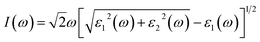Efficient photo-degradation of dyes using CuWO4 nanoparticles with electron sacrificial agents: a combination of experimental and theoretical exploration
Xinjian Xie†
a,
Mengyin Liu†b,
Changhong Wangb,
Lei Chenb,
Jianping Xuc,
Yahui Chengb,
Hong Dongb,
Feng Lub,
Wei-Hua Wangb,
Hui Liub and
Weichao Wang*b
aSchool of Material Science and Engineering, Hebei University of Technology, Tianjin 300130, China
bDepartment of Electronics and Key Laboratory of Photo-Electronic Thin Film Devices and Technology of Tianjin, Nankai University, Tianjin 300071, China. E-mail: weichaowang@nankai.edu.cn; Fax: +86 22 23509930; Tel: +86 22 23509930
cInstitute of Material Physics, Key Laboratory of Display Materials and Photoelectric Devices, Ministry of Education, Tianjin University of Technology, Tianjin 300384, China
First published on 9th December 2015
Abstract
CuWO4 is a promising photocatalytic material, responding in the visible light range, to enhance the utilization of solar energy. Here, CuWO4 nanoparticles have been synthesized via a polyol-mediated synthesis method and subsequently characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-Vis Spectrophotometery combined with theoretical density functional theory (DFT) calculations. For the as-prepared CuWO4 samples, a strong adsorption capacity for the organic pollutant MB rather than photodegradation has been observed. The first-principle calculation with Heyd–Scuseria–Ernzerhof (HSE) screened coulomb hybrid functional results indicate that localization of hybridization of O 2p-orbitals and Cu 3d-orbitals, large electron effective mass and more positive conduction band edge of CuWO4 lead to low carrier mobility and thus the high recombination of excited carriers. Meanwhile, the optical absorption spectrum of experimental observation is consistent with theoretical calculations of pristine CuWO4, demonstrating few defects inhibiting light absorption. To avoid the high rate of recombination of the excited carriers, electron sacrificial agents (H2O2, Na2S2O8) are utilized to suppress the recombination. The photocatalytic activity is thus largely improved.
Introduction
With the increasing problem of environmental pollution, it is important to develop effective technology to clean waste water. Various techniques including biodegradation,1 ultrafiltration,2 ion exchange3 and etc.4–6 have been utilized to achieve this objective. Among these technologies, advanced oxidation processes (AOP)7 stand out as a promising way to clean water. The AOP differ from conventional physical and biological water treatment processes as the AOP generate strong oxidant hydroxyl radicals (·OH) to degrade toxic and refractory pollutants (organic carbon) into simple and harmless inorganic molecules (carbon dioxide and water) without producing secondary pollutants. The ·OH generation can be initiated by primary oxidants (H2O2 or O3),8 energy sources (UV-light, ultrasonic)9,10 or catalysts.11 Semiconductor materials could absorb solar energy and excite carries to generate oxidant for further photocatalytic degradation of organic pollutants. For a superior photocatalyst, the band edges should align up with water redox levels. Specifically, the top of valence band (EV) is required more positive than the redox potential ·OH/H2O (∼2.5 V vs. NHE).12 When solar light is incident on a semiconductor material, the photo-generated electrons and holes react with water and form oxidants (O2−, H2O2 and O3) and essentially produce ·OH.13,14The semiconductor materials have been used to generate oxidants for several years15,16 since the photocatalytic splitting of water was discovered on the TiO2 electrodes by Fujishima and Honda in 1972.17 To date, anatase TiO2 dominates the photocatalysis market owing to its low cost, non-toxicity, highly catalytic activity and chemical stability.18,19 Whereas, TiO2 with a band gap of 3.2 eV displays a low efficiency (∼5%) of utilizing solar energy. In order to ultimately harvest solar energy, it is important to continue searching for visible light driven photocatalysts.20,21
CuWO4, as a ternary narrow band gap semiconductor (Eg ∼ 2.2 eV), is an ideal high-efficiency semiconductor photocatalyst due to its absorption of visible-light22,23 with reasonable valence band alignments with ·OH/H2O energy level. Moreover, the catalytic performances could also be influenced by other factors such as crystallinity, defects and interface, which introduce various electronic structures including band tails,24 defect states,25 and interfaces states.26 These states significantly impact on carrier mobility,27 carrier's recombination,28 electronic conductivity29 and etc.,30 resulting in the variations of the catalytic activity. Without full understanding of the microscopic electronic structures, it would be difficult to further improve the photocatalytic performance. Here, we combined theoretical calculations and experimental methods to link the electronic structures and the catalytic performance of CuWO4. Based on the DFT calculations, the catalytic activity has been improved with the presence of electron capture agents. In such a way, these findings provide insights into further promising photocatalyst design.
