Microemulsion-mediated synthesis of sedum rubrotinctum shaped Cu2O architecture with efficient sunlight driven photocatalytic activity
Lu-feng Yangab,
De-qing Chu*ab,
Li-min Wang*bc,
Ge Gea and
Hui-lou Suna
aCollege of Environment and Chemical Engineering, Tianjin Polytechnic University, Tianjin 300387, P. R. China. E-mail: dqingchu@163.com; Fax: +86-22-83955762; Tel: +86-22-83955762
bState Key Laboratory of Hollow-Fiber Membrane Materials and Membrane Processes, Tianjin 300387, P. R. China
cCollege of Materials Science and Engineering, Tianjin Polytechnic University, Tianjin 300387, P. R. China. E-mail: wanglimin@tjpu.edu.cn
First published on 17th December 2015
Abstract
A novel Cu2O sedum rubrotinctum shaped architecture has been successfully synthesized in a water/oil (w/o) microemulsion. The obtained Cu2O sample was evaluated for its ability in the degradation of methylene blue under sunlight irradiation. It was found that a degradation of 98.94% was achieved in 90 min irradiation time.
As an important p-type semiconductor, Cu2O has a direct small band gap of 2.17 eV1 and is considered to be a promising material in solar cell conversion2–4 and photocatalysis areas.5 Up to now, Cu2O has been prepared by several different methods, such as electrodeposition,6 thermal relaxation,7 sonochemical method,8 vacuum evaporation,9 and liquid-phase reduction.10 Meanwhile, various morphologies of Cu2O, such as nanowires,11 octahedra,12 hollow spheres,13 nanocubes,14 and nanorods15 have been prepared. However, it remains a great challenge to develop simple and feasible approaches for the shape-controlled synthesis of well-defined Cu2O architectures.
Among all the techniques used for preparing metallic nanoparticles, such as gas evaporation, laser vaporization, ionized beam deposition, sol–gel, freeze-drying, and so on, the water-in-oil (w/o) microemulsion technique has been widely used because of the precise control of the size and shape of the nanoparticles and the narrow size distributions achieved by this technique.16,17 Microemulsion is thermodynamically stable and isotropous on a molecular scale and can solubilize proper solution. Water-in-oil (w/o) microemulsion is a transparent and isotropic liquid medium with nanosized water pools dispersed in a continuous phase and stabilized by surfactant and co-surfactant molecules at the water/oil interface. These water pools offer ideal microreactors for the formation of nanoparticles. These nanosized water pools have been widely used as spatially constrained microreactors for controlled synthesis of nanoparticles with desired narrow size distribution. It has been proven that the use of solvothermal method for the synthesis of nanomaterials can not only decrease reaction temperature but also improve the crystallinity of the products. On the other hand, the microemulsion-mediated solvothermal method, as a combination of the two methods mentioned above, possesses all the merits of both and has already been approved as an effective tool to fabricate inorganic nanocrystals with uniform morphology, narrow size distribution and good crystallinity.18–20
In this study, we reported a simple and novel method for the preparation of 3D sedum rubrotinctum shaped Cu2O architecture by a facile microemulsion-mediated method, combined with subsequent solvothermal treatment. As far as we know, there is no report about the synthesis of unique Cu2O crystal with 3D sedum rubrotinctum shaped architecture. Besides, about 15% of the total world production of dyes is lost during the dyeing process and is released in the textile effluents.21 Release of those toxic and colored waste waters in the ecosystem is a dramatic source of non-aesthetic pollution, eutrophication and perturbations in the aquatic life. Thus it is urgent to seek an economic and efficient way to degrade these organic pollutants in water. Photocatalytic technology was proved to be an efficient pathway to degrade these organic pollutants.22 According to reports, various morphologies of Cu2O display excellent photocatalytic ability in the degradation of organic contaminants.23–27 The photocatalytic activity of the as-prepared Cu2O sedum rubrotinctum shaped sample was also investigated for the degradation of methylene blue (MB) under direct sunlight irradiation. Our result also indicated that the as-prepared sedum rubrotinctum shaped Cu2O architecture shows great potential as a sunlight driven photocatalyst, otherwise, pure TiO2 and CdS catalyst take effect only under UV light. It is anticipated that this convenient synthesis route can be applied as a general method for the preparation of other functional inorganic nanomaterials.
