Two-dimensional FTIR correlation spectroscopy reveals chemical changes in dissolved organic matter during the biodrying process of raw sludge and anaerobically digested sludge†
Abstract
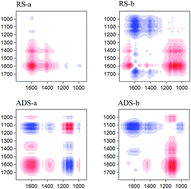
Chemical changes in dissolved organic matter (DOM) during the biodrying of raw sludge (RS) and anaerobically digested sludge (ADS) were analyzed by various techniques, e.g., two-dimensional FTIR correlation spectroscopy (2D FTIR COS). The results showed that the RS and ADS matrices achieved the desired biodrying performance after 18 days. Biodrying caused a decrease in the dissolved organic carbon content and an increase in the molecular weight, aromaticity, and fluorescent intensity of DOM in the sludge matrices. Compared with the RS matrix, the organic matter of the ADS was more biostable, resulting in a lower biodrying performance. The asynchronous map of 2D FTIR COS analysis showed the changes in the heteropolysaccharide first, followed by the protein-like groups in the matrices during the biodrying process, which was contrary to the previous results from anaerobic digestion. They supply the first evidence for the complementarities of anaerobic and aerobic processes in sludge organic compound degradation. 2D FTIR COS analysis is a feasible technique to explore the degradation characteristics of individual organic matters during the sludge treatment.

 Please wait while we load your content...
Please wait while we load your content...