In this work, CuWO4 was prepared by polyol-mediated synthesis method. The synthesized samples are characterized by the X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM) and UV-Vis Spectrophotometer. As-prepared powder displays a strong adsorption capacity of organic pollutant MB rather than photodegradation. First-principle calculations point out that the disadvantage of localized band edge states of hybridization of O 2p-orbitals and Cu 3d-orbitals causes the high recombination of excited carrier. On the other hand, the experimental optical absorption spectrum of sample is consistent with theoretical calculation of pristine CuWO4 which indicates no problem for our synthesized samples to absorb light. In the presence of electronic sacrificial agent, the excited holes are survived and thus CuWO4 displayed a high photocatalytic performance to degrade the MB dye.
Experiment and calculation method
Polyol mediated synthesis method
Copper nitrate (Cu(NO3)2·3H2O, AR, purity ≥ 99.5%) and sodium tungstate (Na2WO4·2H2O, AR, purity ≥ 99.5%) were used to prepared CuWO4. Copper nitrate (1 mmol) was dissolved in 50 mL diethylene glycol (DEG) and stirred by magnetic stirring at room temperature. Sodium tungstate (1 mmol) was dissolved in 1 mL deionized water and was injected into copper nitrate solution quickly with magnetic stirring. After stirring 3 minutes (min), the products were separated from these suspensions via high-speed centrifuge at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min and washed by sequential centrifugation/redispersion from/in ethanol for 5 times to remove redundant impurity and DEG completely, and then dried in the oven at 70 °C for 12 hours. In the annealed process, temperature was kept at 500 °C for 1 hour in the air.
000 rpm for 5 min and washed by sequential centrifugation/redispersion from/in ethanol for 5 times to remove redundant impurity and DEG completely, and then dried in the oven at 70 °C for 12 hours. In the annealed process, temperature was kept at 500 °C for 1 hour in the air.
Evaluation of photocatalytic activity
The photocatalytic ability of CuWO4 was evaluated by degradation of MB. The 40 mg CuWO4 powder was dispersed in methylene blue solution (100 mL, 10 mg L−1) in a beaker. Subsequently, it was stirred about 30 min in dark in order to reach adsorption–desorption equilibrium. A xenon lamp without filter has been used as the light source and the light intensity remains at 20 mW cm−2 measured by an irradiance meter (Model: FZ-A, Beijing Normal University, China) to reduce solution evaporation. During photogradation of MB solution, the magnetic stirring was kept running. The photodegradation was quantified by monitoring the concentration of MB at its maximum of absorption (664 nm). Commercially available Degussa P25 (nanoscale TiO2 powder; 80% anatase, 20% rutile; particle diameter: 25–30 nm) as a reference was used to compare with the photocatalytic performance of CuWO4.Theoretical model and calculation details
The crystal structure of CuWO4 is presented in Fig. 1, containing two formula units with characteristic corner-linked CuO6 and WO6 octahedral. The Jahn–Teller effect of the Cu2+ cation causes a pseudo-tetragonal elongation of the CuO6 octahedral. The ab initio calculations were performed based on the density functional theory (DFT), as implemented in plane-wave based code Vienna ab initio Simulation Package (VASP). The generalized gradient approximation with the Perdew–Burke–Ernzerhof version of the exchange–correlation potential was employed.31 A large energy cutoff of 550 eV was adopted. Brillouin zone was sampled by the set of (5 × 4 × 5) k-points for CuWO4 to balance calculation efficiency and accuracy. The convergence criterion for energy is chosen as 10−4 eV and