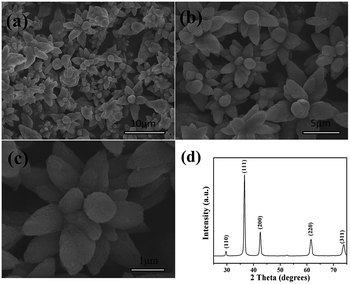
Fig. 1 presents SEM and XRD images of the as-prepared sedum rubrotinctum shaped Cu2O. A panoramic morphology of the product is displayed in Fig. 1(a), indicating the high uniformity. A magnified SEM image showing the close observation of the architecture is given in Fig. 1(b). It reveals that the detailed morphology of Cu2O products is well-defined sedum rubrotinctum shaped architecture with diameters in the range of 3.6–4.2 μm. A close-up view of the sedum rubrotinctum shaped sample in Fig. 1(c) demonstrates the “petals” of the sedum rubrotinctum shaped crystal are about 200 nm in diameters and 1 μm in length, and they regularly contacted with each other into sedum rubrotinctum shaped architecture.
The crystal phase of Cu2O is characterized by XRD, and the data are shown in Fig. 1(d). The characteristic peaks of the cubic phase of Cu2O crystals (JCPDS 05-0667) are observed. No peaks of impurity were detected in the XRD pattern, indicating the formation of pure Cu2O under the experimental condition.
Generally, specific surface area and pore diameter play a part in determining the adsorbent and catalysis properties of the active materials. To further confirm the surface structure of the sedum rubrotinctum shaped Cu2O architecture, N2 adsorption–desorption measurements were performed. The N2 adsorption–desorption and BJH desorption isotherms of the sedum rubrotinctum shaped Cu2O architecture are shown in Fig. 2. As can be seen from the adsorption curve, the N2 adsorption quantity increases monotonically with the increasing pressure, this phenomenon usually appears in porous and hollow materials.28 The specific surface area of the sedum rubrotinctum shaped Cu2O architecture is calculated to be 19.8 m2 g−1 from the BET result, which shows a relatively high surface area. The broad pore size distribution with a single modal centered at 21.1 nm is shown through the desorption isotherm (seen as the inset of Fig. 2).
 | ||
| Fig. 2 N2 adsorption–desorption isotherm and BJH pore size distribution plots (inset) of the sedum rubrotinctum shaped Cu2O product. | ||
Optical absorption behavior is one of the important fundamental properties in revealing the energy structures and applications in photocatalysis. Fig. 3(a) shows the UV-visible absorbance spectra of the as-prepared sedum rubrotinctum shaped Cu2O architecture. Two strong absorption peaks in the UV region are observed at wavelengths of about 435 and 325 nm for the as-synthesized sample. The broader one around 435 nm should attribute to the intrinsic band gap absorption, and the sharp one at 325 nm may result from the residual Triton X-100 absorption peak. The direct optical band gap energy (Eg) of the Cu2O sedum rubrotinctum shaped architecture can be calculated from the 434 nm absorption peak. To estimate the optical absorption edges for the sedum rubrotinctum shaped Cu2O architecture, (αhν)1/n versus hν curves were plotted (Fig. 3(b)) for n values (n = 1/2), a direct optical transition.29 Here, α is the optical absorption coefficient calculated from the absorption spectra, and hν is the incident photon energy. The Eg was determined by extrapolating the linear portion of the curve to zero, and the calculated value of Eg for sedum rubrotinctum shaped Cu2O is 2.21 eV, which is a little larger than the literature value of 2.17 eV for Cu2O bulk crystal.30 The increase in the band gap may be attributed to quantum confinement effects.31 Since the band gap of the Cu2O sedum rubrotinctum shaped sample was so narrow, electrons at valence were easily excited under visible light. Therefore, Cu2O could effectively absorb sunlight and might have potential applications in photocatalysis.
