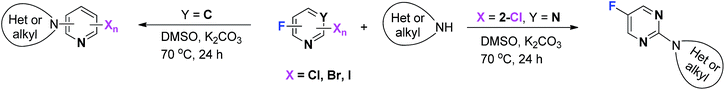Metal-free site-selective C–N bond-forming reaction of polyhalogenated pyridines and pyrimidines†
Lei
Wang
,
Ning
Liu
* and
Bin
Dai
*
School of Chemistry and Chemical Engineering, Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi University, North 4th Road, Shihezi, Xinjiang 832003, China. E-mail: ninglau@163.com; dbinly@126.com; Fax: +86-993-205-7270; Tel: +86-993-205-7157
First published on 21st September 2015
Abstract
This paper presents a metal-free method for highly site-selective C–N bond-forming reaction of polyhalogenated pyridines and pyrimidines. The preferred coupling site can be tuned from the fluorine group bearing the N-heterocyclic ring to the chlorine group when changing from the pyridine ring to the pyrimidine ring. A wide array of halogenated pyridines preferentially reacted with amines at the fluorine group of the pyridine ring to generate monosubstituted halogenated pyridines with high selectivities. Different halogen atoms at various positions were produced by the pyridine ring that performed well under mild conditions. Halogenated pyrimidines underwent highly selective coupling at the chloride group with a wide range of amines having broad substrate applicability and moderate to good yields. The selectivity of the polyfluoropyridines in the developed reaction system was also tested, and the result indicated that the reaction occurred site-selectively at the ortho-position of the nitrogen ring. This reaction accommodated a wide range of halogenated groups. Thus, a wide range of chloro-, bromo-, iodo-, and fluoropyridines were generated, which have a wide utility for organic synthesis.
Introduction
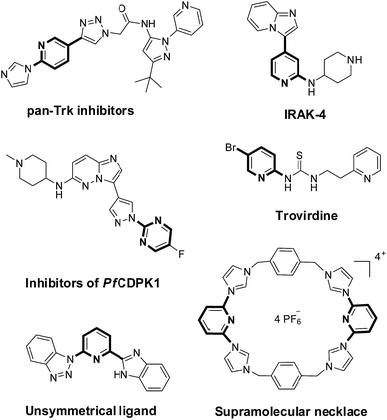
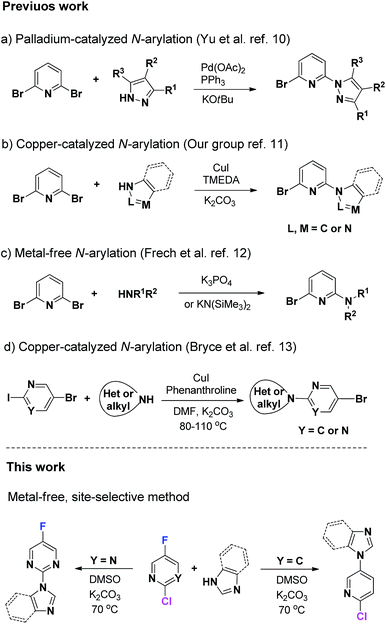
Polysubstituted heteroarenes are important building blocks for bioactive medicinal molecules, advanced materials and organometallic catalysts.1 In particular, polysubstituted pyridines and pyrimidines are used as inhibitors of anti-HIV (e.g., trovirdine),2 enzymes (e.g., IRAK-4),3 pan-Trk-inhibitors,4 and PfCDPK1.5 These compounds are also key in the construction of supramolecular frame6 and dye-sensitized solar cells,7 as well as precursors of organometallic catalysts (Scheme 1).8The substituents bearing N-heterocycles often differ. Therefore, controlling the selectivity of halogen atoms on N-heterocycles is crucial to construct polysubstituted N-heteroarenes.9 Yu et al.10 were the first to prepare 2-bromo-6-(pyrazol-1-yl)pyridines from 2,6-dibromopyridine and pyrazoles through palladium-catalyzed Buchwald–Hartwig coupling (Scheme 2, method a). We have recently found a low-cost and environment-friendly copper catalyst and developed a copper-catalyzed synthesis method to prepare 2-bromo-6-substituent-pyridines (Scheme 2, method b).11 A metal-free method is an alternative strategy to access these key compounds. Meanwhile, Frech et al.12 have developed a metal-free method to prepare 6-bromopyridine-2-amines (Scheme 2, method c). However, these methods are currently limited to 2,6-dibromopyridine.
Considering the importance of pyridines with substituents at other positions, Bryce et al.13 have reported a method to prepare brominated N-heterocyclic substituted pyridines or pyrimidines through a copper-catalyzed C–N bond-forming reaction (Scheme 2, method d). However, in a copper-catalyzed system, the selectivity of halogens bearing the N-heteroaromatic ring cannot be regulated by the type of N-heteroaromatic ring. Imidazole prefers to react with the iodine atom of either 5-bromo-2-iodopyridine or 5-bromo-2-iodopyrimidine.
Selective C–F bond activation has become a current area of interest.14 In this paper, we report a metal-free method for the highly site-selective coupling of halogenated pyridines or pyrimidines with amines through C–F bond activation (Scheme 2). Changing the type of the N-heteroaromatic ring switches the preferred coupling site from the fluorine atom to the chlorine atom. Products are generated because an active halide site has a wide functionality for organic synthesis through a series of subsequent transformations.
Results and discussion
Substituted halogenated pyridines are key structural units in a multitude of pharmaceuticals, agrochemicals and advanced materials.15The coupling reaction between 5-bromo-2-fluoropyridine and pyrazole was chosen as the model reaction to optimize the reaction conditions. A range of reaction conditions, including temperature, solvent, and base, was investigated (Table 1).
| Entry | T (°C) | Solvent | Base | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 5-bromo-2-fluoropyridine (0.5 mmol), pyrazole (1.0 mmol), base (1.0 mmol) in solvent (2 mL), 24 h. DMSO = dimethylsulfoxide, DMA = N,N-dimethylacetamide, DMF = N,N-dimethylformamide, NMP = N-methyl-2-pyrrolidone. Isolated yield, data in parentheses correspond to isolated yield of 1ca. | ||||
| 1 | 50 | DMSO | K2CO3 | 39 (0) |
| 2 | 60 | DMSO | K2CO3 | 67 (0) |
| 3 | 70 | DMSO | K2CO3 | 95 (trace) |
| 4 | 90 | DMSO | K2CO3 | 93 (6) |
| 5 | 110 | DMSO | K2CO3 | 53 (45) |
| 6 | 70 | DMF | K2CO3 | 58 (trace) |
| 7 | 70 | DMA | K2CO3 | 67 (7%) |
| 8 | 70 | NMP | K2CO3 | 47 (trace) |
| 9 | 70 | DMSO | K3PO4·3H2O | 77 (11) |
| 10 | 70 | DMSO | KOH | 26 (40) |
| 11 | 70 | DMSO | KOtBu | 0 (53) |
| 12 | 70 | DMSO | NaHCO3 | 68 (12) |
| 13 | 70 | DMSO | Na2CO3 | 70 (19) |
| 14 | 70 | DMSO | NaOH | 11 (68) |
| 15 | 70 | DMSO | NaOtBu | 0 (33) |
| 16 | 70 | DMSO | Cs2CO3 | 21 (45) |
The effect of temperature on the coupling of 5-bromo-2-fluoropyridine with pyrazole was first examined. The reaction rate increased at reaction temperatures ranging from 50 °C to 70 °C (Table 1, entries 1–3). However, the monosubstituted product underwent second coupling and conversion into the disubstituted product at temperatures above 70 °C (Table 1, entries 4 and 5). Evaluation of various solvents showed that DMSO was superior to DMF, DMA, and NMP (Table 1, entries 3, and 6–8). Evaluation of bases exhibited that weak bases (Table 1, entries 3, 9, 12, and 13) had better selectivity than strong bases (Table 1, entries 10, 11, and 14, 15), with K2CO3 exhibiting the best results (Table 1, entry 3). Despite being a weak base, Cs2CO3 still yielded disubstituted products (Table 1, entry 16). Inorganic base combined with dipolar aprotic solvents such as DMSO, form a superbasic media.16 The extraordinary basicity of Cs2CO3/DMSO may accelerate the deprotonation of azole. However, other weak bases exhibit different catalytic effects from Cs2CO3, which indicates that base acts not only as a deprotonation agent but also exerts additional effects on reaction rates.14b
A reaction system using di- or multihalogenated pyridine and a range of amines was explored under the optimized reaction conditions (Table 2).
Halogen atom substituted position on the pyridine ring showed evident effects on the reaction rate. The 2-fluoropyridine with bromide substitution at the meta position to the nitrogen ring showed higher reactivity than that with bromide substitution at the para or ortho position to the nitrogen ring. Thus, 5-bromo-2-fluoropyridine and 3-bromo-2-fluoropyridine easily reacted with amines to provide the corresponding products in excellent yield (Table 2, 1a–d). However, 4-bromo-2-fluoropyridine and 2-bromo-6-fluoropyridine were weakly reactive in this system and provided moderate product yields (Table 2, 1e and 1f). The electronic effect of the bromide group bearing the 3-fluoropyridine ring was also observed. The 3-fluoropyridine with bromide substitution at the meta position to the nitrogen ring showed higher reactivity than those with bromide substitution at the ortho position to the nitrogen ring. Therefore, 5-bromo-3-fluoropyridine successfully converted them into the desired product in high yields (Table 2, 1g). However, 2-bromo-5-fluoropyridine and 2-bromo-3-fluoropyridine produced low conversion (Table 2, 1h and 1i). This result suggests that the position of the bromide group on the pyridine ring is crucial to the reactivity of fluorinated pyridine.
The iodinated and chlorinated fluoropyridines were also investigated for their reactivity in the developed metal-free system. This method showed high selectivity and reactivity, and the nucleophilic substitution occurred site-selectively at the fluorine substituent of the pyridine ring (Table 2, 1j–s).
The electronic effect of the nitro-substituent of benzimidazole on regioselectivity was examined. The nitro-substituent evidently affected regioselectivity and produced a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1t/1u.
1 mixture of 1t/1u.
Trihalogenated pyridines were also examined for selectivity and reactivity in the developed metal-free reaction system. For example, 3-bromo-5-chloro-2-fluoropyridine underwent high conversion and produced satisfactory results with minimal amounts of by-products (Table 2, 1v).
The type of N-heterocyclic ring was also examined to extend the scope of the reaction system. Interestingly, replacing the pyridine ring with a pyrimidine ring resulted in reversed selectivity. The chlorine atom on the pyrimidine ring had evidently higher reactive activity than the fluorine atom.
The scope of the reaction system using 5-fluoro-2-chloropyrimidine and a range of amines was explored.
The coupling of 5-fluoro-2-chloropyrimidine with a range of azole and indole derivatives proceeded smoothly to produce moderate to good yields (Table 2, 2a–h). Steric hindrance was not evident in the developed metal-free system. 2-Methylbenzimidazole and 2-methylimidazole successfully reacted with 5-fluoro-2-chloropyrimidine to provide good results (Table 2, 2i and 2j). 5-Fluoro-2-chloropyrimidine easily reacted with n-pentylamine to generate the desired product with a 96% yield (Table 2, 2k).
Some starting materials are tautomeric compounds. Thus, their regioselectivity on the coupling reaction was examined. The electronic effect of the methyl- and nitro-substituents only slightly affected regioselectivity, resulting in an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2n/2o or 2p/2q. By contrast, 5-methylimidazole showed high regioselectivity and 5-methyl-substituted products were predominantly formed, producing 1
1 mixture of 2n/2o or 2p/2q. By contrast, 5-methylimidazole showed high regioselectivity and 5-methyl-substituted products were predominantly formed, producing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixtures of 2l/2m.
5 mixtures of 2l/2m.
The main factors responsible for the high site-selectivity of the chloride group bearing pyrimidine were investigated by employing 5-bromo-2-chloropyrimidine instead of 5-fluoro-2-chloropyrimidine (Table 2, 2q–v). The preferred reaction site was also the chloride group, which is consistent with the obtained result when 5-fluoro-2-chloropyrimidine was employed. The reactivity of 2-chloropyrimidine was investigated to eliminate the interference of halogen on the pyridine ring. 2-Chloropyrimidine readily reacted to yield the desired product (Table 2, 2x). Thus, the selectivity of the reaction was dominated by two nitrogen bearing pyrimidine rings rather than by the halide group-bearing the pyrimidine ring.
6-Substituted 2-bromopyridines are important core structures of various agrochemical and pharmaceutical intermediates. They are generally synthesized using 2,6-dibromopyridine.10–12 Herein, the developed metal-free method was applied to synthesize 6-substituted 2-bromopyridines using 2-bromo-6-fluropyridine (Table 3).
The developed metal-free method exhibited higher selectivity than our previously reported copper-catalyzed approach11 and yielded a monosubstituted product with minimal amounts of disubstituted products. Steric and electronic effects on the amines were not evident on reactivity and regioselectivity in the developed reaction system.
We explored the C–N coupling of 2,6-difluoropyridine with benzimidazole under metal-free reaction conditions because it is less costly and more readily available than that of 2-bromo-6-fluoropyridine. The selectivity of the pyridine fluorine atom was efficiently controlled.
The N-arylation of 2,6-difluoropyridine with benzimidazole was selected as the model reaction to optimize the reaction conditions, in which a range of reaction conditions, including solvent, temperature, base, and molar ratio of substrates, was evaluated. The optimal reaction conditions for the N-arylation of 2,6-difluoropyridine with benzimidazole were found to be a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of 2,6-difluoropyridine and benzimidazole, 2.0 equiv. K2CO3 in DMSO at 90 °C (Table 4).
1 molar ratio of 2,6-difluoropyridine and benzimidazole, 2.0 equiv. K2CO3 in DMSO at 90 °C (Table 4).
| Entry | T (°C) | Solvent | Base | Yield (%) |
|---|---|---|---|---|
a Reaction conditions: 2,6-difluropyridine (0.75 mmol), benzimidazole (0.5 mmol), base (1.0 mmol) in solvent (4 mL), 6 h. Isolated yield data in parentheses correspond to isolated yield of 2,6-di(benzimidazol-1-yl)pyridine.
b Molar ratio of 2,6-difluropyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzimidazole = 0.5 benzimidazole = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75.
c Molar ratio of 2,6-difluropyridine 0.75.
c Molar ratio of 2,6-difluropyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzimidazole = 0.5 benzimidazole = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5.
d Molar ratio of 2,6-difluropyridine 0.5.
d Molar ratio of 2,6-difluropyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzimidazole = 1.0 benzimidazole = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. 0.5.
|
||||
| 1b | 90 | DMSO | K2CO3 | 34 (57) |
| 2c | 90 | DMSO | K2CO3 | 49 (29) |
| 3 | 90 | DMSO | K 2 CO 3 | 85 (11) |
| 4d | 90 | DMSO | K2CO3 | 83 (9) |
| 5 | 70 | DMSO | K2CO3 | 51 (trace) |
| 6 | 110 | DMSO | K2CO3 | 70 (26) |
| 7 | 90 | DMF | K2CO3 | 68 (13) |
| 8 | 90 | DMA | K2CO3 | 75 (19) |
| 9 | 90 | NMP | K2CO3 | 73 (22) |
| 10 | 90 | DMSO | K3PO4·3H2O | 54 (7) |
| 11 | 90 | DMSO | KOH | 52 (25) |
| 12 | 90 | DMSO | KOtBu | 65 (34) |
| 13 | 90 | DMSO | NaHCO3 | 70 (17) |
| 14 | 90 | DMSO | Na2CO3 | 69 (13) |
| 15 | 90 | DMSO | HCOONa | 59 (16) |
| 16 | 90 | DMSO | NaOH | 57 (27) |
| 17 | 90 | DMSO | NaOtBu | 37 (43) |
| 18 | 90 | DMSO | Cs2CO3 | 66 (38) |
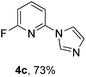
Azole and indole derivatives were tested and afforded the corresponding products in good to excellent yields (Table 5, 4a–g). 4-Azabenzimidazole showed less reactivity and produced a lower yield than other azole (Table 5, 4h). The steric effect of azole in the developed reaction system was not observed and 2-methylbenzimidazole and 2-methylimidazole afforded good to excellent product yields (Table 5, 4i and 4j).
The electronic effect of imidazole and indole significantly influenced the reaction rate. The 5-methoxy-1H-indole bearing electron-donating group afforded high conversion with a good product yield (Table 5, 4l). However, the 4-nitro-1H-imidazole bearing electron-withdrawing group was less reactive and afforded a low product yield, even after the reaction temperature was increased to 110 °C (Table 5, 4k). n-Pentylamine was successfully coupled with 2,6-difluoropyridine to afford the desired product in 86% yield (Table 5, 4m).
Fluorinated heteroaromatic molecules are extremely important building blocks in organic chemistry,17 especially with fluorinated substituted pyridine compounds, which are important molecular scaffolds for drug discovery.18
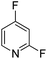
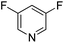
The site-selectivities of difluoro and multifluoropyridine were investigated. The reaction occurred site-selectively at the ortho position of the nitrogen ring and exclusively produced ortho-substituted pyridine in good to excellent yields (Table 6).
| Entry | Substrates | Monoproducts | Dimerisation product |
|---|---|---|---|
| a Reaction conditions: halogenated pyridine (0.5 mmol), amine (1.0 mmol), K2CO3 (1.0 mmol) in DMSO (2 mL), 90 °C, 24 h. Isolated yield. | |||
| 1 |