the maximum Hellmann–Feynman force acting on each atom is less than 0.01 eV Å−1 in ionic relaxation calculations. The calculated lattice constants are a = 4.78 Å, b = 6.00 Å, c = 4.93 Å; α = 92.77°, β = 93.51°, γ = 81.48°. Considering that GGA usually overestimates the lattice constant slightly,32,33 the calculated results are in good agreement with the previous results using the GGA functional (a = 4.84 Å, b = 6.05 Å, c = 4.94 Å)34 and the experimental values (a = 4.69 Å, b = 5.83 Å, c = 4.88 Å).35 For electronic structure calculations, it is well known that DFT underestimates the band gap, thus the electronic structure with the PBE relaxed structure was calculated using the Heyd–Scuseria–Ernzerhof (HSE)36 hybrid functional, in which a portion (16%) of Hartree–Fock exchange was mixed with the PBE functional to produce a band gap of ∼2.2 eV which is highly consistent with experimental observations.37 | ||
| Fig. 1 The crystal structure of CuWO4, the green, blue and red balls represent Cu, W and O atoms, respectively. Arrows represent the spin directions of Cu. | ||
Results and discussion
Due to the high polarity of multidentate alcohols such as ethylene glycol, diethylene glycol or glycerol, polyol-mediated synthesis is considered as an effective way to control nucleation and growth of nanoparticles, stabilize the particle surface and avoid agglomeration.38,39 In this work, we adopted this method to synthesize CuWO4.The as-prepared CuWO4 powder shows green color (inset in Fig. 2) and the according XRD peaks (Fig. 2) are broadened which indicates the amorphous phase of the sample power. After heating the sample under 500 °C for 1 hour, the color turned into dark grey (inset in Fig. 2) and the XRD pattern displays the formation of the pure phase of CuWO4 (Fig. 2). The color change before and after sample annealing results from the typical quantum size effect.
Fig. 3(a)–(c) describe morphologies of as-prepared sample. Irregular nanoparticles have been observed by SEM (Fig. 3(a)), the grain size varying from 10–20 nm has been verified by TEM (Fig. 3(b) and (c)) and the specific surface area is expected to be large. The selected area electron diffraction (SAED) (Fig. 3(c)) shows the blurry spots corresponding to low degree of crystallinity of as-prepared CuWO4. For the annealed sample, SEM and TEM (Fig. 3(d)–(f)) show that the particle size significantly grows up to ∼50 nm and nanoparticles seriously reunite due to regrowth and recrystallization during the annealed process. On the other hand, clear spots of SAED (Fig. 3(f)) also identify the improvement of crystallinity after the sample was annealed.
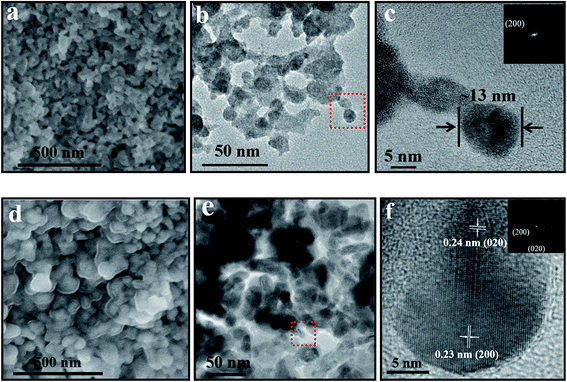 | ||
| Fig. 3 SEM, TEM images of as-prepared sample (a) and (b) and annealed sample (d) and (e); (c) and (f) are magnified high-resolution TEM images of red boxes in (b) and (e), respectively. | ||
For the photodegradation of MB, the as-prepared powder displays a strong adsorption capacity of organic pollutant MB within the first 10 min (Fig. 4(a)). No photodegradation phenomenon has been observed during this period. When continuing increasing observing time larger than 10 min, no more adsorption is displayed since as-prepared CuWO4 has reached its adsorption equilibrium. For the annealed sample, the particle size significantly grows up to ∼50 nm and nanoparticles reunite seriously which have been observed by SEM and TEM (Fig. 3(d)–(f)). Consequently, the decrease of the surface areas leads to the decrease of MB adsorption, as shown in Fig. 4(b). Also, the annealed sample displays no photocatalytic behavior. Little variation of the photocatalytic behaviors for the as-prepared sample and the annealed one proves that particle size is not the key to impact the catalytic activity for this case.