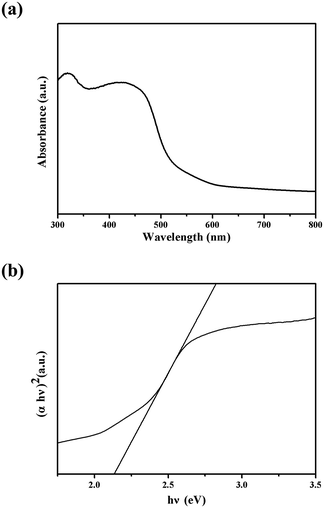 | ||
| Fig. 3 (a) The UV-vis diffuse reflectance spectra for Cu2O sedum rubrotinctum shaped architecture; (b) the (αhν)2 vs. hν plots for estimation of the band gap energies. | ||
X-ray photoelectron spectroscopy (XPS) studies were conducted to further confirm the oxidation state of copper and to verify the formation of Cu2O. Fig. 4(a) shows the Cu 2p core-level spectrum. The peak positions in the spectrum correspond to the binding energy of cuprous oxide. The spin–orbit coupling in copper results in two peaks centered at 933.1 and 953 eV from the 2p3/2 and 2p1/2 core levels.32 The XPS results, in conjunction with the XRD studies, firmly establish the formation of the pure Cu2O and remove the possibility of CuO.
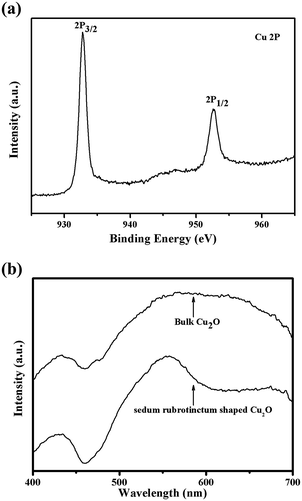 | ||
| Fig. 4 (a) XPS spectrum of sedum rubrotinctum shaped Cu2O. The peak positions establish the presence of copper as Cu2O; (b) photoluminescence spectra of sedum rubrotinctum shaped Cu2O and bulk Cu2O. | ||
Photoluminescence spectroscopy (PL) is an effective tool to study the electronic properties of the samples.33 The photoluminescence spectra of semiconductor materials are usually used to study the recombination of electron and hole. Low peak intensity means low recombination rate of electron and hole.34 Fig. 4(b) shows the photoluminescence spectra of sedum rubrotinctum shaped Cu2O and bulk Cu2O. It can be seen that the peak intensity of sedum rubrotinctum shaped Cu2O is significantly lower than that of bulk Cu2O. That is to say, sedum rubrotinctum shaped Cu2O has lower recombination rate of electron and hole, so it has higher separation rate of electron–hole pairs. As a result, sedum rubrotinctum shaped Cu2O has higher photocatalytic activity. Photoluminescence spectrum in Fig. 4(b) clearly also shows strong emission bands at 554 nm, an obviously blue-shift emission as compared to the bulk PL spectrum which shows a maximum peak around 570 nm. It may be argued that the blue-shift was caused due to the quantum confinement of exciton photogenerated inside the sedum rubrotinctum shaped Cu2O architecture.
The photocatalytic activity of the as-synthesized sedum rubrotinctum shaped Cu2O photocatalyst was evaluated by the degradation of MB under sunlight irradiation. MB concentration was reduced steadily in the presence of the as-synthesized Cu2O sample (Fig. 5(a)) under sunlight irradiation. The time-dependent absorption spectra of MB solution containing Cu2O sedum rubrotinctum shaped photocatalyst during the irradiation are depicted in Fig. 5(a), from which we can see that under our experimental condition, a degradation of 98.94% was achieved in 90 min irradiation time. The decoloration of the solution can be due to the destruction of the dye chromogen. As no new absorption peak is observed, the MB has been decomposed. By monitoring the MB absorption peak at 664 nm, plots of the degradation ratio versus reaction time were obtained for different catalyst samples (the as-prepared sedum rubrotinctum shaped Cu2O and bulk Cu2O) under identical condition (Fig. 5(b)). Fig. 5(b) clearly shows that the Cu2O sedum rubrotinctum shaped architecture is an effective photocatalyst for the direct degradation of MB and its photocatalytic performance is better than that of bulk Cu2O. The result further confirmed the activity of the as-prepared Cu2O photocatalyst.
For comparison, we also conducted the experiments MB direct photolysis (experiment under illumination and no catalyst) and adsorption (dark experiment) over the sedum rubrotinctum shaped Cu2O photocatalyst. Fig. 5(b) shows the comparative results of the changes of degradation rate with reaction time in adsorption, photolysis and photocatalytic process. The results showed that 6.0% and 16.9% of MB were removed for adsorption and photolysis respectively after 90 min. It can be clearly seen that both adsorption and photolysis achieved equilibrium and had no positive influence on the photocatalytic rate. Control experiments showed that MB degradation was negligible in the dark and/or very slow under sunlight in the absence of catalyst.