|

|
None |
| 2 |
|

|
None |
| 3 |

|

|
None |
| 4 |

|
|

|
| 5 |
|

|
None |
| 6 |

|

|

|
| 7 |

|

|
None |
| 8 |

|

|
None |
| 9 |

|
Trace |

|
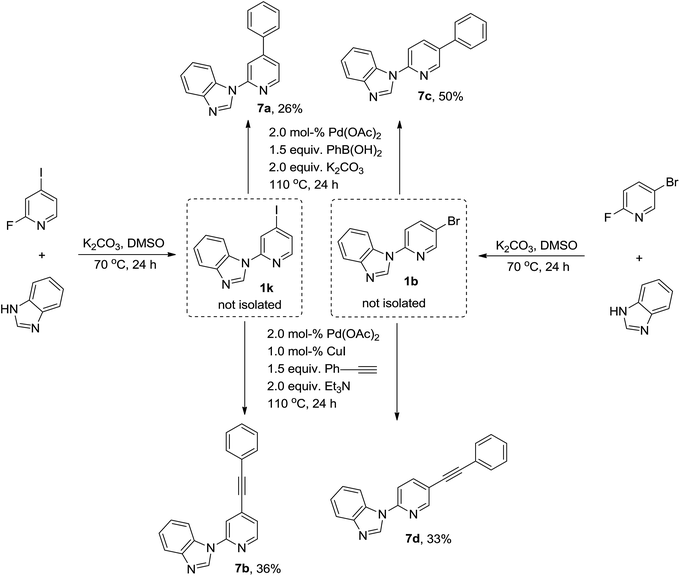
Halogens, such as chlorine, bromine, and iodine, on the N-heteroaromatic ring are well tolerated in this metal-free N-arylation. Thus, this system provides an opportunity to functionalize halogenated pyridines or pyrimidines further and convert them into useful pharmaceutical, agrochemical, and advanced material intermediates through a series of chemical transformations. In this study, we chose 4-iodo-2-fluoropyridine and 5-bromo-2-fluoropyridine as model substrates to explore their efficiency and selectivity through successive SNAr substitution reactions and transition-metal-catalyzed cross-coupling reaction without an intervening purification step. 4-Iodo-2-fluoropyridine and 5-bromo-2-fluoropyridine successively reacted in one-pot with benzimidazole and phenylboronic acid or phenylacetylene through nucleophilic substitution reaction palladium-catalyzed Suzuki coupling or Sonogashira coupling, resulting in valuable intermediates (Scheme 3).
Conclusions
We developed a metal-free method for highly site-selective synthesis of substituted pyridines and pyrimidines. The proposed reaction system showed high selectivity and reactivity for halogenated pyridines. Moreover, nucleophilic substitution site-selectively occurred at the fluorine substituent bearing the pyridine ring. A wide range of substituted pyridines bearing mono-halogen or multi-halogen group was obtained. The effect of the position of the bromine group on the pyridine ring was evident. Fluorinated pyridines bearing bromine group at the 3-position to the nitrogen ring were much more reactive than those bearing bromine group at the 2- and 4-position to the nitrogen ring. The preferred coupling site was switched from the fluorine atom to the chlorine atom when changing from the pyridine ring to pyrimidine ring. The developed metal-free reaction system was highly efficient and selective for the coupling of 5-fluoro-2-chloropyrimidine with a range of amines bearing various functional groups. Site-selectivity was also observed with the coupling reaction between the polyfluoropyridines and amines in the developed reaction system. The reaction was found to occur preferentially at the ortho-position of the nitrogen ring. The major advantage of this process is that the selectivity of halogen atoms on N-heteroaromatic ring for C–N coupling reaction could be completely controlled. This characteristic provides a range of substituted pyridines and pyrimidines bearing fluorine, chloride, bromide, and iodine groups. The resulting halogenated N-heteroaromatic compounds could be converted into valuable intermediates through classical transition-metal-catalyzed Suzuki coupling and Sonogashira coupling.Experimental
General experimental methods
All reactions were performed in glass tubes under air atmosphere. All reagents were purchased from commercial sources used without additional purification. All solvents were AR grade. NMR spectra were recorded on a Bruke Avance III HD 400 spectrometer using TMS as internal standard (400 MHz for 1H NMR, 100 MHz for 13C NMR and 376 MHz for 19F NMR). The mass data of the compounds were collected on a Bruker ultrafleXtreme mass spectrometer.General procedure for metal-free N-arylation
A mixture of halogenated pyridines, amines, and base in solvent was allowed to react under air atmosphere. The reaction mixture was heated to 70 °C or 90 °C for 24 h. After reaction, the reaction mixture was added to brine (15 mL) and extracted three times with dichloromethane (3 × 15 mL). The solvent was concentrated under vacuum and the product was isolated by short chromatography on a silica gel (200–300 mesh) column.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (106 mg, 95%), mp = 145–146 °C; 1H NMR (400 MHz, CDCl3): δ 8.55 (dd, J = 2.4 Hz, J = 0.4 Hz, 1H), 8.37 (s, 1H), 7.95 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H), 7.62 (t, J = 1.6 Hz, 1H), 7.31 (dd, J = 8.8 Hz, J = 0.8 Hz, 1H), 7.23 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.09, 147.67, 141.46, 134.94, 130.89, 117.71, 116.09, 113.45, ppm; HRMS (MALDI): m/z calcd for C8H6BrN3 [M + H]+ 223.9818, found 223.9825.
1): a white solid (106 mg, 95%), mp = 145–146 °C; 1H NMR (400 MHz, CDCl3): δ 8.55 (dd, J = 2.4 Hz, J = 0.4 Hz, 1H), 8.37 (s, 1H), 7.95 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H), 7.62 (t, J = 1.6 Hz, 1H), 7.31 (dd, J = 8.8 Hz, J = 0.8 Hz, 1H), 7.23 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.09, 147.67, 141.46, 134.94, 130.89, 117.71, 116.09, 113.45, ppm; HRMS (MALDI): m/z calcd for C8H6BrN3 [M + H]+ 223.9818, found 223.9825.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (122 mg, 89%), mp = 163–164 °C; 1H NMR (400 MHz, CDCl3): δ 8.66 (d, J = 2.4 Hz, 1H), 8.57 (s, 1H), 8.05–7.99 (m, 2H), 7.89–7.87 (m, 1H), 7.50 (J = 8.4 Hz, J = 0.4 Hz, 1H), 7.43–7.37 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.42, 148.51, 144.57, 141.46, 141.05, 131.87, 124.50, 123.60, 120.77, 117.64, 115.24, 112.67, ppm; HRMS (MALDI): m/z calcd for C12H8BrN3 [M + H]+ 273.9974, found 273.9972.
1): a white solid (122 mg, 89%), mp = 163–164 °C; 1H NMR (400 MHz, CDCl3): δ 8.66 (d, J = 2.4 Hz, 1H), 8.57 (s, 1H), 8.05–7.99 (m, 2H), 7.89–7.87 (m, 1H), 7.50 (J = 8.4 Hz, J = 0.4 Hz, 1H), 7.43–7.37 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.42, 148.51, 144.57, 141.46, 141.05, 131.87, 124.50, 123.60, 120.77, 117.64, 115.24, 112.67, ppm; HRMS (MALDI): m/z calcd for C12H8BrN3 [M + H]+ 273.9974, found 273.9972.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (106 mg, 95%), mp = 69–70 °C; 1H NMR (400 MHz, CDCl3): δ 8.53 (dd, J = 2.8 Hz, J = 0.8 Hz, 1H), 8.47 (dd, J = 2.0 Hz, J = 1.2 Hz, 1H), 7.93–7.92 (m, 2H), 7.76 (s, 1H), 6.49 (dd, J = 2.4 Hz, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.25, 148.88, 142.39, 141.17, 127.10, 117.02, 113.80, 108.17, ppm; HRMS (MALDI): m/z calcd for C8H6BrN3 [M + H]+ 223.9818, found 223.9825.
1): a white solid (106 mg, 95%), mp = 69–70 °C; 1H NMR (400 MHz, CDCl3): δ 8.53 (dd, J = 2.8 Hz, J = 0.8 Hz, 1H), 8.47 (dd, J = 2.0 Hz, J = 1.2 Hz, 1H), 7.93–7.92 (m, 2H), 7.76 (s, 1H), 6.49 (dd, J = 2.4 Hz, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.25, 148.88, 142.39, 141.17, 127.10, 117.02, 113.80, 108.17, ppm; HRMS (MALDI): m/z calcd for C8H6BrN3 [M + H]+ 223.9818, found 223.9825.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid, mp = 162 °C; 1H NMR (400 MHz, CDCl3): δ 8.79 (d, J = 2.4 Hz, 1H), 8.59 (d, J = 2.8 Hz, 1H), 8.19 (dd, J = 8.8 Hz, J = 2.4 Hz, 1H), 8.11 (d, J = 8.8 Hz, 1H), 7.97 (d, J = 2.4 Hz, 1H), 7.81–7.78 (m, 2H), 6.56 (t, J = 2.0 Hz, 1H), 6.52 (t, J = 2.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.61, 142.25, 141.88, 138.57, 134.83, 129.58, 127.11, 126.79, 112.81, 108.39, 108.07, ppm; HRMS (MALDI): m/z calcd for C11H9N5 [M + H]+ 212.0931, found 212.0932.
1): a white solid, mp = 162 °C; 1H NMR (400 MHz, CDCl3): δ 8.79 (d, J = 2.4 Hz, 1H), 8.59 (d, J = 2.8 Hz, 1H), 8.19 (dd, J = 8.8 Hz, J = 2.4 Hz, 1H), 8.11 (d, J = 8.8 Hz, 1H), 7.97 (d, J = 2.4 Hz, 1H), 7.81–7.78 (m, 2H), 6.56 (t, J = 2.0 Hz, 1H), 6.52 (t, J = 2.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.61, 142.25, 141.88, 138.57, 134.83, 129.58, 127.11, 126.79, 112.81, 108.39, 108.07, ppm; HRMS (MALDI): m/z calcd for C11H9N5 [M + H]+ 212.0931, found 212.0932.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (76 mg, 68%), mp = 62–63 °C; 1H NMR (400 MHz, CDCl3): δ 8.49 (s, 1H), 8.33 (d, J = 8.4 Hz, 1H), 7.65 (t, J = 1.6 Hz, 1H), 7.62–7.61 (m, 1H), 7.43 (dd, J = 5.2 Hz, J = 1.6 Hz, 1H), 7.25 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.72, 135.05, 134.84, 130.88, 130.86, 125.26, 116.08, 115.6, ppm; HRMS (MALDI): m/z calcd for C8H6BrN3 [M + H]+ 223.9818, found 223.9816.
1): a white solid (76 mg, 68%), mp = 62–63 °C; 1H NMR (400 MHz, CDCl3): δ 8.49 (s, 1H), 8.33 (d, J = 8.4 Hz, 1H), 7.65 (t, J = 1.6 Hz, 1H), 7.62–7.61 (m, 1H), 7.43 (dd, J = 5.2 Hz, J = 1.6 Hz, 1H), 7.25 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.72, 135.05, 134.84, 130.88, 130.86, 125.26, 116.08, 115.6, ppm; HRMS (MALDI): m/z calcd for C8H6BrN3 [M + H]+ 223.9818, found 223.9816.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (85 mg, 76%), mp = 92–94 °C; 1H NMR (400 MHz, CDCl3): δ 8.32 (s, 1H), 7.67 (t, J = 8.0 Hz, 1H), 7.61 (s, 1H), 7.41 (dd, J = 8.0 Hz, 1H), 7.31 (dd, J = 8.0 Hz, 1H), 7.19 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.81, 141.01, 140.91, 134.99, 131.01, 126.04, 116.12, 110.60, ppm; HRMS (EI): m/z calcd for C8H6BrN3 [M]+ 222.9740, found 222.9737.
1): a white solid (85 mg, 76%), mp = 92–94 °C; 1H NMR (400 MHz, CDCl3): δ 8.32 (s, 1H), 7.67 (t, J = 8.0 Hz, 1H), 7.61 (s, 1H), 7.41 (dd, J = 8.0 Hz, 1H), 7.31 (dd, J = 8.0 Hz, 1H), 7.19 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.81, 141.01, 140.91, 134.99, 131.01, 126.04, 116.12, 110.60, ppm; HRMS (EI): m/z calcd for C8H6BrN3 [M]+ 222.9740, found 222.9737.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (115 mg, 84%), mp = 169 °C; 1H NMR (400 MHz, CDCl3): δ 8.82 (s, 2H), 8.17 (s, 1H), 8.08 (t, J = 2.4 Hz, 1H), 7.95–7.91 (m, 1H), 7.57–7.53 (m, 1H), 7.44–7.40 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.26, 144.03, 143.11, 141.54, 133.78, 133.73, 133.13, 124.56, 123.64, 121.13, 121.08, 109.86, ppm; HRMS (MALDI): m/z calcd for C12H8BrN3 [M + H]+ 273.9974, found 273.9972.
1): a white solid (115 mg, 84%), mp = 169 °C; 1H NMR (400 MHz, CDCl3): δ 8.82 (s, 2H), 8.17 (s, 1H), 8.08 (t, J = 2.4 Hz, 1H), 7.95–7.91 (m, 1H), 7.57–7.53 (m, 1H), 7.44–7.40 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.26, 144.03, 143.11, 141.54, 133.78, 133.73, 133.13, 124.56, 123.64, 121.13, 121.08, 109.86, ppm; HRMS (MALDI): m/z calcd for C12H8BrN3 [M + H]+ 273.9974, found 273.9972.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (59 mg, 43%), mp = 214 °C; 1H NMR (400 MHz, CDCl3): δ 8.64 (dd, J = 2.4 Hz, J = 0.8 Hz, 1H), 8.14 (s, 1H), 7.95–7.90 (m, 1H), 7.79–7.73 (m, 2H), 7.51–7.48 (m, 1H), 7.43–7.38 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 145.29, 143.98, 141.54, 140.94, 133.75, 133.24, 132.62, 129.27, 124.53, 123.57, 121.04, 109.79, ppm; HRMS (MALDI): m/z calcd for C12H8BrN3 [M + H]+ 273.9974, found 273.9970.
1): a white solid (59 mg, 43%), mp = 214 °C; 1H NMR (400 MHz, CDCl3): δ 8.64 (dd, J = 2.4 Hz, J = 0.8 Hz, 1H), 8.14 (s, 1H), 7.95–7.90 (m, 1H), 7.79–7.73 (m, 2H), 7.51–7.48 (m, 1H), 7.43–7.38 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 145.29, 143.98, 141.54, 140.94, 133.75, 133.24, 132.62, 129.27, 124.53, 123.57, 121.04, 109.79, ppm; HRMS (MALDI): m/z calcd for C12H8BrN3 [M + H]+ 273.9974, found 273.9970.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (131 mg, 97%), mp = 116 °C; 1H NMR (400 MHz, CDCl3): δ 8.34 (s, 1H), 8.14 (d, J = 5.2 Hz, 1H), 7.75 (s, 1H), 7.61–7.60 (m, 2H), 7.21 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.24, 135.03, 131.05, 131.02, 121.45, 116.02, 107.15, ppm; HRMS (MALDI): m/z calcd for C8H6IN3 [M + H]+ 271.9679, found 271.9675.
1): a white solid (131 mg, 97%), mp = 116 °C; 1H NMR (400 MHz, CDCl3): δ 8.34 (s, 1H), 8.14 (d, J = 5.2 Hz, 1H), 7.75 (s, 1H), 7.61–7.60 (m, 2H), 7.21 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.24, 135.03, 131.05, 131.02, 121.45, 116.02, 107.15, ppm; HRMS (MALDI): m/z calcd for C8H6IN3 [M + H]+ 271.9679, found 271.9675.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (128 mg, 95%), mp = 143 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H), 8.30 (d, J = 5.2 Hz, 1H), 8.07 (d, J = 7.2 Hz, 1H), 8.01 (s, 1H), 7.90 (d, J = 7.2 Hz, 1H), 7.70 (dd, J = 5.2 Hz, J = 1.6 Hz, 1H), 7.46–7.38 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.06, 149.47, 144.59, 141.08, 131.82, 130.86, 124.52, 123.65, 123.18, 120.78, 112.75, 107.14, ppm; HRMS (MALDI): m/z calcd for C12H8IN3 [M + H]+ 321.9836, found 321.9831.
