found to promote rate enhancement of the iodination reaction and dramatic increase in the yield depending upon the nature of the boronic acid was observed. The mechanistic study revealed that depending upon the nature of the substrate, either the classical ipso substitution or copper catalysed iododeborylation pathway overall dominates the present iodination reaction. The features such as mild reaction conditions, operational simplicity, high to excellent yields, excellent functional group compatibility and low catalyst loading make this method potentially useful in organic synthesis.
Introduction
Development of an efficient, mild and regioselective protocol for the synthesis of aryl halides, in particular aryl iodides is much sought after as they are highly versatile synthetic intermediates widely used in transition metal catalyzed carbon–carbon and carbon–heteroatom bond formation.1 Aryl iodides are also used as precursors for the synthesis of various hypervalent iodine reagents.2 Another important utility of iodoarenes is in the area of labeling of biological targets with short lived radioisotopes for nuclear imaging by positron emission tomography. For instance, Kim et al.3 investigated the possibility of using iodine labeled hypericin derivatives for imaging malignant gliomas with PET and SPECT. Moreover, mild and regioselective iodination of arenes is of particular importance from the perspective of iodine labelled diagnostic and therapeutic agents.4Due to the low reactivity of iodine and related iodinating agents than the corresponding chloro and bromo analogues, synthesis of functionalized aryl iodides is more challenging.5 Generally, iodoarenes are synthesized by the oxidative iodination of arenes with variety of oxidizing agents6 and using classical Sandmeyer reaction7 involving regioselective iodination of arenediazonium salts under acidic conditions.
However, these methods suffer from the limitations such a use of hazardous oxidizing agents, harsh reaction conditions, poor yields, and regioselectivity. Consequently, many alternative methods for the synthesis of aryl iodides have been developed. These include organocatalytic variants of Sandmeyer reaction,8 the use of mercury9 and thallium10 compounds etc., however, these methods also involve the use of toxic reagents. The copper catalyzed synthesis of iodoarenes directly from arenes or aryl bromides as an attractive alternative for the above methods has been reported by Buchwald and co-workers.11
In contrast to many air-sensitive, tend to be easily hydrolysed organoboron compounds,12 boronic acids [R–B(OH)2], are usually crystalline solids, stable to air and moisture and are relatively of low toxicity [benzene boronic acid:13 LD50, oral-rat = 740 mg kg−1] and environmental impact. Apart from being versatile building blocks in organic synthesis, boronic acids find numerous applications such as in the field of material science, biotechnology, medicinal chemistry and supramolecular chemistry.14 The recent advancement in the transition metal catalyzed Miyaura borylation15 and iridium catalysed C–H activation strategies,16 the large number of boronic acids could be readily synthesized without using haloarenes, an unacclaimed perception among scientific community, has advance the field of organoboron chemistry further.
In recent years, ipso substitution of organoboron compounds, in particular, of arylboronic acids/esters/tetrafluoroborate, in which boronic or related group is replaced by entering functional group has emerged a as powerful tool for the regioselective functionalization of arenes. This strategy has been successfully employed for the synthesis of arylazides,17 phenols,17a,18 arylsulfones,17a,19 aryl halides,17a,20,21 nitroarenes17a,22 and amines.17a,23 Based on similar strategy, very recently, we have reported an efficient and green protocol for the ipso iodination of arylboronic acids using CTAB/I2 in aqueous media.24 As compared to other ipso substitutions (ipso nitration, azidation, hydroxylation and amination), relatively the reports on the ipso iodination of arylboronic acids are scares. Moreover, many reported methods for the ipso iodination in the literature17a,19a,f,24 are either performed at high temperature or required large excess of additives/special ligand and high catalyst loading and some of them are having limited substrate scope.24 Therefore, the general, milder and inexpensive alternative is highly desired.
The guiding principle of ipso substitution is that it generally requires the concomitant activation of both the organoboron compounds (via Lewis acid–base type interaction) and electrophilic species (for instance, iodinating agents) which help ipso substitution to occur readily. We reasoned that the activation of I2 with mild base such as morpholine at room temperature would generate in situ N-iodomorpholinium iodide (NIMI). The activation of boronic acid by iodide (I−) ion giving more nucleophilic boron species and its subsequent ipso attack with highly electrophilic N-iodomorpholinium ion could lead to the mild and efficient protocol for ipso iodination of boronic acids at room temperature.
In the present paper, in continuation of our interest in exploring boronic acids as green catalysts or reagents in organic synthesis,24,25 we report herein the copper catalysed an efficient, mild, and general approach for the regioselective synthesis of iodoarenes via ipso iodination of boronic acids using N-iodomorpholinium iodide as iodinating agent at low catalyst loading.
Results and discussion
The iodinating agent used here, N-iodomorpholinium iodide26 was generated in situ from I2 and morpholine. Initially, the ipso iodination of 4-ethylphenyl boronic acid using morpholine and I2 in methanol was considered as a model reaction. A series of experiments were carried to optimize various reaction parameters such as effect of morpholine: I2 ratio, catalyst loading, temperature, solvent and reaction time (Scheme 1).The results are summarized in Table 1. As can be seen from our results, in the absence of morpholine and using I2 alone, the reaction hardly proceeded despite of continuing the reaction for 24 h at room temperature, (Table 1, and entry 1) and use of even higher temperature did not facilitate the reaction (Table 1, entry 2). However, using stoichiometric amount of morpholine and little excess of I2, the above reaction proceeded smoothly but to give low yield, (Table 1, entry 3). In order to improve the product yield, we decided to probe the effect of copper catalyst on this reaction. Thus using catalytic amount (5 mol%) of CuI, considerable increase in the yield of the above reaction was observed (Table 1, entries 3 vs. 4). Under same reaction conditions but using higher temperature, a good yield of corresponding iodoproduct could be obtained within short reaction time (Table 1, entry 5). Use of equimolar amount of morpholine to I2 did not give much favorable result even in the presence of 10 mol% of the catalyst. On the other hand, using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 equiv. of morpholine and I2 and in the presence of 10 mol% of catalyst, 68% yield was obtained at 40 °C within 24 h.
2.4 equiv. of morpholine and I2 and in the presence of 10 mol% of catalyst, 68% yield was obtained at 40 °C within 24 h.
| Entry | Morpholine (equiv.) | I2 (equiv.) | CuI (mol%) | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 0.2 mmol of 4-ethylphenyl boronic acid in 1.5 ml of methanol for the time indicated in table.b Isolate yields by column chromatography.c Under N2 atmosphere.d The boronic acid was completely consumed but no iodoproduct was detected.e 2.0 equiv. of KI or NaI was used as iodinating agent. | ||||||
| 1 | None | 1.2 | None | rt | 24 | 0 |
| 2 | None | 1.2 | None | 65 | 10 | Trace |
| 3 | 1.0 | 1.2 | None | rt | 24 | 32 |
| 4 | 1.0 | 1.2 | 5 | rt | 24 | 40 |
| 5 | 1.0 | 1.2 | 5 | 65 | 10 | 68 |
| 6 | 1.0 | 1.0 | 10 | 65 | 4 | 39 |
| 7 | 1.0 | 2.4 | 10 | 40 | 24 | 68 |
| 8 | 1.0 | 2.4 | 5 | 65 | 4 | 50 |
| 9 | 2.0 | 2.4 | None | rt | 24 | 67 |
| 10 | 2.0 | 2.4 | 5 | rt | 24 | 75 |
| 11 | 2.0 | 2.4 | 5 | 65 | 4 | 74 |
| 12 | 2.0 | 2.4 | 5 | rt | 24 | 62c |
| 13 | 2.0 | 2.4 | 100 | rt | 1 | 65 |
| 14 | 0.2 | 1.0 | None | 40 | 24 | 12 |
| 15 | 0.2 | 2.4 | None | 40 | 24 | 28 |
| 16 | 0.2 | 2.4 | 5 | 40 | 24 | 30 |
| 17 | None | 2.4 | 5 | 40 | 24 | 29 |
| 18 | None | None | 100 | rt | 5 | ndd |
| 19 | None | None | 5 | rt | 24 | 0e |
Despite of several attempts, the yield was still not as good as the process to be called practical. Therefore, to improve the yield further, we decided to use further higher amount of morpholine and iodine. We were delighted to see that using twofold amount of these reagents, more than two fold increases in the yield was observed, (Table 1, entries 3 vs. 9). In the presence of catalytic amount of copper iodide, however, the same reaction furnished 75% yield of the corresponding iodoproduct, (Table 1, and entry 10). Almost similar result was obtained when above reaction was performed at 65 °C but within short reaction time (Table 1, entries 10 vs. 11). These results clearly indicate the striking effect of temperature on the present iodination reaction. Use of inert (N2) atmosphere found to be detrimental for the present reaction as it gave less favourable result, (Table 1, compare entries 11 and 12). As anticipated, using stoichiometric amount of copper catalyst, reaction reached to completion within less than hour but with lower yield even than the uncatalyzed reaction (Table 1, compare entries 9 and 13).
To check whether the reaction can be proceeded even using catalytic amount of morpholine, the model reaction was performed again but this time using catalytic amount of morpholine. As can be seen from our results that the use of catalytic amount of morpholine and little excess of I2 could promote the ipso iodination but to give very low yield, (Table 1, entry 14). Use of twofold excess of I2, however, resulted into more than twofold increase in the yield under same reaction conditions (Table 1, entry 15). We also checked the effect of catalytic amount of morpholine and CuI in the model reaction. Unfortunately, under these conditions the reaction furnished very low yield of the iodoproduct (Table 1, entry 16). Thus the use of catalytic amount of morpholine alone or in combination with CuI did not provide the acceptable results and hence no further attempts of optimizing the catalytic study were made.
Also to check whether the CuI alone or CuI/I2 system could facilitate the ipso iodination of boronic acid, two independent experiments were carried out. One involving copper iodide alone and another one involving catalytic CuI and excess of I2. While CuI/I2 as an iodinating agent found to facilitate the ipso iodination albeit in low yield (Table 1, entry 17), the use of CuI alone did not give any ipso product even in the presence of stoichiometric amount of former (Table 1, entry 18). Finally, the use of alternative iodinating agents such as KI and NaI did not furnish any ipso product (Table 1, entry 19). Thus 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 equiv. of morpholine to I2 ratio was found to be optimal for the success of the reaction.
2.4 equiv. of morpholine to I2 ratio was found to be optimal for the success of the reaction.
We also investigated the effect of different copper salts such as CuCl, CuCN, Cu(OAc)2, CuSO4, and CuO on model reaction using optimized reaction conditions as described above (Table 1, entry 10). The results are summarised in Table 2.
| Entry | Copper salt | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 0.2 mmol of 4-ethylphenyl boronic acid, 0.4 mmol of morpholine, 0.48 mmol of I2 in the presence of 5 mol% of copper salt in 1.5 ml of methanol at room temperature for 24 h.b Isolated yield by column chromatography. | ||
| 1 | None | 67 |
| 2 | CuI | 75 |
| 3 | CuCl | 71 |
| 4 | CuCN | 72 |
| 5 | Cu(OAc)2 | 65 |
| 6 | CuSO4·5H2O | 55 |
| 7 | Cu2O | 71 |
Among various copper salts screened, CuI was found to be the most effective catalyst giving highest yield of the iodoproduct (Table 2, entry 2). The copper salts such as CuCl (Table 2, entry 3), CuCN (Table 2, entry 4) and CuO (Table 2, entry 7) were also found to be effective catalysts, however they furnished lower yields as compared to CuI under same reaction conditions. The other catalysts such as CuSO4 and Cu(OAc)2 found to have no effect on the iodination reaction as they furnished lower yields even than the uncatalyzed reaction (Table 2, compare entries 1, 5 & 6). The reactions involving these two copper salts resulted into relatively complex reaction mixture as compared to other catalysts which probably might be reason behind the low yields obtained using them.
Initially, the methanol was deliberately chosen as a solvent due to high solubility of the salt, N-iodomorpholinium iodide in methanol.26 We also investigated the effect of various solvents such as DCM, acetonitrile, acetone, DMF and THF on the model reaction.
The results are shown in Table 3. As can be seen from our results, amongst all the solvent tested, the methanol was found to be best solvent (Table 3, entry 1). The DCM was found to be another effective solvent for the present reaction but gave little lower yield than methanol (Table 3, entry 2). The acetonitrile (Table 3, entry 3) and DMF (Table 3, entry 4) furnished moderate and low yield respectively and thus proved to be less effective solvents than methanol and DCM. Acetone (Table 3, entry 5) on the other hand found to be fruitless solvent for the present reaction giving only 5% yield. Finally, THF was found to have detrimental effect on the reaction as it gave complex reaction mixture, though the starting material was found to be completely consumed as indicated by TLC (Table 3, entry 6).
| Entry | Solvent | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 0.2 mmol of 4-ethylphenyl boronic acid, 0.4 mmol of morpholine, 0.48 mmol of I2 in the presence of 5 mol% of CuI in 1.5 ml of solvent at room temperature for 24 h.b Isolated yields by column chromatography.c A complex reaction mixture was formed. | ||
| 1 | MeOH | 75 |
| 2 | DCM | 72 |
| 3 | MeCN | 58 |
| 4 | DMF | 36 |
| 5 | Acetone | 5 |
| 6 | THF | —c |
From the detail optimization study, it was found that the CuI had little effect on the present iodination reaction. The other catalysts found to have either lower effect that CuI or less effective even than uncatalyzed reaction. Thus, initial study could not provide the clear information as to the exact role of copper catalyst in the reaction.