The photocatalytic property in the degradation of MB suggested that the as-prepared Cu2O sedum rubrotinctum shaped architecture is an excellent photocatalyst and has the potential in the application of photodegradation of MB which is a typical organic pollutant. And its better photocatalytic ability may arise from the particular morphology and large surface area of the as-synthesized Cu2O sample. Usually, the photocatalytic activity of a catalyst is dependent on its crystallinity, particle size, surface area and unique structure.35–39 In our work, the high specific surface area in the Cu2O sedum rubrotinctum shaped product results in more unsaturated surface coordination sites exposed to the solution. Therefore, the sedum rubrotinctum shaped sample provides more active reaction sites. All of these factors could improve the photocatalytic performance.
The stability and reusability of catalysts are very important issues for practical applications. In order to test the stability of the catalyst, recycling experiments were carried out. As can be seen in Fig. 6(a), the catalyst showed strong photocatalytic ability in the degradation of MB for five cycles. The catalyst was separated by centrifugation after every run and washed with deionized water and absolute ethanol for several times. We observed a decrease in reactivity after every run, which may be because of the mass loss of catalyst during washing and centrifugation.40
In the photocatalytic oxidation (PCO) process, a series of reactive oxygen species, such as h+, ˙OH, or ˙O2−, are supposed to be involved. Isopropanol (IPA), potassium iodide (KI), and benzoquinone (BQ) were scavengers of ˙OH, h+, and ˙O2−, respectively.41,42 In order to investigate high-efficiency of the as-prepared sedum rubrotinctum shaped Cu2O for the degradation of MB, we conducted the reactive species trapping experiments at optimized conditions after irradiation for 90 min and the corresponding results are shown in Fig. 6(b). Without using any scavenger, the degradation of MB on the sedum rubrotinctum shaped Cu2O was found to be 98.94%. In the presence of BQ, IPA and KI, the degree of degradation diminished to 82.3%, 46.7% and 32.8%, respectively. It can be seen that the addition of BQ in the MB solution has little effect on the photocatalytic activity of sedum rubrotinctum shaped Cu2O, suggesting that ˙O2− does not play a key role for the degradation of MB. On the contrary, the photocatalytic degradation of MB is obviously inhibited after the addition of IPA and KI. On the basis of these results, it can be concluded that ˙OH and h+ are the main oxygen active species for sedum rubrotinctum shaped Cu2O in the MB solution under direct sunlight irradiation.
To further investigate the band structure, the band edges of Cu2O were evaluated by Mulliken electronegativity theory:
| ECB = χ − EC − 0.5Eg | (1) |
| EVB = ECB − Eg | (2) |
 | ||
| Fig. 7 Schematic of photo-activation of sedum rubrotinctum shaped Cu2O photocatalyst and degradation of MB. | ||
Based on the experimental results, mechanism for the degradation of MB dye can be proposed. As evident from the result of dark adsorption experiment, although the Cu2O photocatalyst was hierarchically structured, maximum degree of adsorption that could be achieved was 6.0% by the sedum rubrotinctum shaped Cu2O architecture. The lower extent of adsorption may be due to same charges of Cu2O and the MB. Cu2O surface is positively charged at pH below its high PZC value (pH = 9.5). Moreover, MB dye is also positively charged. At neutral pH of the reaction, it is this factor which is responsible for electrostatic repulsion of MB dye molecules on the surface of Cu2O crystal, thereby indicating the degradation of MB dye was solely due to oxidative degradation in presence of Cu2O photocatalyst under sunlight illumination. The possible mechanism of photo-activation is shown in Fig. 7. Upon exposure to the sunlight, Cu2O was excited to generate the electron–hole pairs. Electron was excited to Cu2O conduction band and the hole was present in the Cu2O valance band. Additionally, these electrons further reacted with O2 adsorbed on the surface of catalyst to generate active ˙O2−. At the same time, h+ in the valence band (VB) of Cu2O combined with H2O to produce active ˙OH. These reactive radical species of ˙O2−, h+ and ˙OH were so reactive that they could efficiently degrade MB into CO2, H2O and less intermediates by rupturing the cyclic structures of MB molecules.