1): a white solid (128 mg, 95%), mp = 143 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H), 8.30 (d, J = 5.2 Hz, 1H), 8.07 (d, J = 7.2 Hz, 1H), 8.01 (s, 1H), 7.90 (d, J = 7.2 Hz, 1H), 7.70 (dd, J = 5.2 Hz, J = 1.6 Hz, 1H), 7.46–7.38 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.06, 149.47, 144.59, 141.08, 131.82, 130.86, 124.52, 123.65, 123.18, 120.78, 112.75, 107.14, ppm; HRMS (MALDI): m/z calcd for C12H8IN3 [M + H]+ 321.9836, found 321.9831.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (84 mg, 94%), mp = 150–151 °C; 1H NMR (400 MHz, CDCl3): δ 8.48 (d, J = 5.6 Hz, 1H), 8.06 (s, 1H), 7.42–7.39 (m, 2H), 7.32–7.28 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 153.34, 151.38, 145.49, 134.94, 131.88, 116.77, 114.75, 113.33, ppm; HRMS (MALDI): m/z calcd for C8H6ClN3 [M + H]+ 180.0323, found 180.0314.
1): a white solid (84 mg, 94%), mp = 150–151 °C; 1H NMR (400 MHz, CDCl3): δ 8.48 (d, J = 5.6 Hz, 1H), 8.06 (s, 1H), 7.42–7.39 (m, 2H), 7.32–7.28 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 153.34, 151.38, 145.49, 134.94, 131.88, 116.77, 114.75, 113.33, ppm; HRMS (MALDI): m/z calcd for C8H6ClN3 [M + H]+ 180.0323, found 180.0314.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (75 mg, 65%), mp = 200–201 °C; 1H NMR (400 MHz, CDCl3): δ 8.66 (d, J = 2.4 Hz, 1H), 8.13 (s, 1H), 7.96–7.91 (m, 1H), 7.87 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.60 (d, J = 8.8 Hz), 7.51–7.48 (m, 1H), 7.44–7.39 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.71, 144.89, 143.93, 141.58, 134.07, 133.31, 132.17, 125.48, 124.51, 123.57, 121.03, 109.78, ppm; HRMS (MALDI): m/z calcd for C12H8ClN3 [M + H]+ 230.0480, found 230.0473.
1): a white solid (75 mg, 65%), mp = 200–201 °C; 1H NMR (400 MHz, CDCl3): δ 8.66 (d, J = 2.4 Hz, 1H), 8.13 (s, 1H), 7.96–7.91 (m, 1H), 7.87 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.60 (d, J = 8.8 Hz), 7.51–7.48 (m, 1H), 7.44–7.39 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.71, 144.89, 143.93, 141.58, 134.07, 133.31, 132.17, 125.48, 124.51, 123.57, 121.03, 109.78, ppm; HRMS (MALDI): m/z calcd for C12H8ClN3 [M + H]+ 230.0480, found 230.0473.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (50 mg, 43%), mp = 96–97 °C; 1H NMR (400 MHz, CDCl3): δ 8.88 (dd, J = 2.8 Hz, J = 0.4 Hz, 1H), 8.27 (d, J = 1.2 Hz, 1H), 8.09 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.85 (dt, J = 8.4 Hz, J = 0.8 Hz, 1H), 7.74 (dd, J = 8.4 Hz, J = 0.8 Hz, 1H), 7.54–7.50 (m, 2H), 7.33–7.29 (m, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.42, 142.76, 138.70, 137.03, 136.12, 132.32, 128.05, 125.71, 124.87, 122.31, 121.75, 109.81, ppm; HRMS (MALDI): m/z calcd for C12H8ClN3 [M + H]+ 230.0480, found 230.0483.
1): a white solid (50 mg, 43%), mp = 96–97 °C; 1H NMR (400 MHz, CDCl3): δ 8.88 (dd, J = 2.8 Hz, J = 0.4 Hz, 1H), 8.27 (d, J = 1.2 Hz, 1H), 8.09 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.85 (dt, J = 8.4 Hz, J = 0.8 Hz, 1H), 7.74 (dd, J = 8.4 Hz, J = 0.8 Hz, 1H), 7.54–7.50 (m, 2H), 7.33–7.29 (m, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.42, 142.76, 138.70, 137.03, 136.12, 132.32, 128.05, 125.71, 124.87, 122.31, 121.75, 109.81, ppm; HRMS (MALDI): m/z calcd for C12H8ClN3 [M + H]+ 230.0480, found 230.0483.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (32 mg, 36%), mp = 120–122 °C; 1H NMR (400 MHz, CDCl3): δ 8.54 (d, J = 2.8 Hz, 1H), 7.90 (s, 1H), 7.73 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.50 (d, J = 8.8 Hz, 1H), 7.29 (s, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.10, 142.43, 135.52, 133.01, 131.7, 131.38, 125.22, 118.09, ppm; HRMS (MALDI): m/z calcd for C8H6ClN3 [M + H]+ 180.0323, found 180.0320.
1): a white solid (32 mg, 36%), mp = 120–122 °C; 1H NMR (400 MHz, CDCl3): δ 8.54 (d, J = 2.8 Hz, 1H), 7.90 (s, 1H), 7.73 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.50 (d, J = 8.8 Hz, 1H), 7.29 (s, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.10, 142.43, 135.52, 133.01, 131.7, 131.38, 125.22, 118.09, ppm; HRMS (MALDI): m/z calcd for C8H6ClN3 [M + H]+ 180.0323, found 180.0320.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (46 mg, 51%), mp = 110–111 °C; 1H NMR (400 MHz, CDCl3): δ 8.76 (d, J = 2.8 Hz, 1H), 8.05 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.95 (d, J = 2.4 Hz, 1H), 7.78 (d, J = 1.6 Hz, 1H), 7.44 (dd, J = 8.8 Hz, J = 0.8 Hz, 1H), 6.54 (t, J = 2.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.49, 142.24, 139.84, 135.74, 129.36, 126.82, 124.72, 108.79, ppm; HRMS (MALDI): m/z calcd for C8H6ClN3 [M + H]+ 180.0323, found 180.0323.
1): a white solid (46 mg, 51%), mp = 110–111 °C; 1H NMR (400 MHz, CDCl3): δ 8.76 (d, J = 2.8 Hz, 1H), 8.05 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.95 (d, J = 2.4 Hz, 1H), 7.78 (d, J = 1.6 Hz, 1H), 7.44 (dd, J = 8.8 Hz, J = 0.8 Hz, 1H), 6.54 (t, J = 2.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.49, 142.24, 139.84, 135.74, 129.36, 126.82, 124.72, 108.79, ppm; HRMS (MALDI): m/z calcd for C8H6ClN3 [M + H]+ 180.0323, found 180.0323.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (48 mg, 37%), mp = 156–157 °C; 1H NMR (400 MHz, CDCl3): δ 8.61 (d, J = 2.8 Hz, 1H), 7.99 (s, 1H), 7.84 (dd, J = 8.8 Hz, J = 2.8 Hz, 1H), 7.64 (s, 1H), 7.56 (d, J = 8.4 Hz, 1H), 7.25 (s, 1H), 2.40 (s, 3H), 2.38 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.26, 144.64, 144.63, 142.54, 140.75, 133.82, 132.57, 132.47, 131.74, 125.39, 120.87, 109.92, 20.64, 20.26, ppm; HRMS (MALDI): m/z calcd for C14H12ClN3 [M + H]+ 258.0793, found 258.0788.
1): a white solid (48 mg, 37%), mp = 156–157 °C; 1H NMR (400 MHz, CDCl3): δ 8.61 (d, J = 2.8 Hz, 1H), 7.99 (s, 1H), 7.84 (dd, J = 8.8 Hz, J = 2.8 Hz, 1H), 7.64 (s, 1H), 7.56 (d, J = 8.4 Hz, 1H), 7.25 (s, 1H), 2.40 (s, 3H), 2.38 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.26, 144.64, 144.63, 142.54, 140.75, 133.82, 132.57, 132.47, 131.74, 125.39, 120.87, 109.92, 20.64, 20.26, ppm; HRMS (MALDI): m/z calcd for C14H12ClN3 [M + H]+ 258.0793, found 258.0788.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (51 mg, 42%), mp = 118–119 °C; 1H NMR (400 MHz, CDCl3): δ 8.50 (d, J = 2.8 Hz, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.74 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H), 7.61 (d, J = 8.4 Hz, 1H), 7.35–7.24 (m, 2H), 7.11 (d, J = 8.0 Hz, 1H), 2.55 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.60, 151.05, 147.92, 142.56, 137.10, 136.03, 131.93, 125.48, 123.35, 123.16, 119.44, 109.30, 14.36, ppm.
1): a white solid (51 mg, 42%), mp = 118–119 °C; 1H NMR (400 MHz, CDCl3): δ 8.50 (d, J = 2.8 Hz, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.74 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H), 7.61 (d, J = 8.4 Hz, 1H), 7.35–7.24 (m, 2H), 7.11 (d, J = 8.0 Hz, 1H), 2.55 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.60, 151.05, 147.92, 142.56, 137.10, 136.03, 131.93, 125.48, 123.35, 123.16, 119.44, 109.30, 14.36, ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (32 mg, 33%), mp = 142–143 °C; 1H NMR (400 MHz, CDCl3): δ 8.43 (d, J = 2.8 Hz, 1H), 7.65 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.50 (d, J = 8.4 Hz, 1H), 7.10 (d, J = 1.2 Hz, 1H), 7.02 (d, J = 1.2 Hz, 1H), 2.40 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.01, 146.22, 144.82, 135.48, 133.62, 128.62, 124.96, 120.46, 13.65, ppm.
1): a white solid (32 mg, 33%), mp = 142–143 °C; 1H NMR (400 MHz, CDCl3): δ 8.43 (d, J = 2.8 Hz, 1H), 7.65 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 7.50 (d, J = 8.4 Hz, 1H), 7.10 (d, J = 1.2 Hz, 1H), 7.02 (d, J = 1.2 Hz, 1H), 2.40 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.01, 146.22, 144.82, 135.48, 133.62, 128.62, 124.96, 120.46, 13.65, ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow solid (67 mg, 49%), mp = 286–287 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.97 (s, 1H), 8.89 (dd, J = 2.8 Hz, J = 0.8 Hz, 1H), 8.49 (d, J = 2.0 Hz, 1H), 8.36 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 8.24 (dd, J = 8.8 Hz, J = 2.0 Hz, 1H), 8.02 (d, J = 8.8 Hz, 1H), 7.87 (d, J = 8.8 Hz, 1H), ppm; HRMS (MALDI): m/z calcd for C12H7ClN4O2 [M + H]+ 275.0330, found 275.0328.
1): a pale yellow solid (67 mg, 49%), mp = 286–287 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.97 (s, 1H), 8.89 (dd, J = 2.8 Hz, J = 0.8 Hz, 1H), 8.49 (d, J = 2.0 Hz, 1H), 8.36 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 8.24 (dd, J = 8.8 Hz, J = 2.0 Hz, 1H), 8.02 (d, J = 8.8 Hz, 1H), 7.87 (d, J = 8.8 Hz, 1H), ppm; HRMS (MALDI): m/z calcd for C12H7ClN4O2 [M + H]+ 275.0330, found 275.0328.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow solid (11 mg, 8%), mp = 236–238 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.91 (s, 1H), 8.86 (d, J = 2.8 Hz, 1H), 8.68 (d, J = 2.0 Hz, 1H), 8.31 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 8.25 (dd, J = 8.8 Hz, J = 2.4 Hz, 1H), 7.85 (t, J = 8.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.09, 147.78, 145.99, 144.00, 143.57, 137.86, 136.16, 132.00, 125.85, 119.67, 116.56, 111.99, ppm; HRMS (MALDI): m/z calcd for C12H7ClN4O2 [M + H]+ 275.0330, found 275.0328.
1): a pale yellow solid (11 mg, 8%), mp = 236–238 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.91 (s, 1H), 8.86 (d, J = 2.8 Hz, 1H), 8.68 (d, J = 2.0 Hz, 1H), 8.31 (dd, J = 8.4 Hz, J = 2.8 Hz, 1H), 8.25 (dd, J = 8.8 Hz, J = 2.4 Hz, 1H), 7.85 (t, J = 8.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.09, 147.78, 145.99, 144.00, 143.57, 137.86, 136.16, 132.00, 125.85, 119.67, 116.56, 111.99, ppm; HRMS (MALDI): m/z calcd for C12H7ClN4O2 [M + H]+ 275.0330, found 275.0328.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (110 mg, 85%), mp = 106–107 °C; 1H NMR (400 MHz, CDCl3): δ 8.47 (d, J = 2.0 Hz, 1H), 8.17 (s, 1H), 8.13 (d, J = 2.4 Hz, 1H), 7.55 (t, J = 1.2 Hz, 1H), 7.23 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 146.59, 145.95, 142.73, 137.13, 131.08, 129.52, 119.16, 112.89, ppm; HRMS (MALDI): m/z calcd for C8H5BrClN3 [M + H]+ 257.9428, found 257.9428.
1): a white solid (110 mg, 85%), mp = 106–107 °C; 1H NMR (400 MHz, CDCl3): δ 8.47 (d, J = 2.0 Hz, 1H), 8.17 (s, 1H), 8.13 (d, J = 2.4 Hz, 1H), 7.55 (t, J = 1.2 Hz, 1H), 7.23 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 146.59, 145.95, 142.73, 137.13, 131.08, 129.52, 119.16, 112.89, ppm; HRMS (MALDI): m/z calcd for C8H5BrClN3 [M + H]+ 257.9428, found 257.9428.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (103 mg, 97%), mp = 192 °C; 1H NMR (400 MHz, CDCl3): δ 9.02 (s, 1H), 8.64 (s, 2H), 8.52 (d, J = 7.6 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.46–7.38 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.67, 154.08, 152.26, 146.26, 146.04, 144.76, 141.75, 131.62, 124.75, 123.89, 120.43, 115.14, ppm; 19F NMR (376 MHz, CDCl3): δ −142.10, ppm; HRMS (MALDI): m/z calcd for C11H7FN4 [M + H]+ 215.0728, found 215.0734.
1): a white solid (103 mg, 97%), mp = 192 °C; 1H NMR (400 MHz, CDCl3): δ 9.02 (s, 1H), 8.64 (s, 2H), 8.52 (d, J = 7.6 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.46–7.38 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.67, 154.08, 152.26, 146.26, 146.04, 144.76, 141.75, 131.62, 124.75, 123.89, 120.43, 115.14, ppm; 19F NMR (376 MHz, CDCl3): δ −142.10, ppm; HRMS (MALDI): m/z calcd for C11H7FN4 [M + H]+ 215.0728, found 215.0734.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (71 mg, 86%), mp = 155–156 °C; 1H NMR (400 MHz, CDCl3): δ 8.59–8.58 (m, 1H), 7.86 (t, J = 1.2 Hz, 1H), 7.20 (t, J = 1.2 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 157.33, 154.73, 146.53, 146.31, 136.17, 130.82, 116.75, ppm; 19F NMR (376 MHz, CDCl3): δ −141.36, ppm; HRMS (MALDI): m/z calcd for C7H5FN4 [M + H]+ 165.0571, found 165.0571.