Intrinsically, two critical factors would govern the photocatalytic activity, i.e. carrier conductivity and optical absorption. For the former one, it is governed by the band edge shapes of CuWO4. The later one is influenced by light absorption spectrum. In order to access the failure mechanism of the photocatalytic performance of CuWO4, electronic structure and optical spectrum should be calculated.
With the advantage of the start-of-art supercomputer and computational algorithm, it is now feasible to access the fundamental electronic structures at the atomic level. In this work, we employed density functional theory (DFT) to explore the electronic structure and optical spectrum of the CuWO4.
Fig. 5 illustrates the calculated band structure along high symmetric k-point B (0.5, 0, 0) – Γ (0, 0, 0) – F (0, 0.5, 0) – Q (0, 0.5, 0.5) – G (0, 0, 0.5) in the first Brillouin zone. The conduction band minimum (CBM) and valence band maximum (VBM) are located at Γ k-point and Q k-point, respectively, indicating an indirect band gap. The gap value is ∼2.2 eV which is highly consistent with experimental result.37 Also, in the bottom region of conduction band at Γ point, the band is rather flat. By extracting the curvature of the CBM and VBM, we obtained a large the effective mass of electron and hole (m*e = 59 m0, m*h = 57 m0, m0 is free electron mass), resulting in the poor electron and hole conductivity, respectively.23
 | ||
| Fig. 5 The band structure diagram (left panel) and the spin-dependent density of states (right panel) for CuWO4. Fermi level is set at zero eV in both panels. | ||
Fig. 5 also shows the normalized total and projected density of states (PDOS). The spin-up states are identical to the spin-down ones. Thus, CuWO4 presents an anti-ferromagnetic (AFM) ground state with the magnetic moment of 0.68 μB for Cu atoms and 0.05 μB for O atoms, being consistent with experiment observations of μCu = 0.67 μB, μO = 0.06 μB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 and PBE + U calculation of μCu = 0.74 μB, μO = 0.07 μB.40
35 and PBE + U calculation of μCu = 0.74 μB, μO = 0.07 μB.40
In Fig. 5, the O-2p and Cu-3d orbitals hybrid and dominate the band edges. More specifically, valance band maximum is mainly composed of the orbital hybridization of oxygen 2p-orbitals and copper 3d-orbitals, leading to the more positive position of the VBM,41 and the strong oxidizing property of the excited holes. Therefore, we speculate that O and Cu could be the activity sites for photocatalysis. The excited hole either directly reacts with organic pollutant molecular to decompose it or reacts with OH− to produce strong oxidant hydroxyl radicals. Hybridization of Cu-3d and O-2p results in more positive CBM and narrow band gap which improves the visible-light absorption. Nevertheless, Hybridization of Cu-3d and O-2p also lead to more positive conduction band edge of CuWO4 compared to H+/H2 reduction level in solution.42 In other words, there is no excited electron acceptor in this photocatalytic system, as a result, excited electrons will gather on the CuWO4 surface and cause more severe surface recombination. Moreover, the localized states of hybridization of O 2p and Cu 3d orbitals could lead to the lower carrier mobility, high recombination rate of excited carrier and essentially result in low efficient photocatalytic behavior.
In addition to the material intrinsic electronic properties, optical adsorption is another important factor to govern the photocatalytic activity. For the absorption spectrum of bulk material, it is formatted as:
ε2 and ε1 are imaginary part and real part of dielectric function, respectively, ω is angular frequency. In order to obtain the absorption spectrum, dielectric constant was calculated via DFT. Fig. 6(a) and (b) show the calculated results of the imaginary part and real part of dielectric function for CuWO4 along three Cartesian directions, respectively. The optical anisotropy is due to low crystal symmetry and the peculiarities in the crystal structure, i.e., the existence of the bridge-oxygen ions connecting with neighboring CuO6 and WO6 complexes. Based on the calculated dielectric function curves, we obtained the theoretical absorption spectrum, as shown in Fig. 6(c). The light absorption begins about 2.0 eV, corresponding to the electron excitation from the VBM to the CBM (Fig. 6(c)). Experimentally, the as-prepared and annealed CuWO4 has been used to test the absorption spectrum by UV-Vis Spectrophotometer. For the as-prepared sample, the localized absorption peak appeared at 1.5 eV as the subgap absorption,43 which originates from bonding defects induced located states in the forbidden energy gap.44 This specific subgap absorption helps to improve the visible-light absorption. Compared the theoretical and pure annealed sample absorption spectrum, it is found that they are consistent with each other.45 This means the light absorption of sample is close to the theoretical value attributed to few defects and impurities hindrance light absorption.46,47 In other words, our sample shows bulk-like absorption behavior without impacting by defect levels.
