Iridium nanoparticles supported in polymeric membranes: a new material for hydrogenation reactions†
Abstract
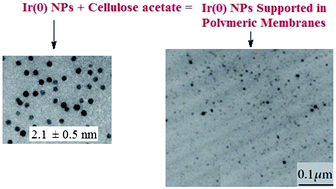
Iridium nanoparticles (Ir(0) NPs) of 2.1 ± 0.5 nm were synthesized from [Ir(cod)Cl]2 (cod = 1,5-cyclooctadiene) in the ionic liquid (IL) 1-n-butyl-3-methylimidazolium hexafluorophosphate [BMI.PF6]. The Ir(0) NPs were subsequently supported on polymeric membranes using cellulose acetate (CA) in acetone and the IL 1-n-butyl-3-methylimidazolium bis(trifluoromethane sulfonyl)imide [BMI.N(Tf)2]. Polymeric membranes with thicknesses of 20 μm were prepared with 10 mg of Ir(0) NPs. The polymeric membranes were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), and transmission electron microscopy (TEM). The analysis showed that the Ir(0) NPs are homogeneously distributed over the entire membrane, which has a compact structure. The presence of small Ir(0) NPs induced increases in the surface areas of the polymeric membranes. The presence of the IL in the membrane structure increases the separation between the cellulose macromolecules, which results in greater flexibility and durability of the polymeric membranes. The CA/LI/Ir(0) combination exhibits excellent synergistic effects that increase the activity of the catalyst in hydrogenation reactions.

 Please wait while we load your content...
Please wait while we load your content...