1): a white solid (71 mg, 86%), mp = 155–156 °C; 1H NMR (400 MHz, CDCl3): δ 8.59–8.58 (m, 1H), 7.86 (t, J = 1.2 Hz, 1H), 7.20 (t, J = 1.2 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 157.33, 154.73, 146.53, 146.31, 136.17, 130.82, 116.75, ppm; 19F NMR (376 MHz, CDCl3): δ −141.36, ppm; HRMS (MALDI): m/z calcd for C7H5FN4 [M + H]+ 165.0571, found 165.0571.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (54 mg, 66%), mp = 93 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 2H), 8.51 (d, J = 2.4 Hz, 1H), 7.82 (s, 1H), 6.49 (dd, J = 2.4 Hz, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 157.33, 154.74, 152.07, 146.56, 146.34, 143.75, 129.25, 108.84, ppm; 19F NMR (376 MHz, CDCl3): δ −142.21, ppm; HRMS (MALDI): m/z calcd for C7H5FN4 [M + H]+ 165.0571, found 165.0571.
1): a white solid (54 mg, 66%), mp = 93 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 2H), 8.51 (d, J = 2.4 Hz, 1H), 7.82 (s, 1H), 6.49 (dd, J = 2.4 Hz, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 157.33, 154.74, 152.07, 146.56, 146.34, 143.75, 129.25, 108.84, ppm; 19F NMR (376 MHz, CDCl3): δ −142.21, ppm; HRMS (MALDI): m/z calcd for C7H5FN4 [M + H]+ 165.0571, found 165.0571.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (55 mg, 51%), mp = 136 °C; 1H NMR (400 MHz, CDCl3): δ 8.72–8.69 (m, 3H), 8.33 (d, J = 0.8 Hz, 1H), 7.82 (dt, J = 8.0 Hz, J = 1.2 Hz, 1H), 7.61–7.56 (m, 1H), 7.38–7.34 (m, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.39, 154.09, 154.06, 153.80, 146.35, 146.13, 139.20, 138.98, 128.51, 126.37, 123.28, 121.09, 115.11, ppm; 19F NMR (376 MHz, CDCl3): δ −143.95, ppm; HRMS (MALDI): m/z calcd for C11H7FN4 [M + H]+ 215.0728, found 215.0730.
1): a white solid (55 mg, 51%), mp = 136 °C; 1H NMR (400 MHz, CDCl3): δ 8.72–8.69 (m, 3H), 8.33 (d, J = 0.8 Hz, 1H), 7.82 (dt, J = 8.0 Hz, J = 1.2 Hz, 1H), 7.61–7.56 (m, 1H), 7.38–7.34 (m, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.39, 154.09, 154.06, 153.80, 146.35, 146.13, 139.20, 138.98, 128.51, 126.37, 123.28, 121.09, 115.11, ppm; 19F NMR (376 MHz, CDCl3): δ −143.95, ppm; HRMS (MALDI): m/z calcd for C11H7FN4 [M + H]+ 215.0728, found 215.0730.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (71 mg, 87%), mp = 122–123 °C; 1H NMR (400 MHz, CDCl3): δ 8.50 (s, 2H), 7.73 (t, J = 2.4 Hz, 2H), 6.36 (d, J = 2.4 Hz, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.59, 154.02, 150.32, 146.04, 145.82, 119.26, 112.14, ppm; 19F NMR (376 MHz, CDCl3): δ −145.17, ppm; HRMS (MALDI): m/z calcd for C8H6FN3 [M + H]+ 164.0619, found 164.0620.
1): a white solid (71 mg, 87%), mp = 122–123 °C; 1H NMR (400 MHz, CDCl3): δ 8.50 (s, 2H), 7.73 (t, J = 2.4 Hz, 2H), 6.36 (d, J = 2.4 Hz, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.59, 154.02, 150.32, 146.04, 145.82, 119.26, 112.14, ppm; 19F NMR (376 MHz, CDCl3): δ −145.17, ppm; HRMS (MALDI): m/z calcd for C8H6FN3 [M + H]+ 164.0619, found 164.0620.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (116 mg, 96%), mp = 225–226 °C; 1H NMR (400 MHz, CDCl3): δ 8.90 (d, J = 3.2 Hz, 1H), 863–8.61 (m, 2H), 8.26 (d, J = 4.8 Hz, 1H), 7.61 (s, 1H), 2.46 (s, 3H), 2.42 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.49, 153.91, 152.31, 152.28, 146.08, 145.86, 143.30, 141.00, 133.80, 132.71, 130.02, 120.45, 115.23, 20.60, 20.20, ppm; 19F NMR (376 MHz, CDCl3): δ −142.81, ppm; HRMS (MALDI): m/z calcd for C13H11FN4 [M + H]+ 243.1041, found 243.1041.
1): a white solid (116 mg, 96%), mp = 225–226 °C; 1H NMR (400 MHz, CDCl3): δ 8.90 (d, J = 3.2 Hz, 1H), 863–8.61 (m, 2H), 8.26 (d, J = 4.8 Hz, 1H), 7.61 (s, 1H), 2.46 (s, 3H), 2.42 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.49, 153.91, 152.31, 152.28, 146.08, 145.86, 143.30, 141.00, 133.80, 132.71, 130.02, 120.45, 115.23, 20.60, 20.20, ppm; 19F NMR (376 MHz, CDCl3): δ −142.81, ppm; HRMS (MALDI): m/z calcd for C13H11FN4 [M + H]+ 243.1041, found 243.1041.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (77 mg, 86%), mp = 113–115 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 2H), 7.81 (d, J = 1.6 Hz, 1H), 6.99 (d, J = 1.6 Hz, 1H), 2.81 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.84, 154.23, 152.30, 146.43, 146.13, 145.91, 127.68, 118.68, 17.82, ppm; 19F NMR (376 MHz, CDCl3): δ −142.28, ppm; HRMS (MALDI): m/z calcd for C8H7FN4 [M + H]+ 179.0728, found 179.0734.
1): a white solid (77 mg, 86%), mp = 113–115 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 2H), 7.81 (d, J = 1.6 Hz, 1H), 6.99 (d, J = 1.6 Hz, 1H), 2.81 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.84, 154.23, 152.30, 146.43, 146.13, 145.91, 127.68, 118.68, 17.82, ppm; 19F NMR (376 MHz, CDCl3): δ −142.28, ppm; HRMS (MALDI): m/z calcd for C8H7FN4 [M + H]+ 179.0728, found 179.0734.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (81 mg, 71%), mp = 110–111 °C; 1H NMR (400 MHz, CDCl3): δ 8.72 (s, 2H), 8.19–8.16 (m, 1H), 7.76–7.73 (m, 1H), 7.36–7.31 (m, 2H), 2.95 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.67, 154.06, 153.06, 153.03, 152.47, 146.22, 146.00, 142.46, 133.83, 123.65, 123.62, 119.11, 113.88, 18.31, ppm; 19F NMR (376 MHz, CDCl3): δ −141.52, ppm; HRMS (MALDI): m/z calcd for C12H9FN4 [M + H]+ 229.0884, found 229.0881.
1): a white solid (81 mg, 71%), mp = 110–111 °C; 1H NMR (400 MHz, CDCl3): δ 8.72 (s, 2H), 8.19–8.16 (m, 1H), 7.76–7.73 (m, 1H), 7.36–7.31 (m, 2H), 2.95 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 156.67, 154.06, 153.06, 153.03, 152.47, 146.22, 146.00, 142.46, 133.83, 123.65, 123.62, 119.11, 113.88, 18.31, ppm; 19F NMR (376 MHz, CDCl3): δ −141.52, ppm; HRMS (MALDI): m/z calcd for C12H9FN4 [M + H]+ 229.0884, found 229.0881.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (88 mg, 96%), mp = 39–40 °C; 1H NMR (400 MHz, CDCl3): δ 8.17 (s, 2H), 5.23 (br, 1H), 3.36 (dd, J = 12.8 Hz, J = 6.8 Hz, 2H), 1.65–1.57 (m, 2H), 1.41–1.32 (m, 4H), 0.93–0.90 (m, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.59, 159.58, 153.06, 150.61, 145.50, 145.29, 42.03, 29.19, 29.09, 22.40, 13.96, ppm; 19F NMR (376 MHz, CDCl3): δ −156.65 to −156.67, ppm; HRMS (MALDI): m/z calcd for C9H14FN3 [M + H]+ 184.1245, found 184.1246.
1): a white solid (88 mg, 96%), mp = 39–40 °C; 1H NMR (400 MHz, CDCl3): δ 8.17 (s, 2H), 5.23 (br, 1H), 3.36 (dd, J = 12.8 Hz, J = 6.8 Hz, 2H), 1.65–1.57 (m, 2H), 1.41–1.32 (m, 4H), 0.93–0.90 (m, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.59, 159.58, 153.06, 150.61, 145.50, 145.29, 42.03, 29.19, 29.09, 22.40, 13.96, ppm; 19F NMR (376 MHz, CDCl3): δ −156.65 to −156.67, ppm; HRMS (MALDI): m/z calcd for C9H14FN3 [M + H]+ 184.1245, found 184.1246.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7. Purification by flash chromatography (petroleum ether/EtOAc = 2
4.7. Purification by flash chromatography (petroleum ether/EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (63 mg, 71%), mp = 129–131 °C; 1H NMR (400 MHz, CDCl3): δ 8.59 (s, 2H2l), 8.54 (s, 2H2m), 8.45 (d, J = 0.8 Hz, 1H2m), 7.54 (d, J = 1.2 Hz, 1H2m), 7.28 (s, 1H2l), 6.87 (br, 1H2l), 2.57 (d, J = 1.2 Hz, 3H2l), 2.31 (d, J = 1.2 Hz, 3H2m), ppm; 13C NMR (100 MHz, CDCl3): δ 157.09, 154.50, 150.88, 150.85, 146.40, 146.21, 146.18, 145.99, 140.09, 137.49, 135.48, 129.57, 112.90, 13.72, 12.87, ppm; 19F NMR (376 MHz, CDCl3): δ −141.78 (2m), 142.13 (2n), ppm; HRMS (MALDI): m/z calcd for C8H7FN4 [M + H]+ 179.0728, found 179.0726.
1): a white solid (63 mg, 71%), mp = 129–131 °C; 1H NMR (400 MHz, CDCl3): δ 8.59 (s, 2H2l), 8.54 (s, 2H2m), 8.45 (d, J = 0.8 Hz, 1H2m), 7.54 (d, J = 1.2 Hz, 1H2m), 7.28 (s, 1H2l), 6.87 (br, 1H2l), 2.57 (d, J = 1.2 Hz, 3H2l), 2.31 (d, J = 1.2 Hz, 3H2m), ppm; 13C NMR (100 MHz, CDCl3): δ 157.09, 154.50, 150.88, 150.85, 146.40, 146.21, 146.18, 145.99, 140.09, 137.49, 135.48, 129.57, 112.90, 13.72, 12.87, ppm; 19F NMR (376 MHz, CDCl3): δ −141.78 (2m), 142.13 (2n), ppm; HRMS (MALDI): m/z calcd for C8H7FN4 [M + H]+ 179.0728, found 179.0726.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (61 mg, 47%), mp = 256–258 °C; 1H NMR (400 MHz, CDCl3): δ 9.19 (s, 1H), 8.77 (d, J = 2.4 Hz, 1H), 8.74 (s, 2H), 8.69 (d, J = 8.8 Hz, 1H), 8.37 (dd, J = 9.2 Hz, J = 2.4 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 157.25, 154.64, 151.68, 146.74, 146.52, 144.76, 144.60, 135.73, 120.28, 116.91, 115.40, 112.25, ppm; 19F NMR (376 MHz, CDCl3): δ −139.93, ppm; HRMS (MALDI): m/z calcd for C11H6FN5O2 [M + H]+ 260.0578, found 260.0578.
1): a white solid (61 mg, 47%), mp = 256–258 °C; 1H NMR (400 MHz, CDCl3): δ 9.19 (s, 1H), 8.77 (d, J = 2.4 Hz, 1H), 8.74 (s, 2H), 8.69 (d, J = 8.8 Hz, 1H), 8.37 (dd, J = 9.2 Hz, J = 2.4 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 157.25, 154.64, 151.68, 146.74, 146.52, 144.76, 144.60, 135.73, 120.28, 116.91, 115.40, 112.25, ppm; 19F NMR (376 MHz, CDCl3): δ −139.93, ppm; HRMS (MALDI): m/z calcd for C11H6FN5O2 [M + H]+ 260.0578, found 260.0578.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3. Purification by flash chromatography (petroleum ether/EtOAc = 2
1.3. Purification by flash chromatography (petroleum ether/EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (109 mg, 96%), mp = 183 °C; 1H NMR (400 MHz, CDCl3): δ 8.97 (s, 1H2p), 8.95 (s, 1H2q), 8.65 (s, 2H2p), 8.64 (s, 2H2q), 8.38 (d, J = 8.4 Hz, 1H2p), 8.33 (s, 1H2q), 7.73 (d, J = 8.4 Hz, 1H2p), 7.65 (s, 1H2q), 7.25 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H2p), 7.22 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H2q), 2.57 (s, 1H2p), 2.53 (s, 1H2q), ppm; 13C NMR (100 MHz, CDCl3): δ 156.60, 154.01, 152.33, 152.26, 146.20, 145.98, 145.07, 142.87, 141.71, 141.32, 134.83, 133.70, 131.81, 129.63, 126.07, 125.30, 120.29, 119.88, 115.04, 114.61, 21.97, 21.47, ppm; 19F NMR (376 MHz, CDCl3): δ −142.45 (2k), 142.53 (2l), ppm; HRMS (MALDI): m/z calcd for C12H9FN4 [M + H]+ 229.0884, found 229.0883.
1): a white solid (109 mg, 96%), mp = 183 °C; 1H NMR (400 MHz, CDCl3): δ 8.97 (s, 1H2p), 8.95 (s, 1H2q), 8.65 (s, 2H2p), 8.64 (s, 2H2q), 8.38 (d, J = 8.4 Hz, 1H2p), 8.33 (s, 1H2q), 7.73 (d, J = 8.4 Hz, 1H2p), 7.65 (s, 1H2q), 7.25 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H2p), 7.22 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H2q), 2.57 (s, 1H2p), 2.53 (s, 1H2q), ppm; 13C NMR (100 MHz, CDCl3): δ 156.60, 154.01, 152.33, 152.26, 146.20, 145.98, 145.07, 142.87, 141.71, 141.32, 134.83, 133.70, 131.81, 129.63, 126.07, 125.30, 120.29, 119.88, 115.04, 114.61, 21.97, 21.47, ppm; 19F NMR (376 MHz, CDCl3): δ −142.45 (2k), 142.53 (2l), ppm; HRMS (MALDI): m/z calcd for C12H9FN4 [M + H]+ 229.0884, found 229.0883.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (107 mg, 95%), mp = 235–236 °C; 1H NMR (400 MHz, CDCl3): δ 8.74 (s, 2H), 8.59 (s, 1H), 7.85 (s, 1H), 7.19 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.30, 153.09, 136.22, 130.93, 116.60, 116.42, ppm; HRMS (MALDI): m/z calcd for C7H5BrN4 [M + H]+ 224.9770, found 224.9763.
1): a white solid (107 mg, 95%), mp = 235–236 °C; 1H NMR (400 MHz, CDCl3): δ 8.74 (s, 2H), 8.59 (s, 1H), 7.85 (s, 1H), 7.19 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.30, 153.09, 136.22, 130.93, 116.60, 116.42, ppm; HRMS (MALDI): m/z calcd for C7H5BrN4 [M + H]+ 224.9770, found 224.9763.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (133 mg, 97%), mp = 193 °C; 1H NMR (400 MHz, CDCl3): δ 9.02 (s, 1H), 8.78 (s, 2H), 8.50 (d, J = 7.2 Hz, 1H), 7.86 (d, J = 7.2 Hz, 1H), 7.45–7.37 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.01, 154.45, 144.88, 141.64, 131.55, 124.88, 124.07, 120.53, 115.47, 115.41, ppm; HRMS (MALDI): m/z calcd for C11H7BrN4 [M + H]+ 274.9927, found 274.9929.