In order to have the clear insight into the role of the copper catalyst, solvent effect and reaction conditions on the ipso iodination reaction, we thought it is worthwhile to have the optimization study with the electron deficient substrate. To probe the effect of above parameters on the iodination reaction, this time the ipso iodination of 3-nitrophenylboronic acid was considered as model reaction, Scheme 2.
The results are summaries in Table 4.
| Entry | Morpholine (equiv.) | I2 (equiv.) | CuI (mol%) | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 0.2 mmol of 3-nitrophenyl boronic acid in 1.5 ml of methanol for the time indicated in table.b Isolate yields by column chromatography.c Reaction time was not optimised and continued for 24 h. | ||||||
| 1 | 0.2 | 2.4 | 5 | rt | 24 | 21 |
| 2 | 0.2 | 2.4 | 5 | 40 | 24 | 26 |
| 3 | 2.0 | 2.4 | None | rt | 24 | 20 |
| 4 | 2.0 | 2.4 | None | 40 | 24 | 41 |
| 5 | 1.0 | 2.4 | 5.0 | 40 | 24 | 63 |
| 6 | 2.0 | 2.0 | 5.0 | 65 | 4 | 92 |
| 7 | 2.0 | 2.4 | 5.0 | rt | 24 | 94 |
| 8 | 2.0 | 2.4 | 5.0 | 40 | 24 | >99c |
| 9 | None | 2.4 | 5.0 | 40 | 24 | 15 |
As shown in Table 4, reaction can be proceeded using catalytic amount of morpholine to give 21 and 26% yield of the iodoproduct at room temperature and 40 °C respectively (Table 4, entries 1 and 2). To our surprise, however, using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 equiv. of morpholine to I2 ratio but without copper catalyst, the reaction gave only 20% yield of the corresponding iodoproduct at room temperature. Thus in contrast to the results obtained with 4-ethylphenylboronic acid, the significant effect of copper catalyst on the ipso iodination of 3-nitrobenzeneboronic acid could be observed even at catalytic level of morpholine (Table 4, compare entries 1 and 3). Interestingly, using 1
2.4 equiv. of morpholine to I2 ratio but without copper catalyst, the reaction gave only 20% yield of the corresponding iodoproduct at room temperature. Thus in contrast to the results obtained with 4-ethylphenylboronic acid, the significant effect of copper catalyst on the ipso iodination of 3-nitrobenzeneboronic acid could be observed even at catalytic level of morpholine (Table 4, compare entries 1 and 3). Interestingly, using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 equiv. of morpholine to I2 ratio and 5 mol% of CuI, 63% yield (Table 4, entry 5) of the corresponding iodoproduct was obtained. This result is much closer to the yield obtained with 4-ethylphenylboronic acid when 10 mol% of CuI was used (compare Table 1, entry 7 with Table 4, entry 5). Furthermore, using stoichiometric amount of morpholine and I2, but at higher temperature, the reaction reached to completion rapidly to give 92% yield which is much greater than the yield obtained with 4-ethylphenylboronic acid but using 2
2.4 equiv. of morpholine to I2 ratio and 5 mol% of CuI, 63% yield (Table 4, entry 5) of the corresponding iodoproduct was obtained. This result is much closer to the yield obtained with 4-ethylphenylboronic acid when 10 mol% of CuI was used (compare Table 1, entry 7 with Table 4, entry 5). Furthermore, using stoichiometric amount of morpholine and I2, but at higher temperature, the reaction reached to completion rapidly to give 92% yield which is much greater than the yield obtained with 4-ethylphenylboronic acid but using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 equiv. of morpholine and I2 ratio under almost similar conditions (compare Table 1, entry 11 with Table 4, entry 6). To our delight, using 2
2.4 equiv. of morpholine and I2 ratio under almost similar conditions (compare Table 1, entry 11 with Table 4, entry 6). To our delight, using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 equiv. morpholine to I2 ratio and 5 mol% of CuI, excellent and almost quantitative yield of the 3-nitro-1-iodobenzene were obtained at room temperature and 40 °C respectively. All these findings clearly indicates the pronounced effect of copper iodide on the iodination of 3-nitrophenylboronic acid as compared to the 4-ethylphenylboronic acid. Surprisingly, in the absence of morpholine but using CuI/I2 as iodinating agent, much lower yield than that of 4-ethylphenylboronic acid was obtained under exactly same reaction conditions (compare Table 1, entry 15 with Table 4, entry 9). The above results clearly indicates the crucial role of morpholine in the present iodination reaction.
2.4 equiv. morpholine to I2 ratio and 5 mol% of CuI, excellent and almost quantitative yield of the 3-nitro-1-iodobenzene were obtained at room temperature and 40 °C respectively. All these findings clearly indicates the pronounced effect of copper iodide on the iodination of 3-nitrophenylboronic acid as compared to the 4-ethylphenylboronic acid. Surprisingly, in the absence of morpholine but using CuI/I2 as iodinating agent, much lower yield than that of 4-ethylphenylboronic acid was obtained under exactly same reaction conditions (compare Table 1, entry 15 with Table 4, entry 9). The above results clearly indicates the crucial role of morpholine in the present iodination reaction.
It's worth mentioning here that with electron rich 4-ethylphenylboronic acid only 10% increment in the yield while in case of electron deficient 3-nitophenylboronic acid dramatic increment in the yield (94% vs. 20 mol%) in copper catalysed reaction as compared to the catalyst free reaction was observed, (Table 1, entries 6 and 7 vs. Table 4, entries 2 and 6). Explicitly, present iodination reaction is strongly dependence upon the electronic nature of the substituent. It can be concluded that the copper catalyst has little effect on the ipso iodination of electron rich substrate but at the same time it dramatically accelerates the reaction when electron withdrawing substituent is present in the boronic acid.
Similar to 4-ethylphenylboronic acid, we also investigated the effect of different copper salts and solvents on the model reaction in this case also. The results are summarized in Table 5. As can be seen from our results, CuI and CuCl (Table 5, entries 2 and 3) were found to be equally effective catalysts giving near quantitative yield of the corresponding iodoproduct under optimized reaction conditions. In contrast to the results obtained with 4-ethylphenylboronic acid, all the copper salts screened here shown pronounced catalytic effect on the present iodination reaction as compared to the uncatalyzed one giving high to near quantitative yield of the corresponding iodoproduct.
| Entry | Solvent | Copper salt (CuX) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 0.2 mmol of 3-nitrolphenyl boronic acid, 0.4 mmol of morpholine, 0.48 mmol of I2 in the presence of 5 mol% of CuX in 1.5 ml of solvent at 40 °C for 24 h.b Isolated yields by column chromatography. | |||
| 1 | MeOH | CuI | >99 |
| 2 | MeOH | CuCl | 99 |
| 3 | MeOH | Cu2O | 80 |
| 4 | MeOH | CuSO4·H2O | 94 |
| 5 | MeOH | Cu(OAc)2 | 89 |
| 6 | DCM | CuI | 10 |
| 7 | MeCN | CuI | 59 |
| 8 | DMF | CuI | Trace |
| 9 | Acetone | CuI | 6 |
In sharp contrast, the copper salts, Cu(OAc)2 and CuSO4·H2O which were found to be less effective catalysts even than uncatalyzed reaction in case of 4-ethylphenylboronic acid, proved to be highly effective catalysts for iodination of 3-nitophenylboronic acid giving very high and excellent yield respectively (Table 5, entries 4 and 5).
Next, the effect of all the solvents investigated previously also tested here. Once again, methanol found to be the best solvent giving highest yield among all the solvent tested. However, the DCM, acetonitrile, and DMF which were proved to be the effective solvents in earlier study, in the present case, except acetonitrile, they found to be fruitless solvents giving very low or poor yield.
Thus the CuI and methanol were found to be most suitable catalyst and solvent respectively for the substrates, 4-ethyl and 3-nitrophenylboronic acid. It's worth mentioning here that the reaction parameters such as nature of copper catalyst, catalyst loading, morpholine: I2 ratio, temperature and solvent all have strikingly different effect on the ipso iodination of electronically contrasting substrates.
The ipso iodination of both, 4-ethyl and 3-nitobenzeneboronic acid can be proceeded very rapidly with good and excellent yields respectively but at the expense of high catalyst loading and temperature, (Table 1, entry 11 and Table 4, entry 8). However, without necessarily relying on such a drastic conditions, excellent results could be obtained using low catalyst loading and under mild conditions. Thus 1.0 equiv. of boronic acid, 2.0 equiv. morpholine, and 2.4 equiv. of I2 in the presence of 5 mol% CuI in methanol at room temperature for 24 h were considered to be the best reaction conditions and used for further study (Scheme 3).
With optimized reaction conditions at hand, next the scope and generality of the reaction was explored using diverse arylboronic acids. The results are summarized in Table 6. As can be seen from our results, the reaction is general as the structurally diverse boronic acids reacted smoothly under present reaction conditions giving high to excellent yields of the corresponding aryliodides.27 Though electron neutral phenylboronic acid provided moderate yield (∼39%) of the iodoproduct, (Table 6, entry 1), the electron rich and electron deficient boronic acids invariably shown much higher reactivity under optimized conditions as described previously.
| Entry | Boronic acid | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 0.2 mmol of boronic acid, 0.4 mmol of morpholine, 0.48 mmol of I2 in the presence of CuI (5 mol%) in methanol (1.5 ml) at room temperature for 24 h.b Isolated yields by column chromatography.c A complex mixture was obtained. | |||
| 1 | 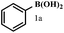 |
 |
39 |
| 2 |  |
 |
75 |
| 3 |  |
 |
90 |
| 4 |  |
 |
87 |
| 5 |  |
 |
95 |
| 6 |  |
 |
—c |
| 7 |  |
 |
85 |
| 8 |  |
 |
53 |
| 9 | 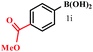 |
 |
82 |
| 10 |  |
 |
92 |
| 11 |  |
 |
85 |
| 12 |  |
 |
93 |
| 13 |  |
 |
90 |
| 14 |  |
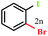 |
78 |
| 15 |  |
 |
96 |
| 16 |  |
 |
95 |
| 17 | 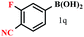 |
 |
81 |
| 18 |  |
 |
94 |
| 19 |  |
 |
83 |
| 20 |  |
 |
28 |
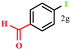
The arylboronic acids bearing alkyl- (Table 6, entries 2 and 3), alkylthio- (Table 6, entries 4, and 5), carbonyl- (Table 6, entries 7 and 8), alkoxy- (Table 6, entry 12), alkoxycarbonyl- (Table 6, entries 9 & 10), amide- (Table 6, entry 11), halide- (Table 6, entries 12–16), cyano- (Table 6, entry 17), nitro- (Table 6, entry 18), sulfonyl- (Table 6, entry 19) etc. participated successfully in the ipso iodination process. Notably, one of the most electron deficient boronic acids, for instance, 3-nitrobenzeneboronic acid had shown exceptionally high reactivity giving near quantitative yield of the iodoproduct using these conditions.
Of particular importance, ortho substituted, sterically hindered boronic acids such as 2-chloro-4-methoxy- (Table 6, entry 12) and 2-bromophenylboronic acid (Table 6, entry 14) also found to be the suitable substrates in the present reaction giving excellent and good yield of the corresponding iodoproducts respectively.
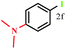
The representative heteroarylboronic acids such pyridine-4-boronic acid and 5-trifluoromethyl-pyridine-3-boronic acid were also investigate under present reaction conditions. The corresponding 3-iodo-5-trifluoromethyl-pyridine was obtained in 28% yield (Table 6, entry 20) and unfortunately, the isolation of product from the reaction of pyridine-4-boronic acid by column chromatography was almost become impossible probably due to rapid formation of corresponding N-oxide and other side products (result not shown). The low yield in case of 5-trifluoromethyl-pyridine-3-boronic acid was mainly due to the complications arised during column chromatography (despite of using triethylamine treated silica) as TLC indicated complete consumption of starting material. For similar reason, highly electron rich, (4-N,N-dimethylamino)phenyl boronic acid (Table 6, entry 6) failed to furnish any ipso product.
Its worth mentioning here that very recently we reported the green protocol for the ipso iodination of arylboronic acids using CTAB/I2 in aqueous media.24 Though the method provides the green alternative for the synthesis of aryl iodides, however, it required high temperature (80 °C) and has limited substrate scope as only electron rich boronic acids were found to be the suitable substrates. As compared to our previous work, the present method involving novel NIMI as iodinating agent is highly versatile as both electron rich as well as electron deficient arylboronic acids and heteroarylboronic acids reacted efficiently to give good to excellent yield of the aryliodides. Furthermore, the method is mild, operationally simple and could be carried out in open atmosphere without the need for special ligand or additives using very low catalyst loading. Thus these two methods have significant differences and the present method provides alternative ipso iodination tool for synthetic applications.
As alkylboronic acids such as methyl- and indanylboronic acids (results not shown) did not react at all under present reaction conditions, it was clear that the presence of aryl group was the prerequisite for the success of the iodination reaction. In general, the reaction was highly regioselective and invariably no formation of other side products was observed.
Finally, we sought to determine the plausible mechanism(s) for the present ipso iodination process. Regarding mechanistic aspect of the present reaction, we carefully noted many crucial facts during our optimization study: (1) the reaction can be proceeded using catalytic amount of morpholine (Table 1, entries 2 and 3 and Table 4, entries 1 and 2) indicates that morpholine might be regenerated during the catalytic cycle, (2) the iodination does not proceed at all even using stoichiometric amount of CuI or more than twofold excess of I2 alone, but using catalytic amount of CuI and excess I2 the ipso iodination proceeded smoothly even at room temperature. We believe that that I2 oxidizes CuI to copper(II) species which must acts as a true catalyst, (3) there is little effect of CuI on the reaction with 4-ethylphenylboronic acid but the effect is pronounced in case of electron deficient 3-nitrophenylboronic acid indicates that CuI plays pivotal role in the catalysis of iodination of 3-nitrophenylboronic acid and related substrates, (4) under inert atmosphere, the yield obtained was more or less similar to that of uncatalyzed reaction (Table 1, entries 15 vs. 10) justifying the need for air atmosphere to facilitate the copper catalysed pathway, (5) the fact that in the absence of morpholine but using CuI and I2 very low yield was obtained (Table 1, entries 15 vs. 17) and using other iodinating agents such as KI or NaI, the reaction did not proceed at all even in the presence of stoichiometric amount of CuI evident the key role of NIMI for the success of the reaction, (6) the transmetallation of organoboron compound to organocopper is not a general reaction. Due to their similar bond energies and electronegativities such a transmetallation of organoboron to organocopper reagents is limited to the preparation of alkenylcopper and to some extent to unfunctionalized alkylcopper compounds.28 This is why the popular Chan–Lam coupling requires either stoichiometric copper catalyst or catalytic copper strictly under oxygen atmosphere or other primary oxidant in the presence of 2–3 equivalent of amine base and special ligand. In case of 3-nitrophenylboronic acid the use of excess morpholine can only proceed the reaction successfully. Therefore, we believe that in the present CuI catalysis such a transmetallation of 3-nitrophenylboronic acid and other electron deficient substrates to the corresponding organocopper reagents must be a slow process and the use of excess morpholine must compensate for the same ultimately giving satisfactory result.
All these findings lead us to speculate that the present ipso iodination reaction can be attributed to the multiple underlying mechanisms such as concomitant metal free ipso-iodination and NIMI/morpholine assisted copper-catalyzed deiodoborylation pathway and depending upon the nature of substrate either of them overall dominates the reaction. On this basis, we proposed the plausible mechanism(s) for the ipso iodination of the boronic acid as outline in Scheme 4.
The N-iodomorpholinium iodide 2 generated from morpholine 1 and I2 acts as actual iodinating agent. The iodide ion activates the boronic acid 3 via Lewis acid–base type interaction leading to the formation of ionic species 4. The ipso attack involving N-iodomorpholinium ion lead to the formation of iodocompound 5 and morpholine is regenerated and reused again. As regard to the copper catalysed deiodoborylation pathway, we believe that the oxidation of CuI 6 with I2 generates Cu(II) species 7 which supposed to be the actual copper catalyst. The transmetallation between 7 and 4 forms the organocuprate 8 which on air oxidation or oxidation with equilibrated O2 in methanol29 results into the formation of aryl copper(III) intermediate 9. The reductive elimination of 9 yield the aryliodides 5 and Cu(I) species is regenerated and reused again. Alternatively, the improvement of the yield in correlation to the added morpholine can also be explained on the basis of participation of morpholine–boronic acid “ate complex” 10 in copper catalysed deiodoborylation pathway (Scheme 4, path C). This also strongly support the need for excess morpholine for the smooth proceeding of the reaction. However, the possibility of stabilization of unstable CuI2 by morpholine as a ligand cannot be ruled out at this point of time.
Experimental section
General information
All the solvents were redistilled before used. Analytical TLCs were performed on Merck silica gel 60F254 plates. The boronic acids were purchased from Johnson Matthey Chem. Ltd and other chemicals were purchased from Sigma Aldrich Chem. Ltd. The IR spectra (ATR mode) were recorded on Brucker IR spectrometer. The 1H and 13C NMR spectra (in CDCl3) were recorded on Agilent 400 or Bruker Avance-II 400 MHz spectrometer. The chemical shift values are on a δ scale and TMS was used as an internal standard. The 19F NMR spectra were recorded in DMSO-d6 on Bruker Avance II 400 MHz spectrometer using CFCl3 as internal standard. Abbreviations used are: s (singlet), d (doublet), t (triplet), dd (double doublet), dt (doublet triplet), m (multiplet). The high-resolution mass spectral analysis was performed on Micromass Q-Tof (ESI-HRMS) and GCMS were recorded on Shimadzu gas chromatograph.General procedure for the synthesis of aryl iodides
To a 10 ml round bottomed flask charged with morpholine (0.4 mmol, 2 equiv.) and I2 (0.48 mmol, 2.4 equiv.) was added methanol (1.5 ml) and the reaction mixture was stirred for 30 minutes at room temperature. Then the appropriate arylboronic acid (0.2 mmol) and CuI (2 mg, 0.01 mmol) was added to it and the mixture was stirred vigorously at room temperature for 24 h. After completion of the reaction (monitored by TLC), the reaction mixture was quenched with 10% sodium thiosulphate solution (to remove excess of iodine) and extracted with ethyl acetate (3 × 10 ml) and the combined organic extract was washed with water and brine. The evaporation of solvent under vacuum after drying over anhy. Na2SO4 followed by column chromatography (petroleum ether (100%) or petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate, 9
ethyl acetate, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave the analytically pure product. (Note: direct column chromatography of crude product adsorbed on silica followed by washing the effluent with Na2S2O3 solution, drying and evaporation of the solvent gave exactly similar results).
1) gave the analytically pure product. (Note: direct column chromatography of crude product adsorbed on silica followed by washing the effluent with Na2S2O3 solution, drying and evaporation of the solvent gave exactly similar results).
Conclusions
In conclusion, we have developed the general and efficient protocol for the regioselective synthesis of aryl iodides via ipso-iodination of arylboronic acids using N-iodomorpholinium iodide as iodinating agent under mild conditions. The method utilizes the readily accessible and cheap reagents, morpholine & I2, copper iodide as catalyst, nonhazardous methanol as a solvent and could be performed in open atmosphere without the need for any special ligand or additives. The mild reaction conditions, operational simplicity, high to excellent yields, excellent functional group compatibility and low catalyst loading are the noteworthy features of the present method which makes it an attractive alternative for the synthesis of valuable aryl iodides. The present method would attract much attention of chemical community at large.Acknowledgements
RHT thanks Department of Science and Technology (DST) Ministry of Science, India for financial support of this project (SR/FT/CS-85/2011), GVB and TGK thank UGC India, for Rajiv Gandhi National Fellowship.Notes and references
- (a) Metal catalyzed cross coupling reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, 1997 Search PubMed; (b) Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize J. Seechurn, C. C. Carin, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed; (c) Palladium Catalysed Cross Coupling Reactions of Organoboron Compounds N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- (a) T. Wirth, M. Ochiai, A. Varvgolis, V. V. Zhdankin, G. F. Koser, H. Tohma and Y. Kita, Topics in Current Chemistry: Hypervalent Iodine Chemistry/Modern Developments in Organic Synthesis, Springer-Verlag, Berlin, 2002, pp. 1–248 Search PubMed; (b) A. Varvoglis, Hypervalent Iodine in Organic Synthesis, Academic Press, London, 1997, pp. 1–223 Search PubMed; (c) P. Stang and V. V. Zhadankin, Chem. Rev., 1996, 96, 1123 CrossRef CAS PubMed; (d) A. Parra and S. Reboredo, Chem.–Eur. J., 2013, 19, 17244 CrossRef CAS PubMed.
- S. W. Kim, J. H. Park, S. D. Yang, M. G. Hur, Y.-S. Kim, J.-S. Chai, Y. S. Kim and K. H. Yu, Bull. Korean Chem. Soc., 2004, 25, 1147–1150 CrossRef CAS.
- (a) M. K. Dewanjee, Radioiodination : Theory, Practice and Biomedical Applications, Kluwer Academic Publishers, Bosten, 1992 Search PubMed; (b) R. Weissleder, Science, 2006, 312, 1168–1171 CrossRef CAS PubMed.
- J. March, Advance Organic Chemistry, Wiley-Interscience, 4th edn, New York, 2000 Search PubMed.
- (a) J. Iskra, S. Stavber and M. Zupan, Synthesis, 2004, 1869–1873 CrossRef CAS; (b) N. C. Ganguly, S. K. Barik and S. Dutta, Synthesis, 2010, 1467–1472 CrossRef CAS; (c) K. M. Sosnowski and L. Skulski, Synthesis, 2006, 1195–1199 Search PubMed; (d) A.-S. Castanet, F. Colobert and P.-E. Broutin, Tetrahedron Lett., 2002, 43, 5047–5048 CrossRef CAS; (e) P. Lulinski and L. Skulski, Bull. Chem. Soc. Jpn., 1997, 70, 1665 CrossRef CAS; (f) P. Lulinski, A. Kryska, M. Sosnowski and L. Skulski, Synthesis, 2004, 441–445 CAS; (g) B. Krassowska-Swiebocka, P. Lulinkski and L. Skulski, Synthesis, 1995, 926 CrossRef CAS; (h) H. O. Wirth, O. Konigstein and W. Kern, Liebigs Ann.Chem., 1977, 42, 4049 Search PubMed; (i) S. Ahmed and S. Razaq, Tetrahedron, 1976, 32, 503 CrossRef; (j) H. Suzuki, K. Nakamura and R. Goto, Bull. Chem. Soc. Jpn., 1966, 39, 128 CrossRef CAS; (k) H. Suzuki, Bull. Chem. Soc. Jpn., 1970, 43, 481 CrossRef CAS; (l) A. Shimizu, K. Yamataka and T. Isoya, Bull. Chem. Soc. Jpn., 1985, 58, 1611 CrossRef CAS; (m) A. Bachki, F. Foabelo and M. Yus, Tetrahedron, 1994, 50, 5139 CrossRef CAS; (n) W.-W. Sy, B. A. Lodge and A. W. By, Synth. Commun., 1990, 20, 877 CrossRef CAS PubMed.
- T. Sandmeyer, Chem. Ber., 1884, 1633 CrossRef PubMed.
- (a) V. D. Filimonov, N. I. Semenischeva, E. A. Krasnokutskaya, A. N. Tretyakov, H. Y. Hwang and K.-W. Chi, Synthesis, 2008, 185–187 CrossRef CAS; (b) V. D. Filimonov and P. Knochel, Synthesis, 2007, 81–84 Search PubMed; (c) A. Hubbard, T. Okazaki and K. K. Laali, J. Org. Chem., 2008, 73, 316–319 CrossRef CAS PubMed.
- R. W. Fish and M. Rosenblum, J. Org. Chem., 1965, 1253 CrossRef CAS.
- A. McKillop, J. D. Hunt, M. J. Zelesko, J. S. Fowler, E. C. Taylor, G. Mc-Gillivray and F. Kienzle, J. Am. Chem. Soc., 1971, 93, 484 Search PubMed.
- A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 14844–14845 CrossRef CAS PubMed.
- A. Pelter, K. Smith and H. C. Brown, Borane Reagents, Academic Press, London, 1998 Search PubMed; K. Smith, Organoboron Chemistry, in Organometallic in Synthesis, ed. M. Schlosser, Wilet Chinchester, 1994 Search PubMed.
- Boron, Metallo-Boron, Compounds and Boranes, ed. R. M. Adams, Wiley, New York, 1964, p. 693, data quoted in Registry of Toxic Effect of Chemicals Substances, NIOSH, 2002 Search PubMed.
- (a) D. G. Hall, Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, 2011, and references cited therein Search PubMed; (b) D. G. Hall, Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, Wiley-VCH, 2nd edn, Weinheim, 2011, and references cited therein Search PubMed; (c) Y. Guan and Y. Zhang, Chem. Soc. Rev., 2013, 42, 8106 RSC; (d) J. N. Cambre and B. S. Sumerlin, Polymer, 2011, 52, 4631 CrossRef CAS PubMed.
- For metal catalyzed borylation reactions: see (a) W. K. Chow, Y. O. Yuen, P. Y. Choy, C. M. So, C. P. Lau, K. W. Wong and F. Y. Kwong, RSC. Adv., 2013, 3, 12518–12539 RSC; (b) T. Ishiyama, M. Murata and N. Miyura, J. Org. Chem., 1995, 60, 7508–7510 CrossRef CAS; (c) A. L. S. Thomson, G. Kabalka, M. R. Akula and J. W. Huffman, Synthesis, 2005, 547–550 Search PubMed; (d) A. Molander, L. N. Cavalcanti and C. Garcia-Garcia, J. Org. Chem., 2013, 78, 6427–6439 CrossRef PubMed; (e) G. A. Molander, S. L. J. Trice and S. M. Kennedy, Org. Chem., 2012, 77, 8678–8688 CrossRef CAS PubMed; (f) W. Tang, S. Keshipeddy, Y. Zhang, X. Wei, J. Savoie, N. D. Patel, N. K. Yee and C. H. Senanayake, Org. Lett., 2011, 13, 1366–1369 CrossRef CAS PubMed; (g) S. Dundik and G. C. Fu, J. Am. Chem. Soc., 2012, 134, 10693–10697 CrossRef PubMed; (h) J. Takagi, A. Kamon, T. Ishiyama and N. Miyaura, Synlett, 2002, 1880–1882 CAS; (i) J. Takagi, K. Takahashi, T. Ishiyama and N. Miaura, J. Am. Chem. Soc., 2002, 124, 8001–8006 CrossRef CAS PubMed; (j) D. G. Hall, Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, 2011, and references cited therein Search PubMed.
- For borylation of arenes via C–H activation see: (a) J. M. Murphy, X. Liao and J. F. Hartwig, J. Am. Chem. Soc., 2007, 129, 15434–15435 CrossRef CAS PubMed; (b) B. Partridge and J. F. Hartwig, Org. Lett., 2013, 15, 140–143 CrossRef CAS PubMed; (c) S. Sebelius, J. Olsson and K. J. Szabo, J. Am. Chem. Soc., 2005, 127, 10478 CrossRef CAS PubMed; (d) V. J. Olsson and K. J. Szabo, Angew. Chem., Int. Ed., 2007, 46, 689 CrossRef PubMed; (e) V. J. Olsson, S. Sebelius, N. Selander and K. J. Szabo, J. Am. Chem. Soc., 2006, 128, 4588 CrossRef CAS PubMed; (f) V. J. Olsson and K. J. Szabo, Org. Lett., 2008, 10, 3129 CrossRef CAS PubMed; (g) D. Hall, Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, 2011, and references cited therein Search PubMed; (h) M. Murata, S. Watanabe and Y. Masuda, J. Org. Chem., 1997, 62, 6458 CrossRef CAS; (i) M. Murata, T. Oyama, S. Watanabe and Y. Masuda, J. Org. Chem., 2000, 65, 164 CrossRef CAS PubMed; (j) C. Kleeberg, L. Dang, Z. Lin and T. B. Marder, Angew. Chem., Int. Ed., 2009, 48, 5350 CrossRef CAS PubMed.
- (a) C.-Z. Tao, X. Cui, A.-X. Liu, L. Liu and Q.-X. Guo, Tetrahedron Lett., 2007, 48, 3525–3529 CrossRef CAS PubMed; (b) S. Mohammed, A. K. Padala, A. Dar, B. Singh, B. Sreedhar, R. A. Vishwakarma and S. B. Bharate, Tetrahedron, 2012, 68, 8156–8162 CrossRef CAS PubMed.
- A. Kar, I. A. Sayeed, W. F. Low, H. M. Kaiser, M. Beller and M. K. Tse, Org. Lett., 2007, 9, 3405 CrossRef CAS PubMed.
- (a) S. Guo, L. Lu and H. Cai, Synlett, 2013, 24, 1712–1714 CrossRef CAS; (b) J. Xu, X. Wang, C. Shao, D. Su, G. Cheng and Y. Hu, Org. Lett., 2010, 12, 1964–1967 CrossRef CAS PubMed; (c) C. Zhu, R. Wang and J. R. Falck, Org. Lett., 2012, 14, 3494–3497 CrossRef CAS PubMed; (d) G. A. Molander and L. N. Cavalcanti, J. Org. Chem., 2011, 76, 623–630 CrossRef CAS PubMed; (e) A. Gogoi and U. Bora, Synlett, 2012, 23, 1079–1081 CrossRef CAS; (f) H.-L. Qi, D.-S. Chen, J.-S. Ye and J.-M. Huang, J. Org. Chem., 2013, 78, 7482–7487 CrossRef CAS PubMed; (g) D.-S. Chen and J.-M. Huang, Synlett, 2013, 24, 499–501 CrossRef CAS; (h) G. K. S. Prakash, S. Chacko, C. Panja, T. E. Thomas, L. Gurung, G. Rasul, T. Mathew and G. A. Olah, Adv. Synth. Catal., 2009, 351, 1567–1574 CrossRef CAS PubMed.
- (a) C. Thiebes, G. K. S. Prakash, N. A. Petasis and G. A. Olah, Synlett, 1998, 141–142 CrossRef CAS; (b) L. Niu, H. Zhang, H. Yang and H. Fu, Synlett, 2014, 25, 995–1000 CrossRef CAS; (c) M. P. Akula, M. L. Yao and G. W. Kabalka, Tetrahedron Lett., 2010, 51, 1170–1171 CrossRef CAS PubMed; (d) F. Tramutola, L. Chiummiento, M. Funicello and P. Lupatteli, Tetrahedron Lett., 2015, 56, 1122–1123 CrossRef CAS PubMed; (e) G. Y. Zhang, G. L. Lv, L. P. Li, F. Chen and J. Cheng, Tetrahedron Lett., 2011, 52, 1993–1995 CrossRef CAS PubMed.
- For the synthesis of aryl halides from organoboron compounds see (a) B. M. Partridge and J. F. Hartwig, Org. Lett., 2013, 15, 140–143 CrossRef CAS PubMed; (b) G. A. Molander and L. N. Cavalcanti, J. Org. Chem., 2011, 76, 7195–7203 CrossRef CAS PubMed; (c) T. Furuya, H. M. Kaiser and T. Ritter, Angew. Chem., Int. Ed., 2008, 47, 5993–5996 CrossRef CAS PubMed; (d) T. Furuya, D. Benitez, E. Tkatchouk, A. E. Strom, P. P. Tang, W. A. Goddard and T. Ritter, J. Am. Chem. Soc., 2010, 132, 3793–3807 CrossRef CAS PubMed; (e) T. Furuya and T. Ritter, Org. Lett., 2009, 11, 2860–2863 CrossRef CAS PubMed; (f) G. W. Kabalka and E. E. Gooch, J. Org. Chem., 1980, 45, 3578 CrossRef CAS; (g) G. W. Kabalka and E. E. Gooch, J. Org. Chem., 1981, 46, 2582 CrossRef CAS; (h) G. W. Kabalka, V. Namboodiri and M. R. Akula, J. Labelled Compd. Radiopharm., 2001, 44, 921 CrossRef CAS PubMed; (i) G. W. Kabalka and A. R. Mereddy, Organometallics, 2004, 23, 4519 CrossRef CAS; (j) R. H. Szumigala, P. N. Devine, D. R. Gauthier and R. P. Volante, J. Org. Chem., 2004, 69, 566 CrossRef CAS PubMed; (k) A. L. S. Thompson, G. W. Kabalka, M. R. Alkula and J. W. Huffman, Synthesis, 2005, 547 CAS.
