Recovery of potassium chloride from blast furnace flue dust
Abstract
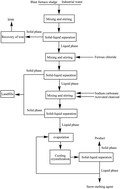
A simple, efficient, economic and environmentally-friendly recovery process for large amounts of potassium chloride from blast furnace flue dust (BF flue dust) with an abundant potassium content is developed. This process is mainly composed of water-leaching, purification, decolorization, vacuum evaporation and cooling crystallization. In this study, the basic properties of blast furnace flue dust were identified by X-ray diffraction (XRD), inductively coupled plasma analysis (ICP), and laser granulometry (LG). The purity of the KCl products was analyzed by ICP combined with the sodium tetraphenylborate (Na-TPB) chemical method and XRD. The particle sizes of the KCl products were characterized by LG and SEM. The results showed that the BF flue dust had a good recovery value with a potassium chloride content of 39.58%. After treating the dust by water-leaching and processing the as-prepared eluent via purification, decolorization and vacuum evaporation, the KCl crystal products were obtained with a yield of 72.77%, 79.52% and 71.09% with 0 °C, 5 °C and 10 °C as the cooling crystallization temperature and 2.27, 2.52 and 2.36 as the mass distribution coefficient, respectively. The KCl crystal products exhibited a narrow particle size distribution with a purity of greater than 96.00%.

 Please wait while we load your content...
Please wait while we load your content...