1): a white solid (133 mg, 97%), mp = 193 °C; 1H NMR (400 MHz, CDCl3): δ 9.02 (s, 1H), 8.78 (s, 2H), 8.50 (d, J = 7.2 Hz, 1H), 7.86 (d, J = 7.2 Hz, 1H), 7.45–7.37 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.01, 154.45, 144.88, 141.64, 131.55, 124.88, 124.07, 120.53, 115.47, 115.41, ppm; HRMS (MALDI): m/z calcd for C11H7BrN4 [M + H]+ 274.9927, found 274.9929.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (104 mg, 92%), mp = 138–139 °C; 1H NMR (400 MHz, CDCl3): δ 8.77 (s, 2H), 8.53 (d, J = 2.8 Hz, 1H), 7.84 (s, 1H), 6.51 (dd, J = 2.8 Hz, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.30, 154.29, 144.09, 129.31, 116.24, 109.11, ppm; HRMS (MALDI): m/z calcd for C7H5BrN4 [M + H]+ 224.9770, found 224.9763.
1): a white solid (104 mg, 92%), mp = 138–139 °C; 1H NMR (400 MHz, CDCl3): δ 8.77 (s, 2H), 8.53 (d, J = 2.8 Hz, 1H), 7.84 (s, 1H), 6.51 (dd, J = 2.8 Hz, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.30, 154.29, 144.09, 129.31, 116.24, 109.11, ppm; HRMS (MALDI): m/z calcd for C7H5BrN4 [M + H]+ 224.9770, found 224.9763.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (52 mg, 46%), mp = 170–172 °C; 1H NMR (400 MHz, CDCl3): δ 8.65 (s, 2H), 7.73 (t, J = 2.4 Hz, 2H), 6.37 (t, J = 2.4 Hz, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 158.81, 119.21, 114.32, 112.48, ppm; HRMS (MALDI): m/z calcd for C8H6BrN3 [M + H]+ 223.9818, found 223.9820.
1): a white solid (52 mg, 46%), mp = 170–172 °C; 1H NMR (400 MHz, CDCl3): δ 8.65 (s, 2H), 7.73 (t, J = 2.4 Hz, 2H), 6.37 (t, J = 2.4 Hz, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 158.81, 119.21, 114.32, 112.48, ppm; HRMS (MALDI): m/z calcd for C8H6BrN3 [M + H]+ 223.9818, found 223.9820.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (110 mg, 90%), mp = 72–73 °C; 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 2H), 5.33 (br, 1H), 3.40–3.35 (m, 2H), 1.65–1.57 (m, 2H), 1.40–1.32 (m, 4H), 0.93–0.90 (m, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 160.70, 158.17, 105.89, 41.74, 29.12, 29.06, 22.41, 14.00, ppm; HRMS (MALDI): m/z calcd for C9H14BrN3 [M + H]+ 244.0444, found 244.0437.
1): a white solid (110 mg, 90%), mp = 72–73 °C; 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 2H), 5.33 (br, 1H), 3.40–3.35 (m, 2H), 1.65–1.57 (m, 2H), 1.40–1.32 (m, 4H), 0.93–0.90 (m, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 160.70, 158.17, 105.89, 41.74, 29.12, 29.06, 22.41, 14.00, ppm; HRMS (MALDI): m/z calcd for C9H14BrN3 [M + H]+ 244.0444, found 244.0437.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (93 mg, 95%), mp = 154 °C; 1H NMR (400 MHz, CDCl3): δ 9.11 (s, 1H), 8.75 (d, J = 4.8 Hz, 2H), 8.61–8.59 (m, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.45–7.36 (m, 2H), 7.19 (t, J = 4.8 Hz, 1H), ppm.
1): a white solid (93 mg, 95%), mp = 154 °C; 1H NMR (400 MHz, CDCl3): δ 9.11 (s, 1H), 8.75 (d, J = 4.8 Hz, 2H), 8.61–8.59 (m, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.45–7.36 (m, 2H), 7.19 (t, J = 4.8 Hz, 1H), ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (102 mg, 74%), mp = 145–148 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H), 8.10 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.76 (t, J = 8.0 Hz, 1H), 7.56 (d, J = 8.0 Hz, 1H), 7.49 (dd, J = 8.0 Hz, J = 4.0 Hz, 1H), 7.45–7.37 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.60, 144.66, 141.15, 140.92, 140.84, 131.79, 125.75, 124.63, 123.72, 120.79, 112.88, 112.14, ppm; HRMS (MALDI): m/z calcd for C12H8BrN3 [M + H]+ 273.9974, found 273.9977.
1): a white solid (102 mg, 74%), mp = 145–148 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H), 8.10 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.76 (t, J = 8.0 Hz, 1H), 7.56 (d, J = 8.0 Hz, 1H), 7.49 (dd, J = 8.0 Hz, J = 4.0 Hz, 1H), 7.45–7.37 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.60, 144.66, 141.15, 140.92, 140.84, 131.79, 125.75, 124.63, 123.72, 120.79, 112.88, 112.14, ppm; HRMS (MALDI): m/z calcd for C12H8BrN3 [M + H]+ 273.9974, found 273.9977.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (107 mg, 78%), mp = 102–105 °C; 1H NMR (400 MHz, CDCl3): δ 8.76 (d, J = 8.0 Hz, 1H), 8.18 (d, J = 4.0 Hz, 1H), 7.98 (dd, J = 8.0 Hz, J = 4.0 Hz, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.63 (t, J = 8.0 Hz, 1H), 7.55 (t, J = 8.0 Hz, 1H), 7.31–7.27 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 153.69, 140.25, 139.29, 138.75, 137.64, 128.42, 126.10, 123.40, 122.98, 120.79, 115.33, 111.52, ppm; HRMS (EI): m/z calcd for C12H8BrN3 [M]+ 272.9896, found 272.9890.
1): a white solid (107 mg, 78%), mp = 102–105 °C; 1H NMR (400 MHz, CDCl3): δ 8.76 (d, J = 8.0 Hz, 1H), 8.18 (d, J = 4.0 Hz, 1H), 7.98 (dd, J = 8.0 Hz, J = 4.0 Hz, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.63 (t, J = 8.0 Hz, 1H), 7.55 (t, J = 8.0 Hz, 1H), 7.31–7.27 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 153.69, 140.25, 139.29, 138.75, 137.64, 128.42, 126.10, 123.40, 122.98, 120.79, 115.33, 111.52, ppm; HRMS (EI): m/z calcd for C12H8BrN3 [M]+ 272.9896, found 272.9890.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (98 mg, 71%), mp = 114–116 °C; 1H NMR (400 MHz, CDCl3): δ 8.59 (d, J = 8.0 Hz, 1H), 8.27 (d, J = 8.0 Hz, 1H), 8.12 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 8.0 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.51–7.46 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.08, 146.74, 140.83, 140.02, 131.24, 129.28, 126.14, 125.25, 119.91, 114.70, 112.58, ppm; HRMS (EI): m/z calcd for C11H7BrN4 [M]+ 273.9849, found 273.9841.
1): a white solid (98 mg, 71%), mp = 114–116 °C; 1H NMR (400 MHz, CDCl3): δ 8.59 (d, J = 8.0 Hz, 1H), 8.27 (d, J = 8.0 Hz, 1H), 8.12 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 8.0 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.51–7.46 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.08, 146.74, 140.83, 140.02, 131.24, 129.28, 126.14, 125.25, 119.91, 114.70, 112.58, ppm; HRMS (EI): m/z calcd for C11H7BrN4 [M]+ 273.9849, found 273.9841.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (85 mg, 76%), mp = 92–94 °C; 1H NMR (400 MHz, CDCl3): δ 8.32 (s, 1H), 7.67 (t, J = 8.0 Hz, 1H), 7.61 (s, 1H), 7.41 (dd, J = 8.0 Hz, 1H), 7.31 (dd, J = 8.0 Hz, 1H), 7.19 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.81, 141.01, 140.91, 134.99, 131.01, 126.04, 116.12, 110.60, ppm; HRMS (EI): m/z calcd for C8H6BrN3 [M]+ 222.9740, found 222.9737.
1): a white solid (85 mg, 76%), mp = 92–94 °C; 1H NMR (400 MHz, CDCl3): δ 8.32 (s, 1H), 7.67 (t, J = 8.0 Hz, 1H), 7.61 (s, 1H), 7.41 (dd, J = 8.0 Hz, 1H), 7.31 (dd, J = 8.0 Hz, 1H), 7.19 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.81, 141.01, 140.91, 134.99, 131.01, 126.04, 116.12, 110.60, ppm; HRMS (EI): m/z calcd for C8H6BrN3 [M]+ 222.9740, found 222.9737.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (68 mg, 61%), mp = 55–58 °C; 1H NMR (400 MHz, CDCl3): δ 8.53 (s, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.73 (s, 1H), 7.65 (t, J = 8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 6.46 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.39, 142.66, 140.68, 139.86, 127.52, 125.19, 110.83, 108.19, ppm; HRMS (EI): m/z calcd for C8H6BrN3 [M]+ 222.9740, found 222.9737.
1): a white solid (68 mg, 61%), mp = 55–58 °C; 1H NMR (400 MHz, CDCl3): δ 8.53 (s, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.73 (s, 1H), 7.65 (t, J = 8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 6.46 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.39, 142.66, 140.68, 139.86, 127.52, 125.19, 110.83, 108.19, ppm; HRMS (EI): m/z calcd for C8H6BrN3 [M]+ 222.9740, found 222.9737.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white viscous solid (79 mg, 58%); 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 4.0 Hz, 1H), 7.64 (t, J = 8.0 Hz, 2H), 7.41 (d, J = 8.0 Hz, 1H), 7.33 (t, J = 8.0 Hz, 2H), 7.22 (t, J = 8.0 Hz, 1H), 6.71 (d, J = 4.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 152.18, 140.47, 140.32, 134.97, 130.60, 125.44, 123.63, 123.55, 121.82, 121.15, 113.50, 112.04, 106.58, ppm; HRMS (EI): m/z calcd for C13H9BrN2 [M]+ 271.9944, found 271.9939.
1): a white viscous solid (79 mg, 58%); 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 4.0 Hz, 1H), 7.64 (t, J = 8.0 Hz, 2H), 7.41 (d, J = 8.0 Hz, 1H), 7.33 (t, J = 8.0 Hz, 2H), 7.22 (t, J = 8.0 Hz, 1H), 6.71 (d, J = 4.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 152.18, 140.47, 140.32, 134.97, 130.60, 125.44, 123.63, 123.55, 121.82, 121.15, 113.50, 112.04, 106.58, ppm; HRMS (EI): m/z calcd for C13H9BrN2 [M]+ 271.9944, found 271.9939.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (155 mg, 96%), mp = 112–113 °C; 1H NMR (400 MHz, CDCl3): δ 8.13 (dq, J = 8.0 Hz, J = 0.8 Hz, 2H), 7.92 (dt, J = 8.0 Hz, J = 0.8 Hz, 2H), 7.77 (t, J = 8.0 Hz, 1H), 7.63 (dd, J = 8.0 Hz, J = 0.8 Hz, 1H), 7.51–7.47 (m, 3H), 7.39–7.35 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.60, 140.92, 140.39, 139.13, 126.50, 124.93, 124.63, 121.56, 120.26, 116.83, 111.43, ppm; HRMS (MALDI): m/z calcd for C17H11BrN2 [M + H]+ 323.0178, found 323.0169.
1): a white solid (155 mg, 96%), mp = 112–113 °C; 1H NMR (400 MHz, CDCl3): δ 8.13 (dq, J = 8.0 Hz, J = 0.8 Hz, 2H), 7.92 (dt, J = 8.0 Hz, J = 0.8 Hz, 2H), 7.77 (t, J = 8.0 Hz, 1H), 7.63 (dd, J = 8.0 Hz, J = 0.8 Hz, 1H), 7.51–7.47 (m, 3H), 7.39–7.35 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.60, 140.92, 140.39, 139.13, 126.50, 124.93, 124.63, 121.56, 120.26, 116.83, 111.43, ppm; HRMS (MALDI): m/z calcd for C17H11BrN2 [M + H]+ 323.0178, found 323.0169.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (101 mg, 70%), mp = 102–104 °C; 1H NMR (400 MHz, CDCl3): δ 7.80 (t, J = 8.0 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.31–7.23 (m, 2H), 2.71 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.35, 149.46, 142.67, 141.33, 140.69, 134.48, 127.18, 123.23, 123.12, 119.39, 118.05, 110.15, 15.54, ppm; HRMS (MALDI): m/z calcd for C13H10BrN3 [M + H]+ 288.0131, found 288.0140.
1): a white solid (101 mg, 70%), mp = 102–104 °C; 1H NMR (400 MHz, CDCl3): δ 7.80 (t, J = 8.0 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.31–7.23 (m, 2H), 2.71 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.35, 149.46, 142.67, 141.33, 140.69, 134.48, 127.18, 123.23, 123.12, 119.39, 118.05, 110.15, 15.54, ppm; HRMS (MALDI): m/z calcd for C13H10BrN3 [M + H]+ 288.0131, found 288.0140.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (68 mg, 57%), mp = 72–74 °C; 1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 1.6 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.37 (dd, J = 8.0 Hz, J = 0.8 Hz, 1H), 7.31 (t, J = 1.2 Hz, 1H), 7.24 (dd, J = 8.0 Hz, J = 0.4 Hz, 1H), 2.28 (d, J = 4.0 Hz, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.85, 140.92, 140.80, 140.27, 134.24, 125.54, 112.34, 110.19, 13.74, ppm; HRMS (MALDI): m/z calcd for C9H8BrN3 [M + H]+ 237.9974, found 237.9980.
1): a white solid (68 mg, 57%), mp = 72–74 °C; 1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 1.6 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.37 (dd, J = 8.0 Hz, J = 0.8 Hz, 1H), 7.31 (t, J = 1.2 Hz, 1H), 7.24 (dd, J = 8.0 Hz, J = 0.4 Hz, 1H), 2.28 (d, J = 4.0 Hz, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.85, 140.92, 140.80, 140.27, 134.24, 125.54, 112.34, 110.19, 13.74, ppm; HRMS (MALDI): m/z calcd for C9H8BrN3 [M + H]+ 237.9974, found 237.9980.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow solid (105 mg, 66%), mp = 201–202 °C; 1H NMR (400 MHz, CDCl3): δ 8.77 (d, J = 2.0 Hz, 1H), 8.68 (s, 1H), 8.36 (dd, J = 2.0 Hz, J = 8.0 Hz, 1H), 8.28 (d, J = 8.0 Hz, 1H), 7.84 (t, J = 8.0 Hz, 1H), 7.58 (t, J = 8.0 Hz, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.79, 144.58, 144.17, 143.72, 141.50, 141.24, 135.93, 126.94, 120.23, 117.19, 113.62, 112.46, ppm; HRMS (MALDI): m/z calcd for C12H7BrN4O2 [M + H]+ 318.9825, found 318.9830.