- (a) G. K. S. Prakash, C. Panja, T. Mathew, V. Surampudi, N. A. Petasis and G. A. Olah, Org. Lett., 2004, 6, 2205–2207 CrossRef CAS PubMed; (b) S. Salzbrunn, J. Simon, G. K. S. Prakash, N. A. Petasis and G. A. Olah, Synlett, 2000, 1485–1487 CAS; (c) S. Manna, S. Maity, S. Rana, S. Agasti and D. Maiti, Org. Lett., 2012, 14, 1736–1739 CrossRef CAS PubMed; (d) X. F. Wu, J. Schranck, H. Neumann and M. Beller, Chem. Commun., 2011, 47, 12462–12463 RSC; (e) S. B. Bharate, R. R. Singh and R. A. Vishwakarma, Tetrahedron Lett., 2012, 53, 5958–5960 CrossRef PubMed.
- (a) S. Voth, J. W. Hollet and J. McCubbin, J. Org. Chem., 2015, 80, 2545–2553 CrossRef CAS PubMed; (b) Q. Xiao, L. Tian, T. Renchang, Y. Xia, D. Qiu, Y. Zhang and J. Wang, Org. Lett., 2012, 14, 4230–4233 CrossRef CAS PubMed; (c) V. Coeffard, X. Moreau, C. Thomassigny and C. Greck, Angew. Chem., Int. Ed., 2013, 52, 5684–5686 CrossRef CAS PubMed; (d) K. W. Anderson, P. M. Mendez, J. Priego and S. L. Buchwald, J. Org. Chem., 2003, 68, 9563–9573 CrossRef CAS PubMed; (e) C. Zhu, G. Li, D. H. Ess, J. R. Falck and L. Kurti, J. Am. Chem. Soc., 2012, 134, 18253–18256 CrossRef CAS PubMed.
- R. H. Tale, G. K. Toradmal, V. B. Gopula, A. H. Rodge, R. P. Pawar and K. M. Patil, Tetrahedron Lett., 2015, 56, 2699–2703 CrossRef CAS PubMed.
- (a) R. H. Tale and R. N. Adude, Tetrahedron Lett., 2006, 47, 7263–7265 CrossRef CAS PubMed; (b) R. H. Tale, A. D. Sagar, H. D. Santan and R. N. Adude, Synlett, 2006, 3, 415–418 CrossRef; (c) A. D. Sagar, R. H. Tale and R. N. Adude, Tetrahedron Lett., 2003, 44, 7061–7063 CrossRef CAS.
- (a) R. V. Rice and G. D. Beal, US Pat. 2,290,710, July 21, 1943; (b) M. Koyama, N. Ohtani, F. Kai, I. Moriguchi and S. Inouye, J. Med. Chem., 1987, 30, 552 CrossRef CAS.
- The analytical data for representative compounds are provided below: 4-ethyliodobenzene 2b: yellow liquid, 75% 1H NMR: δ 1.20 (t, 3H), 2.57 (q, 2H), 6.93 (dd, 2H), 7.57 (dd, H). EI-MS [M]+ = 232. 4-Isopropyl iodobenzene 2c: yellow liquid, 90% 1H NMR: (CDCl3, 400 MHz) δ 1.25 (d, 6H), 2.68 (septet, 1H), 6.98 (dd, 2H), 7.61 (dd, H); EI-MS [M]+ = 246. Isopropyl-4-iodophenyl sulphide 2e: yellow liquid, yield 95% 1H NMR (CDCl3, 400 MHz): δ 1.27 (d, 6H), 3.35 (septet, 1H), 7.10 (dd, 2H), 7.58 (dd, H). 13NMR (CDCl3, 400 MHz): δ 23.00, 36.1, 91.7, 133.2, 135.7, 137.7; GC-MS RT= 11.01[m/z] = 278. HRMS; calcd for: C9H11IS, 277.9626; found 277.962, 4-carboethoxyiodobenzene 2j: yellow liquid, yield 92% 1H NMR (CDCl3, 400 MHz): δ 1.38 (t, 3H), 4.35 (q, 2H), 7.73 (dt, 2H), 7.78 (dt, 2H). 13C NMR (CDCl3, 400 MHz): δ 14.5, 61.2, 100.6, 129.9, 131, 137.6, 166. 2-Chloro-1-iodo-4-methoxybenzene 2l: faint yellow oil, yield 93% 1H NMR (CDCl3, 400 MHz): δ 3.78 (s, 3H), 6.55 (dd, J = 2.97 Hz, 1H), 7.02 (d, J = 2.88 Hz, 1H), 7.67 (d, J = 8.78 Hz, 1H). 1-Bromo-3-iodo-5-methylbenzene 2o: light yellow liquid, yield 96% 1H NMR (CDCl3, 400 MHz): δ 2.28 (s, 3H), 7.29 (s, 1H), 7.46 (s, 1H), 7.65 (s, 1H). HRMS; calcd for: C7H6BrI, 295.8698; found 295.8698. 1-Iodo-4-(methylsulfonyl)benzene 2s: while solid, yield 83% 1H NMR (CDCl3, 400 MHz): δ 3.03 (s, 3H), 7.63 (m, 2H), 7.91 (m, 2H), 13C NMR (CDCl3, 400 MHz): δ 44.4, 101.6, 128.7, 138.6, 140.1. 3-(Trifluoromethyl)-5-iodopyridine 2t: white solid, yield 28% (isolated by column chromatography using trimethyl amine pretreated silica gel) 1H NMR (CDCl3, 400 MHz): δ 8.24 (m, 1H), 8.83 (s, 1H), 9.03 (s, 1H), HRMS (calculated for C6H3NIH = 272.9271); found = 273.9341 (M + H)+.
- (a) Modern Organocopper Chemistry, ed. N. Krause, Wiley-VCH Verlag GmbH, 2002 Search PubMed; (b) N. Miyaura, N. Sasaki, M. Itoh and A. Suzuki, Tetrahedron Lett., 1977, 18, 173 CrossRef.
- F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Synlett, 2012, 23, 2179–2182 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, analytical data and copies of 1H, 13C, 19F NMR; and, HRMS/mass spectra of newly synthesized compounds. See DOI: 10.1039/c5ra18820b |
| This journal is © The Royal Society of Chemistry 2015 |