Conclusions
In summary, 3D sedum rubrotinctum shaped Cu2O architecture has been successfully synthesized via a simple microemulsion-mediated solvothermal route by carefully optimizing the reaction parameters in w/o microemulsion. It is the first time that the excellent 3D sedum rubrotinctum shaped Cu2O crystal has been synthesized so far. It was found that the microemulsion system is a prerequisite for the formation of Cu2O product with 3D sedum rubrotinctum morphology. The simple synthesis approach casts new light on the controllable fabrication of novel Cu2O 3D diversified architectures. Furthermore, the photocatalysis measurements showed that the Cu2O sedum rubrotinctum shaped sample exhibited outstanding photocatalytic property for the degradation of MB under sunlight irradiation mainly due to the unique excellent architecture with a high BET surface area. It was found that a degradation of 98.94% was achieved in 90 min irradiation time. Based on the effect of scavengers, it can be concluded that ˙OH and holes are the main active species for the degradation. On the basis of its efficient photocatalytic activity, sedum rubrotinctum shaped Cu2O architecture shows promise for use in environmental purification of organic pollutants under natural sunlight and extend the scope of the photocatalysts in practical application. Besides, it is also anticipated that this convenient synthesis route can be applied as a general method for the preparation of other inorganic nanomaterials.Experimental
All reagents are analytically pure and purchased from Tianjin Kermel Co. Ltd and used without further purification. A typical synthesis of Cu2O sedum rubrotinctum shaped architecture is as follows: two types of microemulsion solutions were prepared by solubilizing an aqueous cupric acetate monohydrate (Cu(Ac)2·H2O), trisodium citrate dihydrate (Na3C6H5O7·2H2O), and sodium hydroxide (NaOH) mixed solution or a ascorbic acid (H2A) solution into a cyclohexane, Triton X-100, 1-butanol system (according to the mass ratio cyclohexane/Triton X-100/1-butanol = 30/4/2, the molar ratio of water to surfactant fixed at 9.0). After 10 min of vigorous stirring, the above two different microemulsion solutions with equivalent volume were mixed rapidly and stirred for another 30 min. The final aqueous solution concentrations were [Cu(Ac)2] = 0.025 M, [Na3C6H5O7] = 0.025 M, [NaOH] = 0.275 M, and [H2A] = 0.031 M, respectively (the concentrations of these reactants are based on the volume of aqueous solution and not the total volume). The resulting mixture was then loaded into a 100 mL Teflon-lined autoclave, which was sealed and heated at 60 °C for 4 h, and then cooled to room temperature naturally.To break the microemulsion, 20 mL of acetone were added. Breaking the micelles caused the nanoparticles to slowly precipitate, with a powder clearly becoming visible on the bottom of the bottle after 10 min. To remove the organic phase residuals, 40 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetone and ethanol was added to the gelatinous precipitate, mildly sonicated and centrifuged at 12
1 mixture of acetone and ethanol was added to the gelatinous precipitate, mildly sonicated and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Subsequently 20 mL of ethanol and 20 mL of deionized water were added to the dispersion that was sonicated and centrifuged again at 12
000 rpm for 10 min. Subsequently 20 mL of ethanol and 20 mL of deionized water were added to the dispersion that was sonicated and centrifuged again at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The particles were further washed several times with deionised water, and then dried in vacuum at 80 °C for 6 h.
000 rpm for 10 min. The particles were further washed several times with deionised water, and then dried in vacuum at 80 °C for 6 h.
The phase and structure of the products were characterized by using X-ray diffraction (XRD, D8 DISCOVER with GADDS version of BRUCK Company of Germany), and scanning electron microscopy (SEM, Qunanta 200, FEI). The specific surface area (SBET) of the samples was evaluated using the multipoint Brunauer–Emmett–Teller (BET) method. The ultraviolet-visible diffuse reflection absorptive spectra (UV/DRS) were recorded by a UV-vis spectrometer (UV-2700, Shimadzu, Japan) equipped with an integrating sphere accessory in the diffuse reflectance mode (R) and BaSO4 was used as a reference material. X-ray Photoelectron Spectroscopic (XPS) measurements were performed in an ultra-high vacuum chamber (PHI1257) with a base pressure of ∼4 × 10−10 Torr. The XPS spectrometer was equipped with a high resolution hemispherical electron analyzer (279.4 mm diameter with 25 meV resolution) and a Mg (Kα) (hν = 1253.6 eV) X-ray excitation source. All the spectra were referenced to adventitious C (1s) at 284.5 eV binding energy (BE). Photoluminescence spectroscopy (PL) measurements were performed in a photoluminescence spectrometer (PL, F-4500, Japan). The photocatalytic absorbance measurements were performed on a UV-vis spectrometer (TU-1901, Beijing).