On the basis of the theoretical analysis, charge carrier separation would be the key to influence the photocatalytic activity. Thus, promoted separation of the excited carriers would be an effective method to achieve the photocatalytic degradation of MB. In this work, we utilized electron sacrificial agents to examine the charge separation effect on the photocatalytic performance.48 To confirm the electron capture agent's boost effect on enhancing the photodegradation by as-prepared CuWO4, 1 mmol H2O2 or Na2S2O8 was dispersed into the MB solution to check the catalytic activity. Fig. 7(a) indicates that individual H2O2 or Na2S2O8 displays a limited degraded performance. Surprisingly, in as-prepared CuWO4 (40 mg) combined with 1 mmol H2O2 or Na2S2O8, we found that MB decomposes rapidly and the MB concentrations reaches to zero within 60 min (see Fig. 7(a)). As a comparison, the as-prepared CuWO4 with electronic capture agents (H2O2 or Na2S2O8) shows superiority over the same amount of P25 and P25 together with H2O2 rather than Na2S2O8 in weight in terms of the decomposition of MB. In any case, P25 with Na2S2O8 shows the highest efficiency because of the stronger oxidation of Na2S2O8 in regards to H2O2 (Fig. 7(a)). To examine the role of hole to decompose MB, we utilized (NH4)2C2O4 to exhaust the supply of excited holes, and we found that MB degradation with (NH4)2C2O4 is identical with the self-degradations under UV-Vis light. In other words, hole is the key to decompose MB in our case.
 | ||
| Fig. 7 The photodegradation of MB (a) as-prepared CuWO4 sample, P25 and (b) annealed sample added electron sacrificial agents, and only electron sacrificial agent without any catalyst. | ||
In order to access the role of electron sacrificial agent to assist in the catalytic performance of the as-prepared CuWO4, we proposed the following schematic diagram to address how electron sacrificial agents improve the photocatalytic efficiency (Fig. 8(a)).
 | ||
| Fig. 8 (a) Photocatalytic process in the presence of electron sacrificial agents. (b) CuWO4 band alignments with H+/H2, O2/H2O2, O2/H2O and ·OH energy levels. | ||
When the CuWO4 absorbs the photon energy larger than its band gap, the excited electrons (e−) jump from the valence band to the conduction band and form electron–hole pairs. Parts of them recombine in the as-prepared CuWO4 particles and the others move to the surfaces (Fig. 8(a)). Meanwhile, owing to the improper band alignment with water redox potentials, as presented in Fig. 8(b), severe surface recombination occurs as discussed above. Consequently, no enough h+ combines with OH− and forms ·OH because reactive time (about 10−3 s) is much longer than the surface recombining time (about 10−12 s). When the electron sacrificial agent (H2O2 or Na2S2O8) is introduced into MB solution, the excited electrons on surface are captured by electron sacrificial agents. The carrier recombination is thus suppressed and the excited holes have enough time to oxidize the H2O molecules and generate the strong oxidant ·OH. In addition to the role of charge separation from electron sacrificial agents, the strong MB adsorption on the as-prepared CuWO4 surface could be another factor to enhance the photodegradation.
For any photocatalyst, crystallinity, electronic structure and possibly surface charge would be crucial to determine the catalytic performance. In the annealed CuWO4 sample with diameter of ∼50 nm, even in the presence of electron sacrificial agent, very limited catalytic activity was observed in Fig. 7(b). The limited activity could be attributed to the lower adsorption capacity arising from the high crystallinity (Fig. 4(b)). Compared to as-prepared sample, the localized absorption peak at 1.5 eV disappeared in the absorption spectrum of annealed sample (Fig. 6(c)), decreasing the visible-light absorption and photocatalytic efficiency. From the perspective of surface charge, as an n-type semiconductor,49 band will upper bend when contacting with solution.50 As a result, the exited holes tend to transfer to the nanoparticles surface, then directly react with MB or produce ·OH which leads to the decomposition of MB pollutants. For the as-prepared amorphous phase, it provide more active sites for surface photogenerated carriers and prevent them from rapid recombination due to high disorders, thus promoting carrier transfer and photocatalytic reactions.51 After annealing the sample, the particle size increases and thus the active sites significantly reduce. Consequently, the carrier recombination became severe, and the photocatalytic efficiency decreased.