1): a pale yellow solid (105 mg, 66%), mp = 201–202 °C; 1H NMR (400 MHz, CDCl3): δ 8.77 (d, J = 2.0 Hz, 1H), 8.68 (s, 1H), 8.36 (dd, J = 2.0 Hz, J = 8.0 Hz, 1H), 8.28 (d, J = 8.0 Hz, 1H), 7.84 (t, J = 8.0 Hz, 1H), 7.58 (t, J = 8.0 Hz, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.79, 144.58, 144.17, 143.72, 141.50, 141.24, 135.93, 126.94, 120.23, 117.19, 113.62, 112.46, ppm; HRMS (MALDI): m/z calcd for C12H7BrN4O2 [M + H]+ 318.9825, found 318.9830.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale brown solid (109 mg, 72%), mp = 59–60 °C; 1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 4.0 Hz, 1H), 7.61 (t, J = 8.0 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.28 (d, J = 8.0 Hz, 1H), 7.09 (d, J = 4.0 Hz, 1H), 6.96 (dd, J = 8.0 Hz, J = 4.0 Hz, 1H), 6.64 (d, J = 4.0 Hz, 1H), 3.87 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 155.31, 152.18, 140.38, 140.25, 131.34, 130.02, 125.69, 123.14, 114.67, 112.98, 111.35, 106.45, 103.13, 55.72, ppm; HRMS (MALDI): m/z calcd for C14H11BrN2O [M]+ 302.0049, found 302.0054.
1): a pale brown solid (109 mg, 72%), mp = 59–60 °C; 1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 4.0 Hz, 1H), 7.61 (t, J = 8.0 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.28 (d, J = 8.0 Hz, 1H), 7.09 (d, J = 4.0 Hz, 1H), 6.96 (dd, J = 8.0 Hz, J = 4.0 Hz, 1H), 6.64 (d, J = 4.0 Hz, 1H), 3.87 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 155.31, 152.18, 140.38, 140.25, 131.34, 130.02, 125.69, 123.14, 114.67, 112.98, 111.35, 106.45, 103.13, 55.72, ppm; HRMS (MALDI): m/z calcd for C14H11BrN2O [M]+ 302.0049, found 302.0054.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale brown oil (105 mg, 86%); 1H NMR (400 MHz, CDCl3): δ 7.24 (t, J = 8.0 Hz, 1H), 6.69 (d, J = 8.0 Hz, 1H), 6.26 (d, J = 8.0 Hz, 1H), 4.72 (br, 1H), 3.19 (t, J = 8.0 Hz, 2H), 1.63–1.56 (m, 2H), 1.38–1.32 (m, 4H), 0.92–0.89 (m, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.02, 140.24, 139.50, 115.43, 103.89, 42.26, 29.09, 28.95, 22.39, 14.00, ppm; HRMS (MALDI): m/z calcd for C10H15BrN2 [M + H]+ 243.0491, found 243.0490.
1): a pale brown oil (105 mg, 86%); 1H NMR (400 MHz, CDCl3): δ 7.24 (t, J = 8.0 Hz, 1H), 6.69 (d, J = 8.0 Hz, 1H), 6.26 (d, J = 8.0 Hz, 1H), 4.72 (br, 1H), 3.19 (t, J = 8.0 Hz, 2H), 1.63–1.56 (m, 2H), 1.38–1.32 (m, 4H), 0.92–0.89 (m, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.02, 140.24, 139.50, 115.43, 103.89, 42.26, 29.09, 28.95, 22.39, 14.00, ppm; HRMS (MALDI): m/z calcd for C10H15BrN2 [M + H]+ 243.0491, found 243.0490.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (91 mg, 85%), mp = 95–96 °C; 1H NMR (400 MHz, CDCl3): δ 8.57 (s, 1H), 8.10 (d, J = 7.6 Hz, 1H), 8.01–7.95 (m, 1H), 7.86 (d, J = 7.6 Hz, 1H), 7.47–7.35 (m, 3H), 6.92 (d, J = 8.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.90, 161.48, 148.32, 148.17, 144.67, 143.63, 143.55, 141.03, 131.79, 124.55, 123.66, 120.77, 112.90, 110.25, 106.93, 106.58, ppm; 19F NMR (376 MHz, CDCl3): δ −65.55, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0777.
1): a white solid (91 mg, 85%), mp = 95–96 °C; 1H NMR (400 MHz, CDCl3): δ 8.57 (s, 1H), 8.10 (d, J = 7.6 Hz, 1H), 8.01–7.95 (m, 1H), 7.86 (d, J = 7.6 Hz, 1H), 7.47–7.35 (m, 3H), 6.92 (d, J = 8.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.90, 161.48, 148.32, 148.17, 144.67, 143.63, 143.55, 141.03, 131.79, 124.55, 123.66, 120.77, 112.90, 110.25, 106.93, 106.58, ppm; 19F NMR (376 MHz, CDCl3): δ −65.55, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0777.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (70 mg, 66%), mp = 75–76 °C; 1H NMR (400 MHz, CDCl3): δ 8.82 (d, J = 8.0 Hz, 1H), 8.20 (s, 1H), 7.93–7.86 (m, 2H), 7.76 (d, J = 8.0 Hz, 1H), 7.54 (t, J = 8.0 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 6.75 (d, J = 8.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.27, 160.88, 142.74, 142.66, 138.82, 137.61, 128.32, 126.08, 122.93, 120.81, 115.36, 109.66, 109.61, 104.18, 103.82, ppm; 19F NMR (376 MHz, CDCl3): δ −68.63, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0779.
1): a white solid (70 mg, 66%), mp = 75–76 °C; 1H NMR (400 MHz, CDCl3): δ 8.82 (d, J = 8.0 Hz, 1H), 8.20 (s, 1H), 7.93–7.86 (m, 2H), 7.76 (d, J = 8.0 Hz, 1H), 7.54 (t, J = 8.0 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 6.75 (d, J = 8.0 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.27, 160.88, 142.74, 142.66, 138.82, 137.61, 128.32, 126.08, 122.93, 120.81, 115.36, 109.66, 109.61, 104.18, 103.82, ppm; 19F NMR (376 MHz, CDCl3): δ −68.63, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0779.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (61 mg, 75%), mp = 90 °C; 1H NMR (400 MHz, CDCl3): δ 8.33 (s, 1H), 7.93 (q, J = 8.0 Hz, 1H), 7.61 (s, 1H), 7.25–7.20 (m, 2H), 6.88 (dd, J = 8.0 Hz, J = 2.4 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.88, 161.46, 147.49, 143.72, 143.64, 135.09, 131.08, 116.15, 108.68, 108.63, 107.22, 106.87, ppm; HRMS (MALDI): m/z calcd for C8H6FN3 [M + H]+ 164.0619, found 164.0619.
1): a white solid (61 mg, 75%), mp = 90 °C; 1H NMR (400 MHz, CDCl3): δ 8.33 (s, 1H), 7.93 (q, J = 8.0 Hz, 1H), 7.61 (s, 1H), 7.25–7.20 (m, 2H), 6.88 (dd, J = 8.0 Hz, J = 2.4 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.88, 161.46, 147.49, 143.72, 143.64, 135.09, 131.08, 116.15, 108.68, 108.63, 107.22, 106.87, ppm; HRMS (MALDI): m/z calcd for C8H6FN3 [M + H]+ 164.0619, found 164.0619.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (125 mg, 95%), mp = 107–108 °C; 1H NMR (400 MHz, CDCl3): δ 8.14 (dq, J = 7.6 Hz, J = 0.8 Hz, 2H), 8.03 (q, J = 8.4 Hz, 1H), 7.96 (dq, J = 8.4 Hz, J = 0.8 Hz, 2H), 7.58 (ddd, J = 8.0 Hz, J = 2.0 Hz, J = 0.8 Hz, 1H), 7.51–7.47 (m, 2H), 7.39–7.35 (m, 2H), 6.96–6.93 (m, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 164.13, 161.72, 150.30, 150.16, 143.00, 142.92, 139.19, 126.45, 124.61, 121.49, 120.24, 115.15, 115.10, 111.54, 106.18, 105.82, ppm; 19F NMR (376 MHz, CDCl3): δ −65.84, ppm; HRMS (MALDI): m/z calcd for C17H11FN2 [M + H]+ 263.0979, found 263.0978.
1): a white solid (125 mg, 95%), mp = 107–108 °C; 1H NMR (400 MHz, CDCl3): δ 8.14 (dq, J = 7.6 Hz, J = 0.8 Hz, 2H), 8.03 (q, J = 8.4 Hz, 1H), 7.96 (dq, J = 8.4 Hz, J = 0.8 Hz, 2H), 7.58 (ddd, J = 8.0 Hz, J = 2.0 Hz, J = 0.8 Hz, 1H), 7.51–7.47 (m, 2H), 7.39–7.35 (m, 2H), 6.96–6.93 (m, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 164.13, 161.72, 150.30, 150.16, 143.00, 142.92, 139.19, 126.45, 124.61, 121.49, 120.24, 115.15, 115.10, 111.54, 106.18, 105.82, ppm; 19F NMR (376 MHz, CDCl3): δ −65.84, ppm; HRMS (MALDI): m/z calcd for C17H11FN2 [M + H]+ 263.0979, found 263.0978.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow oil (58 mg, 71%); 1H NMR (400 MHz, CDCl3): δ 8.49 (dd, J = 2.8 Hz, J = 0.8 Hz, 1H), 7.91 (q, J = 8.0 Hz, 1H), 7.88–7.85 (m, 1H), 7.76 (d, J = 1.2 Hz, 1H), 6.81 (ddd, J = 7.6 Hz, J = 2.4 Hz, J = 0.8 Hz, 1H), 6.48 (dd, J = 2.8 Hz, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.42, 161.02, 150.00, 143.37, 143.29, 142.64, 127.44, 108.88, 108.84, 108.20, 106.14, 105.78, ppm; 19F NMR (376 MHz, CDCl3): δ −68.29, ppm; HRMS (MALDI): m/z calcd for C8H6FN3 [M + H]+ 164.0619, found 164.0619.
1): a pale yellow oil (58 mg, 71%); 1H NMR (400 MHz, CDCl3): δ 8.49 (dd, J = 2.8 Hz, J = 0.8 Hz, 1H), 7.91 (q, J = 8.0 Hz, 1H), 7.88–7.85 (m, 1H), 7.76 (d, J = 1.2 Hz, 1H), 6.81 (ddd, J = 7.6 Hz, J = 2.4 Hz, J = 0.8 Hz, 1H), 6.48 (dd, J = 2.8 Hz, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.42, 161.02, 150.00, 143.37, 143.29, 142.64, 127.44, 108.88, 108.84, 108.20, 106.14, 105.78, ppm; 19F NMR (376 MHz, CDCl3): δ −68.29, ppm; HRMS (MALDI): m/z calcd for C8H6FN3 [M + H]+ 164.0619, found 164.0619.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a brown oil (63 mg, 78%); 1H NMR (400 MHz, CDCl3): δ 7.83 (q, J = 8.0 Hz, 1H), 7.51 (t, J = 2.4 Hz, 2H), 7.18 (dd, J = 8.0 Hz, J = 2.0 Hz, 1H), 6.74–6.71 (m, 1H), 6.38 (t, J = 2.4 Hz, 2H), ppm; 19F NMR (376 MHz, CDCl3): δ −67.19, ppm; HRMS (MALDI): m/z calcd for C9H7FN2 [M + H]+ 163.0666, found 163.0671.
1): a brown oil (63 mg, 78%); 1H NMR (400 MHz, CDCl3): δ 7.83 (q, J = 8.0 Hz, 1H), 7.51 (t, J = 2.4 Hz, 2H), 7.18 (dd, J = 8.0 Hz, J = 2.0 Hz, 1H), 6.74–6.71 (m, 1H), 6.38 (t, J = 2.4 Hz, 2H), ppm; 19F NMR (376 MHz, CDCl3): δ −67.19, ppm; HRMS (MALDI): m/z calcd for C9H7FN2 [M + H]+ 163.0666, found 163.0671.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (81 mg, 76%), mp = 166 °C; 1H NMR (400 MHz, CDCl3): δ 8.64 (d, J = 8.8 Hz, 1H), 8.22 (d, J = 8.0 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.04 (q, J = 8.0 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.48 (t, J = 8.0 Hz, 1H), 6.96 (dd, J = 8.0 Hz, J = 2.4 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.50, 161.08, 146.74, 143.54, 143.46, 131.33, 129.22, 125.22, 119.96, 114.69, 110.87, 110.83, 107.23, 106.88, ppm; 19F NMR (376 MHz, CDCl3): δ −67.39, ppm; HRMS (MALDI): m/z calcd for C11H7FN4 [M + H]+ 215.0728, found 215.0728.
1): a white solid (81 mg, 76%), mp = 166 °C; 1H NMR (400 MHz, CDCl3): δ 8.64 (d, J = 8.8 Hz, 1H), 8.22 (d, J = 8.0 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.04 (q, J = 8.0 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.48 (t, J = 8.0 Hz, 1H), 6.96 (dd, J = 8.0 Hz, J = 2.4 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.50, 161.08, 146.74, 143.54, 143.46, 131.33, 129.22, 125.22, 119.96, 114.69, 110.87, 110.83, 107.23, 106.88, ppm; 19F NMR (376 MHz, CDCl3): δ −67.39, ppm; HRMS (MALDI): m/z calcd for C11H7FN4 [M + H]+ 215.0728, found 215.0728.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow oil (109 mg, 96%); 1H NMR (400 MHz, CDCl3): δ 8.03–7.96 (m, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.34–7.31 (m, 1H), 7.28–7.20 (m, 2H), 7.01–6.97 (m, 1H), 2.68 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.97, 161.54, 147.83, 147.68, 143.49, 143.41, 142.62, 123.21, 123.07, 119.28, 116.43, 116.38, 110.30, 108.73, 108.37, 15.52, ppm; 19F NMR (376 MHz, CDCl3): δ −65.11, ppm; HRMS (MALDI): m/z calcd for C13H10FN3 [M + H]+ 228.0932, found 228.0929.
1): a pale yellow oil (109 mg, 96%); 1H NMR (400 MHz, CDCl3): δ 8.03–7.96 (m, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.34–7.31 (m, 1H), 7.28–7.20 (m, 2H), 7.01–6.97 (m, 1H), 2.68 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.97, 161.54, 147.83, 147.68, 143.49, 143.41, 142.62, 123.21, 123.07, 119.28, 116.43, 116.38, 110.30, 108.73, 108.37, 15.52, ppm; 19F NMR (376 MHz, CDCl3): δ −65.11, ppm; HRMS (MALDI): m/z calcd for C13H10FN3 [M + H]+ 228.0932, found 228.0929.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow solid (66 mg, 75%), mp = 100 °C; 1H NMR (400 MHz, CDCl3): δ 7.94 (q, J = 8.0 Hz, 1H), 7.30 (s, 1H), 7.22 (d, J = 7.6 Hz, 1H), 7.02 (s, 1H), 6.92 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H), 2.65 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.53, 161.11, 148.83, 145.14, 143.29, 143.21, 128.24, 118.72, 113.19, 113.14, 107.62, 107.27, 15.79, ppm; 19F NMR (376 MHz, CDCl3): δ −65.72, ppm; HRMS (MALDI): m/z calcd for C9H8FN3 [M + H]+ 178.0775, found 178.0767.
1): a pale yellow solid (66 mg, 75%), mp = 100 °C; 1H NMR (400 MHz, CDCl3): δ 7.94 (q, J = 8.0 Hz, 1H), 7.30 (s, 1H), 7.22 (d, J = 7.6 Hz, 1H), 7.02 (s, 1H), 6.92 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H), 2.65 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.53, 161.11, 148.83, 145.14, 143.29, 143.21, 128.24, 118.72, 113.19, 113.14, 107.62, 107.27, 15.79, ppm; 19F NMR (376 MHz, CDCl3): δ −65.72, ppm; HRMS (MALDI): m/z calcd for C9H8FN3 [M + H]+ 178.0775, found 178.0767.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow solid (22 mg, 21%), mp = 174–175 °C; 1H NMR (400 MHz, CDCl3): δ 8.48 (d, J = 1.6 Hz, 1H), 8.31 (d, J = 1.6 Hz, 1H), 8.09 (q, J = 8.0 Hz, 1H), 7.42 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H), 7.08 (dd, J = 8.0 Hz, J = 2.8 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.94, 161.48, 144.55, 144.47, 133.55, 115.99, 109.80, 109.45, 109.32, 109.27, ppm; 19F NMR (376 MHz, CDCl3): δ −64.39, ppm; HRMS (MALDI): m/z calcd for C8H5FN4O2 [M + H]+ 209.0469, found 209.0472.
1): a pale yellow solid (22 mg, 21%), mp = 174–175 °C; 1H NMR (400 MHz, CDCl3): δ 8.48 (d, J = 1.6 Hz, 1H), 8.31 (d, J = 1.6 Hz, 1H), 8.09 (q, J = 8.0 Hz, 1H), 7.42 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H), 7.08 (dd, J = 8.0 Hz, J = 2.8 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.94, 161.48, 144.55, 144.47, 133.55, 115.99, 109.80, 109.45, 109.32, 109.27, ppm; 19F NMR (376 MHz, CDCl3): δ −64.39, ppm; HRMS (MALDI): m/z calcd for C8H5FN4O2 [M + H]+ 209.0469, found 209.0472.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow oil (104 mg, 86%); 1H NMR (400 MHz, CDCl3): δ 8.28 (d, J = 9.2 Hz, 1H), 7.86 (q, J = 8.0 Hz, 1H), 7.68 (d, J = 3.6 Hz, 1H), 7.30 (dd, J = 8.0 Hz, J = 2.4 Hz, 1H), 7.12 (d, J = 2.4 Hz, 1H), 6.99 (ddd, J = 9.2 Hz, J = 2.4 Hz, J = 0.4 Hz, 1H), 6.74 (ddd, J = 8.0 Hz, J = 2.8 Hz, J = 0.4 Hz, 1H), 6.66 (dd, J = 3.6 Hz, J = 0.8 Hz, 1H), 3.90 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.72, 161.32, 155.32, 151.11, 150.96, 142.90, 142.82, 131.38, 130.08, 125.90, 114.76, 112.95, 109.50, 109.46, 106.38, 104.07, 103.71, 103.16, 55.72, ppm; 19F NMR (376 MHz, CDCl3): δ −66.89, ppm; HRMS (MALDI): m/z calcd for C14H11FN2O [M + H]+ 243.0928, found 243.0928.
1): a pale yellow oil (104 mg, 86%); 1H NMR (400 MHz, CDCl3): δ 8.28 (d, J = 9.2 Hz, 1H), 7.86 (q, J = 8.0 Hz, 1H), 7.68 (d, J = 3.6 Hz, 1H), 7.30 (dd, J = 8.0 Hz, J = 2.4 Hz, 1H), 7.12 (d, J = 2.4 Hz, 1H), 6.99 (ddd, J = 9.2 Hz, J = 2.4 Hz, J = 0.4 Hz, 1H), 6.74 (ddd, J = 8.0 Hz, J = 2.8 Hz, J = 0.4 Hz, 1H), 6.66 (dd, J = 3.6 Hz, J = 0.8 Hz, 1H), 3.90 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 163.72, 161.32, 155.32, 151.11, 150.96, 142.90, 142.82, 131.38, 130.08, 125.90, 114.76, 112.95, 109.50, 109.46, 106.38, 104.07, 103.71, 103.16, 55.72, ppm; 19F NMR (376 MHz, CDCl3): δ −66.89, ppm; HRMS (MALDI): m/z calcd for C14H11FN2O [M + H]+ 243.0928, found 243.0928.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a pale yellow oil (78 mg, 86%); 1H NMR (400 MHz, CDCl3): δ 7.44 (d, J = 8.0 Hz, 1H), 6.17 (dd, J = 8.0 Hz, J = 2.4 Hz, 1H), 6.09 (d, J = 7.6 Hz, 1H), 468 (br, 1H), 3.22 (t, J = 7.2 Hz, 2H), 1.63–1.56 (m, 2H), 1.38–1.31 (m, 4H), 0.89 (t, J = 6.8 Hz, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 164.36, 162.02, 158.31, 158.14, 141.71, 141.62, 102.11, 102.07, 95.25, 94.88, 42.16, 29.11, 28.97, 22.39, 13.95, ppm; 19F NMR (376 MHz, CDCl3): δ −70.06, ppm; HRMS (MALDI): m/z calcd for C10H15FN2 [M + H]+ 183.1292, found 183.1288.
1): a pale yellow oil (78 mg, 86%); 1H NMR (400 MHz, CDCl3): δ 7.44 (d, J = 8.0 Hz, 1H), 6.17 (dd, J = 8.0 Hz, J = 2.4 Hz, 1H), 6.09 (d, J = 7.6 Hz, 1H), 468 (br, 1H), 3.22 (t, J = 7.2 Hz, 2H), 1.63–1.56 (m, 2H), 1.38–1.31 (m, 4H), 0.89 (t, J = 6.8 Hz, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 164.36, 162.02, 158.31, 158.14, 141.71, 141.62, 102.11, 102.07, 95.25, 94.88, 42.16, 29.11, 28.97, 22.39, 13.95, ppm; 19F NMR (376 MHz, CDCl3): δ −70.06, ppm; HRMS (MALDI): m/z calcd for C10H15FN2 [M + H]+ 183.1292, found 183.1288.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (102 mg, 96%), mp = 116–117 °C; 1H NMR (400 MHz, CDCl3): δ 8.46 (d, J = 5.6 Hz, 1H), 8.27 (s, 1H), 7.95–7.91 (m, 1H), 7.73–7.69 (m, 1H), 7.48–7.42 (m, 3H), 7.20 (t, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 166.02, 163.63, 149.87, 149.70, 147.70, 147.59, 144.52, 141.13, 132.11, 124.85, 124.02, 121.26, 114.86, 114.81, 110.61, 102.93, 102.52, ppm; 19F NMR (376 MHz, CDCl3): δ −63.75, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0770.
1): a white solid (102 mg, 96%), mp = 116–117 °C; 1H NMR (400 MHz, CDCl3): δ 8.46 (d, J = 5.6 Hz, 1H), 8.27 (s, 1H), 7.95–7.91 (m, 1H), 7.73–7.69 (m, 1H), 7.48–7.42 (m, 3H), 7.20 (t, J = 1.6 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 166.02, 163.63, 149.87, 149.70, 147.70, 147.59, 144.52, 141.13, 132.11, 124.85, 124.02, 121.26, 114.86, 114.81, 110.61, 102.93, 102.52, ppm; 19F NMR (376 MHz, CDCl3): δ −63.75, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0770.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (65 mg, 61%), mp = 115–116 °C; 1H NMR (400 MHz, CDCl3): δ 8.54 (s, 1H), 8.50 (dd, J = 3.2 Hz, J = 0.8 Hz, 1H), 8.01 (dd, J = 6.4 Hz, J = 1.6 Hz, 1H), 7.90 (dd, J = 6.4 Hz, J = 1.6 Hz, 1H), 7.71–7.66 (m, 1H), 7.61 (ddd, J = 8.8 Hz, J = 3.6 Hz, J = 0.8 Hz, 1H), 7.44–7.37 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.08, 156.54, 145.93, 145.90, 144.46, 141.26, 137.42, 137.16, 132.09, 126.13, 125.93, 124.34, 123.40, 120.69, 115.38, 115.33, 112.23, ppm; 19F NMR (376 MHz, CDCl3): δ −129.13, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0775.
1): a white solid (65 mg, 61%), mp = 115–116 °C; 1H NMR (400 MHz, CDCl3): δ 8.54 (s, 1H), 8.50 (dd, J = 3.2 Hz, J = 0.8 Hz, 1H), 8.01 (dd, J = 6.4 Hz, J = 1.6 Hz, 1H), 7.90 (dd, J = 6.4 Hz, J = 1.6 Hz, 1H), 7.71–7.66 (m, 1H), 7.61 (ddd, J = 8.8 Hz, J = 3.6 Hz, J = 0.8 Hz, 1H), 7.44–7.37 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 159.08, 156.54, 145.93, 145.90, 144.46, 141.26, 137.42, 137.16, 132.09, 126.13, 125.93, 124.34, 123.40, 120.69, 115.38, 115.33, 112.23, ppm; 19F NMR (376 MHz, CDCl3): δ −129.13, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0775.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (103 mg, 97%), mp = 137–138 °C; 1H NMR (400 MHz, CDCl3): δ 8.54 (s, 1H), 8.75 (s, 1H), 8.65 (s, 1H), 8.20–8.18 (m, 1H), 7.95–7.92 (m, 1H), 7.70–7.67 (m, 1H), 7.58–7.54 (m, 1H), 7.45–7.40 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 160.05, 158.05, 144.09, 141.59, 140.73, 140.69, 137.75, 137.52, 133.84, 133.79, 133.10, 124.55, 123.63, 121.09, 118.41, 118.21, 109.87, ppm; 19F NMR (376 MHz, CDCl3): δ −123.41, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0775.
1): a white solid (103 mg, 97%), mp = 137–138 °C; 1H NMR (400 MHz, CDCl3): δ 8.54 (s, 1H), 8.75 (s, 1H), 8.65 (s, 1H), 8.20–8.18 (m, 1H), 7.95–7.92 (m, 1H), 7.70–7.67 (m, 1H), 7.58–7.54 (m, 1H), 7.45–7.40 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 160.05, 158.05, 144.09, 141.59, 140.73, 140.69, 137.75, 137.52, 133.84, 133.79, 133.10, 124.55, 123.63, 121.09, 118.41, 118.21, 109.87, ppm; 19F NMR (376 MHz, CDCl3): δ −123.41, ppm; HRMS (MALDI): m/z calcd for C12H8FN3 [M + H]+ 214.0775, found 214.0775.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (40 mg, 32%), mp = 114–115 °C; 1H NMR (400 MHz, CDCl3): δ 8.46 (d, J = 2.8 Hz, 1H), 8.02–7.98 (m, 1H), 7.91–7.87 (m, 1H), 7.77–7.71 (m, 1H), 7.46–7.40 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.06, 148.03, 148.01, 147.98, 147.04, 147.02, 146.88, 146.86, 145.47, 145.44, 145.42, 145.39, 144.63, 144.61, 144.47, 144.45, 144.01, 143.96, 143.71, 143.66, 143.34, 141.37, 141.31, 141.25, 141.13, 141.07, 141.01, 132.13, 124.72, 124.02, 120.61, 118.56, 118.52, 118.36, 118.32, 118.28, 118.12, 118.09, 112.95, 112.93, ppm; 19F NMR (376 MHz, CDCl3): δ −87.44 (dd, J = 30.1, J = 24.1, 1F), −126.79 (dd, J = 30.1, J = 3.8, 1F), 136.29 (dd, J = 22.6, J = 3.8, 1F), ppm; HRMS (MALDI): m/z calcd for C12H6F3N3 [M + H]+ 250.0587, found 250.0587.
1): a white solid (40 mg, 32%), mp = 114–115 °C; 1H NMR (400 MHz, CDCl3): δ 8.46 (d, J = 2.8 Hz, 1H), 8.02–7.98 (m, 1H), 7.91–7.87 (m, 1H), 7.77–7.71 (m, 1H), 7.46–7.40 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.06, 148.03, 148.01, 147.98, 147.04, 147.02, 146.88, 146.86, 145.47, 145.44, 145.42, 145.39, 144.63, 144.61, 144.47, 144.45, 144.01, 143.96, 143.71, 143.66, 143.34, 141.37, 141.31, 141.25, 141.13, 141.07, 141.01, 132.13, 124.72, 124.02, 120.61, 118.56, 118.52, 118.36, 118.32, 118.28, 118.12, 118.09, 112.95, 112.93, ppm; 19F NMR (376 MHz, CDCl3): δ −87.44 (dd, J = 30.1, J = 24.1, 1F), −126.79 (dd, J = 30.1, J = 3.8, 1F), 136.29 (dd, J = 22.6, J = 3.8, 1F), ppm; HRMS (MALDI): m/z calcd for C12H6F3N3 [M + H]+ 250.0587, found 250.0587.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (105 mg, 85%), mp = 142 °C; 1H NMR (400 MHz, CDCl3): δ 8.48 (d, J = 2.8 Hz, 1H), 8.46 (d, J = 2.0 Hz, 1H), 8.02–7.97 (m, 1H), 7.93–7.88 (m, 1H), 7.78 (dd, J = 10.0 Hz, J = 2.0 Hz, 1H), 7.44–7.39 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.06, 148.41, 143.53, 143.40, 143.35, 141.58, 141.47, 137.22, 137.12, 132.38, 129.88, 129.86, 126.16, 125.95, 124.47, 123.77, 120.54, 113.20, 113.18, ppm; 19F NMR (376 MHz, CDCl3): δ −124.26, ppm; HRMS (MALDI): m/z calcd for C12H7ClFN3 [M + H]+ 248.0385, found 248.0385.
1): a white solid (105 mg, 85%), mp = 142 °C; 1H NMR (400 MHz, CDCl3): δ 8.48 (d, J = 2.8 Hz, 1H), 8.46 (d, J = 2.0 Hz, 1H), 8.02–7.97 (m, 1H), 7.93–7.88 (m, 1H), 7.78 (dd, J = 10.0 Hz, J = 2.0 Hz, 1H), 7.44–7.39 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.06, 148.41, 143.53, 143.40, 143.35, 141.58, 141.47, 137.22, 137.12, 132.38, 129.88, 129.86, 126.16, 125.95, 124.47, 123.77, 120.54, 113.20, 113.18, ppm; 19F NMR (376 MHz, CDCl3): δ −124.26, ppm; HRMS (MALDI): m/z calcd for C12H7ClFN3 [M + H]+ 248.0385, found 248.0385.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (115 mg, 95%), mp = 94–95 °C; 1H NMR (400 MHz, CDCl3): δ 8.35 (s, 2H), 7.82–7.73 (m, 2H), 7.20 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.87, 147.22, 145.34, 145.29, 136.88, 136.78, 136.17, 136.07, 130.18, 130.16, 128.91, 128.70, 117.38, 117.32, 116.92, 116.90, ppm; 19F NMR (376 MHz, CDCl3): δ −125.59, ppm; HRMS (MALDI): m/z calcd for C8H5BrFN3 [M + H]+ 241.9724, found 241.9722.
1): a white solid (115 mg, 95%), mp = 94–95 °C; 1H NMR (400 MHz, CDCl3): δ 8.35 (s, 2H), 7.82–7.73 (m, 2H), 7.20 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 149.87, 147.22, 145.34, 145.29, 136.88, 136.78, 136.17, 136.07, 130.18, 130.16, 128.91, 128.70, 117.38, 117.32, 116.92, 116.90, ppm; 19F NMR (376 MHz, CDCl3): δ −125.59, ppm; HRMS (MALDI): m/z calcd for C8H5BrFN3 [M + H]+ 241.9724, found 241.9722.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (52 mg, 30%), mp = 234–235 °C; 1H NMR (400 MHz, CDCl3): δ 8.59 (s, 2H), 7.95 (t, J = 7.2 Hz, 4H), 7.90 (t, J = 8.8 Hz, 1H), 7.46–7.36 (m, 4H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.86, 148.81, 146.21, 146.16, 143.50, 141.39, 141.33, 141.27, 133.35, 133.31, 133.24, 133.20, 132.18, 124.87, 124.16, 120.75, 117.69, 117.46, 117.23, 113.15, ppm; 19F NMR (376 MHz, CDCl3): δ −124.29, ppm; HRMS (MALDI): m/z calcd for C19H11F2N5 [M + H]+ 348.1055, found 348.1054.
1): a white solid (52 mg, 30%), mp = 234–235 °C; 1H NMR (400 MHz, CDCl3): δ 8.59 (s, 2H), 7.95 (t, J = 7.2 Hz, 4H), 7.90 (t, J = 8.8 Hz, 1H), 7.46–7.36 (m, 4H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.86, 148.81, 146.21, 146.16, 143.50, 141.39, 141.33, 141.27, 133.35, 133.31, 133.24, 133.20, 132.18, 124.87, 124.16, 120.75, 117.69, 117.46, 117.23, 113.15, ppm; 19F NMR (376 MHz, CDCl3): δ −124.29, ppm; HRMS (MALDI): m/z calcd for C19H11F2N5 [M + H]+ 348.1055, found 348.1054.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (45 mg, 32%), mp = 111–112 °C; 1H NMR (400 MHz, CDCl3): δ 8.37 (s, 1H), 7.84 (s, 1H), 7.74 (d, J = 1.2 Hz, 1H), 7.35 (s, 1H), 7.28–7.27 (m, 1H), 7.25 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 154.68, 154.65, 152.27, 152.24, 142.21, 142.15, 139.60, 139.54, 137.10, 137.07, 136.92, 136.88, 136.78, 136.74, 136.24, 136.14, 134.08, 133.96, 133.93, 133.80, 131.05, 131.03, 130.64, 119.52, 119.50, 117.37, 117.30, 110.81, 110.78, 110.44, 110.41, ppm; 19F NMR (376 MHz, CDCl3): δ −66.83 (d, J = 26.3, 1F), −139.49 (d, J = 26.3, 1F), ppm; HRMS (MALDI): m/z calcd for C11H6ClF2N5 [M + H]+ 282.0353, found 282.0352.
1): a white solid (45 mg, 32%), mp = 111–112 °C; 1H NMR (400 MHz, CDCl3): δ 8.37 (s, 1H), 7.84 (s, 1H), 7.74 (d, J = 1.2 Hz, 1H), 7.35 (s, 1H), 7.28–7.27 (m, 1H), 7.25 (s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 154.68, 154.65, 152.27, 152.24, 142.21, 142.15, 139.60, 139.54, 137.10, 137.07, 136.92, 136.88, 136.78, 136.74, 136.24, 136.14, 134.08, 133.96, 133.93, 133.80, 131.05, 131.03, 130.64, 119.52, 119.50, 117.37, 117.30, 110.81, 110.78, 110.44, 110.41, ppm; 19F NMR (376 MHz, CDCl3): δ −66.83 (d, J = 26.3, 1F), −139.49 (d, J = 26.3, 1F), ppm; HRMS (MALDI): m/z calcd for C11H6ClF2N5 [M + H]+ 282.0353, found 282.0352.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (35 mg, 26%), mp = 182–184 °C; 1H NMR (400 MHz, CDCl3): δ 8.68–8.66 (m, 2H), 8.13–8.11 (m, 1H), 7.93–7.91 (m, 1H), 7.78 (q, J = 0.8 Hz, 1H), 7.74–7.71 (m, 2H), 7.60–7.51 (m, 4H), 7.46–7.38 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.87, 150.57, 149.84, 144.72, 141.46, 137.39, 132.23, 129.77, 129.38, 127.11, 124.26, 123.34, 120.74, 120.13, 112.62, 112.32, ppm; HRMS (MALDI): m/z calcd for C18H13N3 [M + H]+ 272.1182, found 272.1182.
1): a white solid (35 mg, 26%), mp = 182–184 °C; 1H NMR (400 MHz, CDCl3): δ 8.68–8.66 (m, 2H), 8.13–8.11 (m, 1H), 7.93–7.91 (m, 1H), 7.78 (q, J = 0.8 Hz, 1H), 7.74–7.71 (m, 2H), 7.60–7.51 (m, 4H), 7.46–7.38 (m, 2H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.87, 150.57, 149.84, 144.72, 141.46, 137.39, 132.23, 129.77, 129.38, 127.11, 124.26, 123.34, 120.74, 120.13, 112.62, 112.32, ppm; HRMS (MALDI): m/z calcd for C18H13N3 [M + H]+ 272.1182, found 272.1182.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a yellow solid (53 mg, 36%), mp = 184–186 °C; 1H NMR (400 MHz, CDCl3): δ 8.64 (s, 1H), 8.61 (d, J = 5.2 Hz, 1H), 8.13 (d, J = 7.2 Hz, 1H), 7.91 (d, J = 6.8 Hz, 1H), 7.70 (s, 1H), 7.65–7.60 (m, 2H), 7.48–7.38 (m, 6H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.12, 149.41, 144.69, 141.25, 134.47, 132.04, 132.02, 129.64, 128.62, 124.36, 123.62, 123.46, 121.61, 120.72, 115.88, 112.83, 95.42, 86.08, ppm; HRMS (MALDI): m/z calcd for C20H13N3 [M + H]+ 296.1182, found 296.1182.
1): a yellow solid (53 mg, 36%), mp = 184–186 °C; 1H NMR (400 MHz, CDCl3): δ 8.64 (s, 1H), 8.61 (d, J = 5.2 Hz, 1H), 8.13 (d, J = 7.2 Hz, 1H), 7.91 (d, J = 6.8 Hz, 1H), 7.70 (s, 1H), 7.65–7.60 (m, 2H), 7.48–7.38 (m, 6H), ppm; 13C NMR (100 MHz, CDCl3): δ 150.12, 149.41, 144.69, 141.25, 134.47, 132.04, 132.02, 129.64, 128.62, 124.36, 123.62, 123.46, 121.61, 120.72, 115.88, 112.83, 95.42, 86.08, ppm; HRMS (MALDI): m/z calcd for C20H13N3 [M + H]+ 296.1182, found 296.1182.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (68 mg, 50%), mp = 145 °C; 1H NMR (400 MHz, CDCl3): δ 8.86 (dd, J = 2.4 Hz, J = 0.4 Hz, 1H), 8.67 (s, 1H), 8.15–8.11 (m, 2H), 7.93 (d, J = 7.2 Hz, 1H), 7.69–7.65 (m, 3H), 7.57–7.53 (m, 2H), 7.49–7.39 (m, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.81, 147.62, 144.54, 141.30, 137.24, 136.67, 135.00, 132.15, 129.28, 128.43, 126.97, 124.33, 123.41, 120.66, 114.09, 112.79, ppm; HRMS (MALDI): m/z calcd for C18H13N3 [M + H]+ 272.1182, found 272.1182.
1): a white solid (68 mg, 50%), mp = 145 °C; 1H NMR (400 MHz, CDCl3): δ 8.86 (dd, J = 2.4 Hz, J = 0.4 Hz, 1H), 8.67 (s, 1H), 8.15–8.11 (m, 2H), 7.93 (d, J = 7.2 Hz, 1H), 7.69–7.65 (m, 3H), 7.57–7.53 (m, 2H), 7.49–7.39 (m, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 148.81, 147.62, 144.54, 141.30, 137.24, 136.67, 135.00, 132.15, 129.28, 128.43, 126.97, 124.33, 123.41, 120.66, 114.09, 112.79, ppm; HRMS (MALDI): m/z calcd for C18H13N3 [M + H]+ 272.1182, found 272.1182.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a white solid (49 mg, 33%), mp = 157–159 °C; 1H NMR (400 MHz, CDCl3): δ 8.77 (d, J = 1.6 Hz, 1H), 8.65 (s, 1H), 8.12 (d, J = 7.6 Hz, 1H), 8.03 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H), 7.91 (d, J = 7.2 Hz, 1H), 7.62–7.58 (m, 3H), 7.44–7.38 (m, 5H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.91, 148.51, 144.44, 141.30, 141.15, 131.92, 131.72, 129.02, 128.53, 124.53, 123.67, 122.31, 120.68, 118.59, 113.42, 112.97, 93.41, 85.15, ppm; HRMS (MALDI): m/z calcd for C20H13N3 [M + H]+ 296.1182, found 296.1187.
1): a white solid (49 mg, 33%), mp = 157–159 °C; 1H NMR (400 MHz, CDCl3): δ 8.77 (d, J = 1.6 Hz, 1H), 8.65 (s, 1H), 8.12 (d, J = 7.6 Hz, 1H), 8.03 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H), 7.91 (d, J = 7.2 Hz, 1H), 7.62–7.58 (m, 3H), 7.44–7.38 (m, 5H), ppm; 13C NMR (100 MHz, CDCl3): δ 151.91, 148.51, 144.44, 141.30, 141.15, 131.92, 131.72, 129.02, 128.53, 124.53, 123.67, 122.31, 120.68, 118.59, 113.42, 112.97, 93.41, 85.15, ppm; HRMS (MALDI): m/z calcd for C20H13N3 [M + H]+ 296.1182, found 296.1187.
Acknowledgements
The authors thank the financial support from the National Natural Science Foundation of China (grant number 21466033).Notes and references
- (a) F. Tinnis, E. Stridfeldt, H. Lundberg, H. Adolfsson and B. Olofsson, Org. Lett., 2015, 17, 2688–2691 CrossRef CAS PubMed; (b) K. A. Kumar, P. Kannaboina, D. K. Dhaked, R. A. Vishwakarma, P. V. Bharatam and P. Das, Org. Biomol. Chem., 2015, 13, 1481–1491 RSC; (c) K. K. Sharma, S. Sharma, A. Kudwal and R. Jain, Org. Biomol. Chem., 2015, 13, 4637–4641 RSC; (d) A. Kumar and A. K. Bishnoi, RSC Adv., 2015, 5, 20516–20520 RSC; (e) R. Sivakami, S. G. Babu, S. Dhanuskodi and R. Karvembu, RSC Adv., 2015, 5, 8571–8578 RSC; (f) M. Nasrollahzadeh, S. M. Sajadi and M. Maham, RSC Adv., 2015, 5, 40628–40635 RSC; (g) D. Kim, K. Yoo, S. E. Kim, H. J. Cho, J. Lee, Y. Kim and M. Kim, J. Org. Chem., 2015, 80, 3670–3676 CrossRef CAS PubMed; (h) S. G. Rull, J. F. Blandez, M. R. Fructos, T. R. Belderrain and M. C. Nicasio, Adv. Synth. Catal., 2015, 357, 907–911 CrossRef CAS PubMed; (i) Y. Wang, J. Ling, Y. Zhang, A. Zhang and Q. Yao, Eur. J. Org. Chem., 2015, 4153–4161 CAS; (j) S. H. Kim, M. Kim, J. G. Verkade and Y. Kim, Eur. J. Org. Chem., 2015, 1954–1960 CrossRef CAS PubMed.
- (a) S. Roy, A. J. Zych, R. J. Herr, C. Cheng and G. W. Shipps Jr, Tetrahedron, 2010, 66, 1973–1979 CrossRef CAS PubMed; (b) C. Ahgren, K. Backro, F. W. Bell, A. S. Cantrell, M. Clemens, J. M. Colacino, J. B. Deeter, J. A. Engelhardt and M. Hogberg, Antimicrob. Agents Chemother., 1995, 39, 1329–1335 CrossRef CAS; (c) H. Zhang, L. Vrang, C. Rydergaard, C. Aahgren and B. Oeberg, Antiviral Chem. Chemother., 1996, 7, 221–229 CrossRef CAS PubMed.
- (a) G. M. Buckley, R. Fosbeary, J. L. Fraser, L. Gowers, A. P. Higueruelo, L. A. James, K. Jenkins, S. R. Mack, T. Morgan, D. M. Parry, W. R. Pitt, O. Rausch, M. D. Richard and V. Sabin, Bioorg. Med. Chem. Lett., 2008, 18, 3656–3660 CrossRef CAS PubMed; (b) G. M. Buckley, T. A. Ceska, J. L. Fraser, L. Gowers, C. R. Groom, A. P. Higueruelo, K. Jenkins, S. R. Mack, T. Morgan, D. M. Parry, W. R. Pitt, O. Rausch, M. D. Richard and V. Sabin, Bioorg. Med. Chem. Lett., 2008, 18, 3291–3295 CrossRef CAS PubMed.
- S. J. Stachel, J. M. Sanders, D. A. Henze, M. T. Rudd, H.-P. Su, Y. Li, K. K. Nanda, M. S. Egbertson, P. J. Manley, K. L. G. Jones, E. J. Brnardic, A. Green, J. A. Grobler, B. Hanney, M. Leitl, M.-T. Lai, V. Munshi, D. Murphy, K. Rickert, D. Riley, A. Krasowska-Zoladek, C. Daley, P. Zuck, S. A. Kane and M. T. Bilodeau, J. Med. Chem., 2014, 57, 5800–5816 CrossRef CAS PubMed.
- J. M. Large, S. A. Osborne, E. Smiljanic-Hurley, K. H. Ansell, H. M. Jones, D. L. Taylor, B. Clough, J. L. Green and A. A. Holder, Bioorg. Med. Chem. Lett., 2013, 23, 6019–6024 CrossRef CAS PubMed.
- (a) H.-Y. Gong, B. M. Rambo, E. Karnas, V. M. Lynch, K. M. Keller and J. L. Sessler, J. Am. Chem. Soc., 2011, 133, 1526–1533 CrossRef CAS PubMed; (b) H.-Y. Gong, B. M. Rambo, E. Karnas, V. M. Lynch and J. L. Sessler, Nat. Chem., 2010, 2, 406–409 CrossRef CAS PubMed.
- N. Onozawa-Komatsuzaki, M. Yanagida, T. Funaki, K. Kasuga, K. Sayama and H. Sugihara, Sol. Energy Mater. Sol. Cells, 2011, 95, 310–314 CrossRef CAS PubMed.
- (a) W.-M. Du, P. Wu, Q.-F. Wang and Z.-K. Yu, Organometallics, 2013, 32, 3083–3090 CrossRef CAS; (b) F.-L. Zeng and Z.-K. Yu, Organometallics, 2008, 27, 2898–2901 CrossRef CAS; (c) F. Zeng and Z.-K. Yu, Organometallics, 2008, 27, 6025–6028 CrossRef CAS; (d) F. L. Zeng and Z. K. Yu, J. Org. Chem., 2006, 71, 5274–5281 CrossRef CAS PubMed.
- (a) G. Sandford, R. Slater, D. S. Yufit, J. A. K. Howard and A. Vong, J. Fluorine Chem., 2014, 167, 91–95 CrossRef CAS PubMed; (b) S. Heider, H. Petzold, G. Chastanet, S. Schlamp, T. Rüffer, B. Weber and J.-F. Létard, Dalton Trans., 2013, 42, 8575–8584 RSC; (c) R. Pritchard, C. A. Kilner and M. A. Halcrow, Tetrahedron Lett., 2009, 50, 2484–2486 CrossRef CAS PubMed.
- X.-J. Sun, Z.-K. Yu, S.-Z. Wu and W.-J. Xiao, Organometallics, 2005, 24, 2959–2963 CrossRef CAS.
- L. Wang, N. Liu, B. Dai and H.-C. Hu, Eur. J. Org. Chem., 2014, 6493–6500 CrossRef CAS PubMed.
- J. L. Bolliger, M. Oberholzer and C. M. Frech, Adv. Synth. Catal., 2011, 353, 945–954 CrossRef CAS PubMed.
- J. S. Siddle, A. S. Batsanov and M. R. Bryce, Eur. J. Org. Chem., 2008, 2746–2750 CrossRef CAS PubMed.
- (a) H. Amii and K. Uneyama, Chem. Rev., 2009, 109, 2119–2183 CrossRef CAS PubMed; (b) F. Diness and D. P. Fairlie, Angew. Chem., Int. Ed., 2012, 51, 8012–8016 CrossRef CAS PubMed.
- (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS PubMed; (b) L.-C. Campeau and K. Fagnou, Chem. Soc. Rev., 2007, 36, 1058–1068 RSC; (c) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174–238 CrossRef CAS PubMed; (d) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173–1193 RSC.
- (a) Y. Yuan, I. Thomé, S. H. Kim, D. Chen, A. Beyer, J. Bonnamour, E. Zuidema, S. Chang and C. Bolm, Adv. Synth. Catal., 2010, 352, 2892–2898 CrossRef CAS PubMed; (b) P. Caubère, Chem. Rev., 1993, 93, 2317–2334 CrossRef; (c) T. Ishikawa, Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts, Wiley-VCH, Weinheim, 2009 Search PubMed.
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (b) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed; (c) K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305–321 CrossRef CAS; (d) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- (a) A. Baron, G. Sandford, R. Slater, D. S. Yufit, J. A. K. Howard and A. Vong, J. Org. Chem., 2005, 70, 9377–9381 CrossRef CAS PubMed; (b) G. Sandford, R. Slater, D. S. Yufit, J. A. K. Howard and A. Vong, J. Org. Chem., 2005, 70, 7208–7216 CrossRef CAS PubMed; (c) J. A. Christopher, L. Brophy, S. M. Lynn, D. D. Miller, L. A. Sloan and G. Sandford, J. Fluorine Chem., 2008, 129, 447–454 CrossRef CAS PubMed; (d) C. Fischer, S. Shah, B. L. Hughes, G. N. Nikov, J. L. Crispino, R. E. Middleton, A. A. Szewczak, B. Munoz and M. S. Shearman, Bioorg. Med. Chem. Lett., 2011, 21, 773–776 CrossRef CAS PubMed; (e) Z. Wan, A. Hall, Y. Sang, J.-N. Xiang, E. Yang, B. Smith, G. Yang, H. Yu, J. Wang, L. F. Lau, T. Li, W. Zhao, X. Su, X. Zhang, Y. Zhou, Y. Jin, Z. Tong, Z. Cheng, I. Hussain, J. D. Elliott and Y. Matsuoka, Bioorg. Med. Chem. Lett., 2011, 21, 4832–4835 CrossRef CAS PubMed; (f) X. Huang, R. Aslanian, W. Zhou, X. Zhu, J. Qin, W. Greenlee, Z. Zhu, L. Zhang, L. Hyde, I. Chu, M. Cohen-Williams and A. Palani, ACS Med. Chem. Lett., 2010, 1, 184–187 CrossRef CAS PubMed.
- Y. W. Jo, W. B. Im, J. K. Rhee, M. J. Shim, W. B. Kim and E. C. Choi, Bioorg. Med. Chem., 2004, 12, 5909–5915 CrossRef CAS PubMed.
- R. A. Altman, E. D. Koval and S. L. Buchwald, J. Org. Chem., 2007, 72, 6190–6199 CrossRef CAS PubMed.
- Y.-X. Xie, S.-F. Pi, J. Wang, D.-L. Yin and J.-H. Li, J. Org. Chem., 2006, 71, 8324–8327 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18653f |
| This journal is © The Royal Society of Chemistry 2015 |