The photocatalytic activity of the as-prepared Cu2O sedum rubrotinctum shaped architecture was evaluated according to the photodegradation of methylene blue (MB) aqueous solutions under direct sunlight irradiation. The sunlight experiments were carried out between 11.00 am and 1.00 pm during the months of June (summer season) at Tianjin City. The ambient temperature was between 27 °C and 28.5 °C. In a typical experiment, a sample of as-prepared Cu2O sedum rubrotinctum shaped architecture (25 mg) was dispersed in a 100 mL of MB aqueous solution with the concentration of 10 mg L−1. The temperature of the photoreactor was maintained at 27 °C. Before illumination, the suspension was ultrasonicated for 40 s and then stirred for 30 min in the dark to obtain adsorption equilibrium of MB molecules. And then, the suspension was irradiated with natural sunlight. The suspension was kept up stirring during the whole irradiation process. The suspension was centrifuged at a given time interval. The concentration of MB during the degradation course was detected by a UV-vis spectrophotometer (TU-1901) through recording the absorbance of the characteristic peak of MB at 664 nm. The degradation rate of pollutant was recorded as (C0–C)/C0, where C0 is the concentration before irradiation, and C is the concentration at any sampling time. The photocatalytic behavior of bulk Cu2O was also measured as a reference to that of the synthesized catalyst.
Acknowledgements
We gratefully appreciate assistance with SEM, XRD, BET, UV-vis, XPS and PL analysis from Material Experiment Center of Tianjin Polytechnic University.Notes and references
- M. Shen, T. Yokouchi, S. Koyama and T. Goto, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 13066 CrossRef CAS.
- K. Akimoto, S. Ishizuka, M. Yanagita, Y. Nawa, G. K. Paul and T. Sakurai, Sol. Energy, 2006, 80, 715–722 CrossRef CAS.
- T. Mahalingam, J. S. P. Chitra, J. P. Chu, H. Moon, H. J. Kwon and Y. D. Kim, J. Mater. Sci.: Mater. Electron., 2006, 17, 519–523 CrossRef CAS.
- D. Tryk, A. Fujishima and K. Honda, Electrochim. Acta, 2000, 45, 2363–2376 CrossRef CAS.
- Y. G. Zhang, L. L. Ma, J. L. Li and Y. Yu, Environ. Sci. Technol., 2007, 41, 6264–6269 CrossRef CAS PubMed.
- L. Huang, H. Wang, Z. Wang, A. Mitra, D. Zhao and Y. Yan, Chem. Mater., 2002, 14, 876–880 CrossRef CAS.
- S. Deki, K. Akamatsu, T. Yano, M. Mizuhata and A. Kajinami, J. Mater. Chem., 1998, 8, 1865–1868 RSC.
- R. V. Kumar, Y. Mastai, Y. Diamant and A. Gedanken, J. Mater. Chem., 2001, 11, 1209–1213 RSC.
- H. Yanagimoto, K. Akamatsu, K. Gotoh and S. Deki, J. Mater. Chem., 2001, 11, 2387–2389 RSC.
- S. Ram and C. Mitra, Mater. Sci. Eng., A, 2001, 304, 805–809 CrossRef.
- M. Hafez, F. Al-Marzouki and W. E. Mahmoud, Mater. Lett., 2011, 65, 1868–1870 CrossRef CAS.
- X. Zhang, G. Wang, H. Wu, D. Zhang, X. Zhang, P. Li and H. Wu, Mater. Lett., 2008, 62, 4363–4365 CrossRef CAS.
- H. Zhang, Q. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766–2771 CrossRef CAS.
- L. Gou and C. J. Murphy, Nano Lett., 2003, 3, 231–234 CrossRef CAS.
- Z. Cheng, J. Xu, H. Zhong, X. Chu and J. Song, Mater. Lett., 2011, 65, 1871–1874 CrossRef CAS.
- M. A. López-Quintela, Curr. Opin. Colloid Interface Sci., 2003, 8, 137–144 CrossRef.
- M. A. López-Quintela, C. Tojo, M. Blanco, L. G. Rio and J. Leis, Curr. Opin. Colloid Interface Sci., 2004, 9, 264–278 CrossRef.