Conclusions
In summary, we have investigated the catalytic activity of CuWO4 nanoparticles in experiment, combined with the DFT electronic structures calculations. The first principle calculation results illustrate that more positive conduction band edge position than H+/H2O level, low carrier mobility and high recombination rate of CuWO4 result in an effective adsorption rather than photodegradation of MB for the as-prepared sample. The annealed samples with larger particle size display even a smaller adsorption capability of MB. In the presence of electron sacrificial agents, the photocatalytic efficiency of the as-prepared CuWO4 is significantly improved, due to the suppression of the combination of photo-generation carrier and the enhancement of the formation of ·OH. On the other hand, the annealed sample displays rather low catalytic behavior owing to the low adsorption capability.Acknowledgements
This work is supported by National Natural Science Foundation of China (11304161, 11104148, 51171082, 21573117 and 11404172), 1000-youth talent program in China, Tianjin Natural Science Foundation (13JCYBJC41100, 14JCZDJC37700), the National Basic Research Program of China (973 Program with No. 2014CB931703), Fundamental Research Funds for the Central Universities. We thank the technology support from the Texas Advanced Computing Center (TACC) at the University of Texas at Austin (http://www.tacc.utexas.edu) for providing grid resources that have contributed to the research results reported within this paper.References
- T. Godjevargova, D. Ivanova, Z. Alexieva and N. Dimova, Process Biochem., 2003, 38, 915 CrossRef CAS.
- N. S. Azmi and K. F. M. Yunos, Agriculture and Agricultural Science Procedia, 2014, 2, 257 CrossRef.
- M. F. Rahman, S. Peldszus and W. B. Anderson, Water Res., 2014, 50, 318 CrossRef CAS PubMed.
- C. Dorado, C. A. Mullen and A. A. Boateng, ACS Sustainable Chem. Eng., 2014, 2, 301 CrossRef CAS.
- P. C. Kearney, M. T. Muldoon, C. J. Somich, J. M. Ruth and D. J. Voaden, J. Agric. Food Chem., 1988, 36, 1301 CrossRef CAS.
- G. Han, S. G. Shin, J. K. Lim, M. Jo and S. Hwang, AIP Conf. Proc., 2010, 1251, 209 CrossRef CAS.
- R. Andreozzia, V. Caprioa, A. Insolab and R. Marottac, Catal. Today, 1999, 53, 51 CrossRef.
- B. T. Oh, Y. S. Seo, D. Sudhakar, J. H. Choe, S. M. Lee, Y. J. Park and M. Cho, J. Hazard. Mater., 2014, 279, 105 CrossRef CAS PubMed.
- O. S. Keen, I. Ferrer, E. M. Thurman and K. G. Linden, Chemosphere, 2014, 117, 316 CrossRef CAS PubMed.
- K. Makino, M. M. Mossoba and P. Riesz, J. Phys. Chem., 1983, 87, 1369 CrossRef CAS.
- H. Czili and A. Horváth, Appl. Catal., B, 2008, 81, 295 CrossRef CAS.
- S. Pasternak and Y. Paz, ChemPhysChem, 2013, 14, 2059 CrossRef CAS PubMed.
- R. Marschall and L. Z. Wang, Catal. Today, 2014, 225, 111 CrossRef CAS.
- P. K. J. Robertson, J. M. C. Robertson and D. W. Bahnemann, J. Hazard. Mater., 2012, 211, 161 CrossRef PubMed.
- A. Mills and S. L. Hunte, J. Photochem. Photobiol., A, 1997, 108, 1 CrossRef CAS.
- K. Nakata, T. Ochiai, T. Murakami and A. Fujishima, Electrochim. Acta, 2012, 84, 103 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169 CrossRef CAS.
- J. C. Yu, J. G. Yu, L. Z. Zhang and W. K. Ho, J. Photochem. Photobiol., A, 2002, 148, 263 CrossRef CAS.
- H. L. Wang, T. Deutsch and J. A. Turner, ECS Trans., 2008, 6, 37 CAS.
- J. R. Bolton, S. J. Strickler and J. S. Connolly, Nature, 1985, 316, 495 CrossRef CAS.
- R. L. Perales, J. R. Fuertes, D. Errandonea, D. M. Garcia and A. Segura, EPL, 2008, 83, 37002 CrossRef.
- K. J. Pyper, J. E. Yourey and B. M. Bartlett, J. Phys. Chem. C, 2013, 117, 24726 CAS.
- Y. Y. Kang, Y. Q. Yang, L. C. Yin, X. D. Kang, G. Liu and H. M. Cheng, Adv. Mater., 2015, 27, 4572 CrossRef CAS PubMed.
- C. X. Yang, T. Y. Li, Z. J. Cheng, H. X. Gan and J. Y. Chen, Phys. B, 2012, 407, 844 CrossRef CAS.
- W. C. Wang, S. Y. Chen, P. X. Yang, C. G. Duan and L. W. Wang, J. Mater. Chem. A, 2013, 1, 1078 CAS.
- J. T. Heath, J. D. Cohen, W. N. Shafarman, D. X. Liao and A. A. Rockett, Appl. Phys. Lett., 2002, 80, 4540 CrossRef CAS.
- A. Layek, B. Manna and A. Chowdhury, Chem. Phys. Lett., 2012, 539, 133 CrossRef.
- J. D. W. Camacho and K. J. Stevenson, J. Phys. Chem. C, 2009, 113, 19082 Search PubMed.
- T. Scheidt, E. G. Rohwer, H. M. V. Bergmann and H. Stafast, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 165314 CrossRef.
- J. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- L. Kihlborg and E. Gebert, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1970, 26, 1020 CrossRef CAS.
- A. Kuzmin, A. Kalinko and R. A. Evarestov, Acta Mater., 2013, 61, 371 CrossRef CAS.
- J. R. Fuertes, D. Errandonea, R. L. Perales, A. Segura, J. González, F. Rodríguez, F. J. Manjón, S. Ray, P. R. Hernández, A. Muñoz, Z. Zhu and C. Y. Tu, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 224115 CrossRef.
- J. B. Forsyth, C. Wilkinson and A. I. Zvyagin, J. Phys.: Condens. Matter, 1991, 3, 8433 CrossRef CAS.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207 CrossRef CAS.
- N. Gaillard, Y. C. Chang, A. D. Angelis, S. Higgins and A. A. Braun, Int. J. Hydrogen Energy, 2013, 38, 3166 CrossRef CAS.
- C. Feldmann, Adv. Funct. Mater., 2003, 13, 101 CrossRef CAS.
- P. Schmitt, N. Brem, S. Schunk and C. Feldmann, Adv. Funct. Mater., 2011, 21, 3037 CrossRef CAS.
- M. V. Lalic, Z. S. Popovic and F. R. Vukajlovic, Comput. Mater. Sci., 2011, 50, 1179 CrossRef CAS.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC.
- J. E. Yourey and B. M. Bartlett, J. Mater. Chem., 2011, 21, 7651 RSC.
- M. L. Benkhedir, Defect Levels in the Amorphous Selenium Bandgap, PhD thesis, KU Leuven, 2006, p. 18.
- P. W. Anderson, Phys. Rev., 1958, 109, 1492 CrossRef CAS.
- J. E. Yourey, J. B. Kurtz and B. M. Bartlett, J. Phys. Chem. C, 2012, 116, 3200 CAS.
- F. D. Weerdt and A. T. Collins, Diamond Relat. Mater., 2008, 17, 171 CrossRef.
- E. Burstein, G. Picus, B. Henvis and R. Wallis, J. Phys. Chem. Solids, 1956, 1, 65 CrossRef.
- J. S. Romao, M. S. Hamdy, G. Mul and J. Baltrusaitis, J. Hazard. Mater., 2015, 282, 208 CrossRef CAS PubMed.
- T. Mathew, N. M. Batra and S. K. Arora, J. Mater. Sci., 1992, 27, 4003 CrossRef CAS.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. X. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef CAS PubMed.
- X. B. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746 CrossRef CAS PubMed.
Footnote |
| † These authors contribute equally. |
| This journal is © The Royal Society of Chemistry 2016 |