- M. Cao, C. Hu and E. Wang, J. Am. Chem. Soc., 2003, 125, 11196–11197 CrossRef CAS PubMed.
- M. Cao, X. Wu, X. He and C. Hu, Langmuir, 2005, 21, 6093–6096 CrossRef CAS PubMed.
- Y. Liu and Y. Chu, Mater. Chem. Phys., 2005, 92, 59–63 CrossRef CAS.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J. M. Herrmann, Appl. Catal., B, 2001, 31, 145–157 CrossRef CAS.
- J. M. Herrmann, Catal. Today, 1999, 53, 115–129 CrossRef CAS.
- B. Xin, P. Wang, D. Ding, J. Liu, Z. Ren and H. Fu, Appl. Surf. Sci., 2008, 254, 2569–2574 CrossRef CAS.
- L. Xu, H. Xu, S. Wu and X. Zhang, Appl. Surf. Sci., 2012, 258, 4934–4938 CrossRef CAS.
- X. Lin, R. Zhou, J. Zhang and S. Fei, Appl. Surf. Sci., 2009, 256, 889–893 CrossRef CAS.
- J. Zhang, W. Liu, X. Wang, X. Wang, B. Hu and H. Liu, Appl. Surf. Sci., 2013, 282, 84–91 CrossRef CAS.
- M. C. Huang, T. Wang, W. S. Chang, J. C. Lin, C. C. Wu, I. C. Chen, K. C. Peng and S. W. Lee, Appl. Surf. Sci., 2014, 301, 369–377 CrossRef CAS.
- X. Y. Yang, A. Leonard, A. Lemaire, G. Tian and B. L. Su, Chem. Commun., 2011, 47, 2763–2786 RSC.
- Y. Nakano, S. Saeki and T. Morikawa, Appl. Phys. Lett., 2009, 94, 022111 CrossRef.
- L. L. Ma, J. L. Li, H. Z. Sun, M. Q. Qiu, J. B. Wang, J. Y. Chen and Y. Yu, Mater. Res. Bull., 2010, 45, 961–968 CrossRef CAS.
- Y. Sui, W. Fu, H. Yang, Y. Zeng, Y. Zhang, Q. Zhao, Y. Li, X. Zhou, Y. Leng and M. Li, Cryst. Growth Des., 2009, 10, 99–108 Search PubMed.
- C. H. Kuo, Y. T. Chu, Y. F. Song and M. H. Huang, Adv. Funct. Mater., 2011, 21, 792–797 CrossRef CAS.
- N. Zhang, M. Q. Yang, S. Q. Liu, Y. G. Sun and Y. J. Xu, Chem. Rev., 2015, 115, 10307–10377 CrossRef CAS PubMed.
- H. J. Liu, T. Y. Peng, D. N. Ke, Z. H. Peng and C. H. Yan, Mater. Chem. Phys., 2007, 104, 377–383 CrossRef CAS.
- D. Li and H. Haneda, Chemosphere, 2003, 51, 129–137 CrossRef CAS PubMed.
- C. Han, Z. Chen, N. Zhang, J. C. Colmenares and Y. J. Xu, Adv. Funct. Mater., 2015, 25, 221–229 CrossRef CAS.
- S. Q. Liu, Z. R. Tang, Y. G. Sun, J. C. Colmenares and Y. J. Xu, Chem. Soc. Rev., 2015, 44, 5053–5075 RSC.
- C. Han, M. Q. Yang, B. Weng and Y. J. Xu, Phys. Chem. Chem. Phys., 2014, 16, 16891–16903 RSC.
- N. Zhang, Y. H. Zhang and Y. J. Xu, Nanoscale, 2012, 4, 5792–5813 RSC.
- Y. Q. Shi, K. Q. Zhou, B. B. Wang, S. H. Jiang, X. D. Qian, Z. Gui, R. K. K. Yuen and Y. Hu, J. Mater. Chem. A, 2014, 2, 535–544 CAS.
- Z. H. Chen, W. L. Wang, Z. G. Zhang and X. M. Fang, J. Phys. Chem. C, 2013, 117, 19346–19352 CAS.
- G. T. Li, K. H. Wong, X. W. Zhang, C. Hu, J. C. Yu, R. C. Y. Chan and P. K. Wong, Chemosphere, 2009, 76, 1185–1191 CrossRef CAS PubMed.
- A. Walsh and K. T. Butler, Acc. Chem. Res., 2013, 47, 364–372 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |