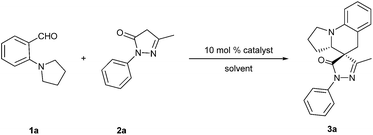
Zinc chloride catalyzed stereoselective construction of spiropyrazolone tetrahydroquinolines via tandem [1,5]-hydride shift/cyclization sequence†
Tuan Zhao,
Huanrui Zhang,
Longchen Cui,
Jingping Qu and
Baomin Wang*
State Key Laboratory of Fine Chemicals, School of Pharmaceutical Science and Technology, Dalian University of Technology, Dalian 116024, P. R. China. E-mail: bmwang@dlut.edu.cn
First published on 5th October 2015
Abstract
A zinc chloride catalyzed tandem 1,5-hydride shift/cyclization process to form spiropyrazolone terahydroquinoline derivatives is developed. A series of new spiropyrazolone derivatives were obtained in good to high yields with good to excellent diastereoselectivities (up to 95% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr). Additionally, the spiropyrazolone derivatives could be converted into the corresponding novel spriopyrazolines.
5 dr). Additionally, the spiropyrazolone derivatives could be converted into the corresponding novel spriopyrazolines.
In recent years, pyrazol-5-one derivatives have drawn great attention, because they play important roles in a broad range of biological profiles including analgesic, antibacterial and antifungal activities, anti-inflammatory effects, CCR3 antagonist activity, antitumor activity and anti-ischemic effects.1 Within this context, spirocyclic pyrazolones represent a promising subset with potential bioactivities.2 For example, a class of 4-spiro-5-pyrazolone was found to be an inhibitor of type-4 phosphodiesterase (Fig. 1).2d Therefore, the development of simple and efficient methods to synthesize pyrazolone derivatives, especially spiropyrazolones, has fascinated more and more researchers, and diverse methods have been reported in the past few years.3 On the other hand, the tetrahydroquinoline skeleton is an important constitutional unit in natural products and synthetic bioactive compounds (Fig. 1).4 Herein we wish to report the first zinc chloride catalyzed protocol for the stereoselective synthesis of spiro[tetrahydroquinoline-4,4′-pyrazolones] via a tandem 1,5-hydride transfer and sequent ring closure reaction.
The functionalization of a C(sp3)–H bond via intramolecular 1,5-hydride transfer/ring closure sequence, namely, the “internal redox process”, has received considerable attention in modern organic chemistry due to its atom- and step-economy.5 Its key feature is the 1,5-hydride shift of the C(sp3)-H bound α to the heteroatom. Subsequent 6-endo cyclization to the cation species affords the corresponding heterocycle.5i,5m,6 Synthetic chemists have paid much effort to explore intensively the internal redox process and many tetrahydroquinoline derivatives were synthesized by this strategy.4c,5i,5m,7 These studies, coupled with the significance of spiropyrazolone scaffolds, encouraged us to develop a new methodology to construct new spiropyrazolones in a simple, efficient, and stereoselective way. Specifically, we envisioned a one-pot reaction sequence involving an intermolecular aldol-type condensation of o-dialkylaminobenzaldehyde with pyrazolone to form the 4-alkylidenepyrazolone, followed by an intramolecular 1,5-hydride transfer/6-endo cyclization process.
We began our study by using 1a with 2a as model substrates for screening of the optimal reaction conditions. An initial control experiment revealed that the planned internal redox process could occur in THF without any acid catalyst, but the substrate could not convert into the desired product 3a completely even after long reaction times (47% yield, 63 h) (Table 1, entry 1). Then with THF as solvent, we screened the catalysts. A series of metal triflate were employed to catalyze the reaction in refluxing THF (Table 1, entries 2–5). Zinc triflate proved to be competent for the formation of the desired product 3a in 76% yield with 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr (Table 1, entry 4), whereas the others could furnish the product in varying yields (Table 1, entries 2, 3 and 5).
6 dr (Table 1, entry 4), whereas the others could furnish the product in varying yields (Table 1, entries 2, 3 and 5).
| Entry | Catalyst (mol%) | Solvent | Time (h) | Yieldb (%) | drc |
|---|---|---|---|---|---|
| a All reactions were carried out with 1 (0.20 mmol), 2 (0.21 mmol) in refluxing solvent (2.0 mL).b Isolated yield.c The dr was determined by 1H NMR of the crude reaction mixture. | |||||
| 1 | — | THF | 63 | 47 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 2 | Cu(OTf)2 (10) | THF | 20 | 44 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 3 | Sc(OTf)3 (10) | THF | 19 | 63 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 4 | Zn(OTf)2 (10) | THF | 44 | 76 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 5 | Yb(OTf)3 (10) | THF | 25 | 71 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 6 | SnCl2·2H2O (10) | THF | 77 | 67 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 7 | FeCl3 (10) | THF | 15 | 64 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 8 | LiCl (40) | THF | 43 | 78 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 9 | ZnCl2 (10) | THF | 20 | 87 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 10 | TsOH (20) | THF | 29 | 84 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 11 | TFA (40) | THF | 45 | 58 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 12 | CSA (10) | THF | 43 | 56 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 13 | ZnCl2 (10) | DCE | 20 | 89 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 14 | ZnCl2 (10) | MeCN | 20 | 87 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 15 | ZnCl2 (10) | EtOH | 20 | 91 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 16 | — | EtOH | 20 | 64 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
Some cheaper Lewis acids were also tested (Table 1, entries 6–9), and to our delight, the same reaction could be completed with zinc chloride after 20 h affording 3a in 87% yield with 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr (Table 1, entry 9). When some Brønsted acids were used in the reaction, the yields were not further improved (Table 1, entries 10–12). Encouraged by the initial results, a range of solvents was tested in the presence of zinc chloride (10 mol%), and the results indicated that the other solvents (EtOH, DCE, MeCN) are also suitable media for the transformation of 1a and 2a to product 3a smoothly with good results (Table 1, entries 13–15). Therefore, in light of multiple factors including the price of the catalyst, reactivity, and stereoselectivity, we decided to use zinc chloride as the best catalyst and EtOH as solvent for further investigation.
7 dr (Table 1, entry 9). When some Brønsted acids were used in the reaction, the yields were not further improved (Table 1, entries 10–12). Encouraged by the initial results, a range of solvents was tested in the presence of zinc chloride (10 mol%), and the results indicated that the other solvents (EtOH, DCE, MeCN) are also suitable media for the transformation of 1a and 2a to product 3a smoothly with good results (Table 1, entries 13–15). Therefore, in light of multiple factors including the price of the catalyst, reactivity, and stereoselectivity, we decided to use zinc chloride as the best catalyst and EtOH as solvent for further investigation.
With the optimized reaction conditions established, the substrate scope of the tandem 1,5-hydride transfer/cyclization reaction was next investigated (Table 2).8 First, we examined the scope of the pyrazolone component. The pyrazolones with various alkyl substituents at 3-position reacted with 2a efficiently to afford the desired spiropyrazolones 3b–g in good to high yields (77–95%) with good to high dr values (89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 to >95
11 to >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Changing the alkyl substituent into aryl substituent led to a slight drop in diastereoselectivities (78
5). Changing the alkyl substituent into aryl substituent led to a slight drop in diastereoselectivities (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 dr), but high yield was still maintained (Table 2, 3f). Substitution on the 1-position was also well tolerated, as 2,6-dichlorophenyl, 1-naphthyl, and non-substitution, all led to the desired products in good yields (73–84%) with high dr values (93
22 dr), but high yield was still maintained (Table 2, 3f). Substitution on the 1-position was also well tolerated, as 2,6-dichlorophenyl, 1-naphthyl, and non-substitution, all led to the desired products in good yields (73–84%) with high dr values (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7–95
7–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (Table 2, 3g–3i).
5) (Table 2, 3g–3i).
Then we used various o-dialkylaminobenzaldehydes to react with 1a to examine the substrate scope. The substrates, bearing halo-substituents at the ortho-, meta- and para-position of the aromatic ring, served to generate the target products in good to high yields (80–95% yield) with high dr values (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 to >95
12 to >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (Table 2, 3j–3m). And we found that with halo-substituents at the ortho- or para-position of the aromatic ring the substrates could convert into the target products within shorter reaction times. Presumably, the rate enhancement could be attributed to the electron-withdrawing effect of the halogen substituents, which facilitates both the condensation step and the hydride shift event. With trifluoromethyl substituent at the meta-position, the corresponding substrate could convert into product 3n smoothly in 85% yield with 90
5) (Table 2, 3j–3m). And we found that with halo-substituents at the ortho- or para-position of the aromatic ring the substrates could convert into the target products within shorter reaction times. Presumably, the rate enhancement could be attributed to the electron-withdrawing effect of the halogen substituents, which facilitates both the condensation step and the hydride shift event. With trifluoromethyl substituent at the meta-position, the corresponding substrate could convert into product 3n smoothly in 85% yield with 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr (Table 2, 3n). Changing o-dialkylaminobenzaldehyde to o-dialkylaminonicotinaldehyde, the product 3o was obtained in 84% yield with 87
10 dr (Table 2, 3n). Changing o-dialkylaminobenzaldehyde to o-dialkylaminonicotinaldehyde, the product 3o was obtained in 84% yield with 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 dr.
13 dr.
To further broaden the scope of this reaction, the related piperidine, morpholine, and piperazine derived substrates were employed in the reaction, and the desired products could be obtained in good to high yields (75–94%) with excellent diastereoselectivities ranging from 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to >95
8 to >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (Table 2, 3p–3r). However, the substrate derived from noncyclic amine could smoothly convert to the desired target product but with a poor dr value (Table 2, 3s).
5 (Table 2, 3p–3r). However, the substrate derived from noncyclic amine could smoothly convert to the desired target product but with a poor dr value (Table 2, 3s).
To demonstrate the practical utility, the reaction of 1b and 2b was performed at 5 mmol scale. The desired product was formed in 95% yield with 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr (Scheme 1). The exact structure and relative configuration of the major isomer of 3t was established by single crystal X-ray analysis.9
11 dr (Scheme 1). The exact structure and relative configuration of the major isomer of 3t was established by single crystal X-ray analysis.9
Based on the above experimental observations and literature precedents, a plausible mechanism for the zinc chloride catalyzed construction of spiropyrazolone tetrahydroquinolines is depicted in the Scheme 2. First, the o-dialkylaminobenzaldehyde reacts with pyrazolone to form the 4-alkylidenepyrazolone via condensation. Then, with the activation of the α,β-unsaturated carbonyl group by zinc chloride,5d,5i,5m,6c,10 the key hydride shift occurs followed by cyclization to give the corresponding spiropyrazolone tetrahydroquinoline with high diastereoselectivity.
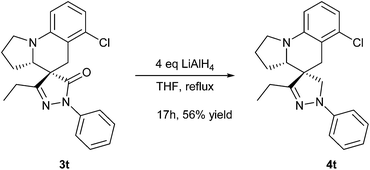
As shown in Scheme 3, we wanted to convert spiropyrazolone tetrahydroquinoline derivative 3t into the related spiropyrazoline tetrahydroquinoline derivative 4t. After some trial and error, we found that the reduction of product 3t by LiAlH4 in refluxing THF could afford the corresponding pyrazoline 4t in 56% yield with the same dr value. Pyrazolines constitute an interesting class of heterocycles due to their effective biological activities such as anticancer, antioxidant, antibacterial, antifungal, antidepressant, antiinflammatory, anticonvulsant, antitumor, and analgesic properties.11 The presence of this core in molecules plays a key role in enhancing the activity. However, to our best knowledge the methodology for the construction of the new class of spiropyrazoline in which the 5-dihydro pyrazoline core is fused with a tetrahydroquinoline moiety at the C4-position remains elusive. Our method provides a way to synthesize this class of spriopyrazoline and offers a platform to form other similar skeletons.
Conclusions
In conclusion, we have developed a direct protocol for the synthesis of the spriopyrazolone tetrahyquinoline skeleton via 1,5-hydride shift/cyclization sequence using zinc chloride as the catalyst in good to high yields with good to excellent diastereoselectivities. The reaction is broad in scope and the product could be obtained in gram scale. Additionally, we revealed that the products could be transformed to a new class of spiropyrazoline. Efforts to realize an enantioselective version of this reaction are ongoing in our laboratory.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21076035, 20972022), the Program for New Century Excellent Talents in University (NCET-11-0053), the Fundamental Research Funds for the Central Universities (DUT13ZD202).Notes and references
- (a) G. Varvounis, Adv. Heterocycl. Chem., 2009, 98, 143–224 CrossRef CAS; (b) S. Bondock, R. Rabie, H. A. Etman and A. A. Fadda, Eur. J. Med. Chem., 2008, 43, 2122–2129 CrossRef CAS PubMed; (c) D. Costa, A. P. Marques, R. L. Reis, J. L. Lima and E. Fernandes, Free Radical Biol. Med., 2006, 40, 632–640 CrossRef CAS PubMed; (d) K. Sujatha, G. Shanthi, N. P. Selvam, S. Manoharan, P. T. Perumal and M. Rajendran, Bioorg. Med. Chem. Lett., 2009, 19, 4501–4503 CrossRef CAS PubMed; (e) R. Brogden, Drugs, 1986, 32, 60–70 CrossRef; (f) C. Pegurier, P. Collart, P. Danhaive, S. Defays, M. Gillard, F. Gilson, T. Kogej, P. Pasau, N. van Houtvin, M. van Thuyne and B. van Keulen, Bioorg. Med. Chem. Lett., 2007, 17, 4228–4231 CrossRef CAS PubMed; (g) J. S. Casas, E. E. Castellano, J. Ellena, M. S. Garcia-Tasende, M. L. Perez-Paralle, A. Sanchez, A. Sanchez-Gonzalez, J. Sordo and A. Touceda, J. Inorg. Biochem., 2008, 102, 33–45 CrossRef CAS PubMed; (h) T.-W. Wu, L.-H. Zeng, J. Wu and K.-P. Fung, Life Sci., 2002, 71, 2249–2255 CrossRef CAS.
- (a) B. Han, W. Huang, W. Ren, G. He, J.-H. Wang and C. Peng, Adv. Synth. Catal., 2015, 357, 561–568 CrossRef CAS PubMed; (b) A. van Loevezijn, J. Venhorst, W. I. Iwema Bakker, C. G. de Korte, W. de Looff, S. Verhoog, J. W. van Wees, M. van Hoeve, R. P. van de Woestijne, M. A. van der Neut, A. J. Borst, M. J. van Dongen, N. M. de Bruin, H. G. Keizer and C. G. Kruse, J. Med. Chem., 2011, 54, 7030–7054 CrossRef CAS PubMed; (c) C. Yang, J. Li, R. Zhou, X. Chen, Y. Gao and Z. He, Org. Biomol. Chem., 2015, 13, 4869–4878 RSC; (d) E. Amata, N. D. Bland, R. K. Campbell and M. P. Pollastri, Tetrahedron Lett., 2015, 56, 2832–2835 CrossRef CAS PubMed.
- (a) A. N. R. Alba, A. Zea, G. Valero, T. Calbet, M. Font-Bardia, A. Mazzanti, A. Moyano and R. Rios, Eur. J. Org. Chem., 2011, 1318–1325 CrossRef CAS PubMed; (b) P. Chauhan, S. Mahajan, C. C. Loh, G. Raabe and D. Enders, Org. Lett., 2014, 16, 2954–2957 CrossRef CAS PubMed; (c) X. Han, W. Yao, T. Wang, Y. R. Tan, Z. Yan, J. Kwiatkowski and Y. Lu, Angew. Chem., Int. Ed., 2014, 53, 5643–5647 CrossRef CAS PubMed; (d) J.-H. Li and D.-M. Du, Chem.–Asian J., 2014, 9, 3278–3286 CrossRef CAS PubMed; (e) J. Liang, Q. Chen, L. Liu, X. Jiang and R. Wang, Org. Biomol. Chem., 2013, 11, 1441–1445 RSC; (f) L. Liu, Y. Zhong, P. Zhang, X. Jiang and R. Wang, J. Org. Chem., 2012, 77, 10228–10234 CrossRef CAS PubMed; (g) P. Sun, C.-Y. Meng, F. Zhou, X.-S. Li and J.-W. Xie, Tetrahedron, 2014, 70, 9330–9336 CrossRef CAS PubMed; (h) B. Wu, J. Chen, M.-Q. Li, J.-X. Zhang, X.-P. Xu, S.-J. Ji and X.-W. Wang, Eur. J. Org. Chem., 2012, 1318–1327 CrossRef CAS PubMed.
- (a) R. M. Kariba, P. J. Houghton and A. Yenesew, J. Nat. Prod., 2002, 65, 566–569 CrossRef CAS PubMed; (b) P. Yates, F. N. MacLachlan, I. D. Rae, M. Rosenberger, A. G. Szabo, C. R. Willis, M. P. Cava, M. Behforouz, M. V. Lakshmikantham and W. Zeiger, J. Am. Chem. Soc., 1973, 95, 7842–7850 CrossRef CAS; (c) G. S. Basarab, V. Galullo, N. DeGrace, S. Hauck, C. Joubran and S. S. Wesolowski, Org. Lett., 2014, 16, 6456–6459 CrossRef CAS PubMed; (d) K. Koketsu, A. Minami, K. Watanabe, H. Oguri and H. Oikawa, Curr. Opin. Chem. Biol., 2012, 16, 142–149 CrossRef CAS PubMed; (e) C.-J. Liu, D.-Y. Liu and L. Xiang, Yaoxue Xuebao, 2010, 45, 9–16 CAS.
- For selected reviews, see: (a) M. C. Haibach and D. Seidel, Angew. Chem., Int. Ed., 2014, 53, 5010–5036 CrossRef CAS PubMed; (b) L. Wang and J. Xiao, Adv. Synth. Catal., 2014, 356, 1137–1171 CrossRef CAS PubMed; (c) B. Peng and N. Maulide, Chem.–Eur. J., 2013, 19, 13274–13287 CrossRef CAS PubMedFor recent examples on internal redox process, see: (d) S. J. Pastine and D. Sames, Org. Lett., 2005, 7, 5429–5431 CrossRef CAS PubMed; (e) S. J. Pastine, K. M. McQuaid and D. Sames, J. Am. Chem. Soc., 2005, 127, 12180–12181 CrossRef CAS PubMed; (f) M. Tobisu and N. Chatani, Angew. Chem., Int. Ed., 2006, 45, 1683–1684 CrossRef CAS PubMed; (g) C. Zhang, C. K. De, R. Mal and D. Seidel, J. Am. Chem. Soc., 2008, 130, 416–417 CrossRef CAS PubMed; (h) K. M. McQuaid and D. Sames, J. Am. Chem. Soc., 2009, 131, 402–403 CrossRef CAS PubMed; (i) S. Murarka, C. Zhang, M. D. Konieczynska and D. Seidel, Org. Lett., 2009, 11, 129–132 CrossRef CAS PubMed; (j) B. Bolte and F. Gagosz, J. Am. Chem. Soc., 2011, 133, 7696–7699 CrossRef CAS PubMed; (k) K. Mori, S. Sueoka and T. Akiyama, J. Am. Chem. Soc., 2011, 133, 2424–2426 CrossRef CAS PubMed; (l) M. C. Haibach, I. Deb, C. K. De and D. Seidel, J. Am. Chem. Soc., 2011, 133, 2100–2103 CrossRef CAS PubMed; (m) Y.-Y. Han, W.-Y. Han, X. Hou, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2012, 14, 4054–4057 CrossRef CAS PubMed; (n) I. D. Jurberg, B. Peng, E. Wostefeld, M. Wasserloos and N. Maulide, Angew. Chem., Int. Ed., 2012, 51, 1950–1953 CrossRef CAS PubMed; (o) Z. W. Jiao, S.-Y. Zhang, C. He, Y.-Q. Tu, S.-H. Wang, F.-M. Zhang, Y.-Q. Zhang and H. Li, Angew. Chem., Int. Ed., 2012, 51, 8811–8815 CrossRef CAS PubMed; (p) M. Wang, ChemCatChem, 2013, 5, 1291–1293 CrossRef CAS PubMed; (q) K. Mori, K. Kurihara and T. Akiyama, Chem. Commun., 2014, 50, 3729–3731 RSC; (r) K. Mori, K. Kurihara, S. Yabe, M. Yamanaka and T. Akiyama, J. Am. Chem. Soc., 2014, 136, 3744–3747 CrossRef CAS PubMed; (s) K. Mori, N. Umehara and T. Akiyama, Adv. Synth. Catal., 2015, 357, 901–906 CrossRef CAS PubMed.
- (a) W. H. N. Nijhuis, W. Verboom, D. N. Reinhoudt and S. Harkema, J. Am. Chem. Soc., 1987, 109, 3136–3138 CrossRef CAS; (b) W. H. N. Nijhuis, W. Verboom, A. A. El-Fadl, G. J. V. Hummel and D. N. Reinhoudt, J. Org. Chem., 1989, 54, 209–216 CrossRef CAS; (c) L. Zhang, L. Chen, J. Lv, J.-P. Cheng and S. Luo, Chem.–Asian J., 2012, 7, 2569–2576 CrossRef CAS PubMed.
- (a) P. Mátyus, O. Éliás, P. Tapolcsányi, Á. Polonka-Bálint and B. Halász-Dajka, Synthesis, 2006, 16, 2625–2639 CrossRef; (b) S. Murarka, I. Deb, C. Zhang and D. Seidel, J. Am. Chem. Soc., 2009, 131, 13226–13227 CrossRef CAS PubMed; (c) K. Mori, S. Sueoka and T. Akiyama, Chem. Lett., 2011, 40, 1386–1388 CrossRef CAS; (d) K. Mori, K. Ehara, K. Kurihara and T. Akiyama, J. Am. Chem. Soc., 2011, 133, 6166–6169 CrossRef CAS PubMed; (e) W.-Y. Han, J. Zuo, Z.-J. Wu, X.-M. Zhang and W.-C. Yuan, Tetrahedron, 2013, 69, 7019–7025 CrossRef CAS PubMed; (f) P. F. Wang, C. H. Jiang, X. Wen, Q. L. Xu and H. Sun, J. Org. Chem., 2015, 80, 1155–1162 CrossRef CAS PubMed; (g) W. Cao, X. Liu, J. Guo, L. Lin and X. Feng, Chem.–Eur. J., 2015, 21, 1632–1636 CrossRef CAS PubMed.
- For detail, see the ESI†.
- ESI†.
- (a) M. Noguchi, H. Yamada and T. Sunagawa, J. Chem. Soc., Perkin Trans. 1, 1998, 3327–3330 RSC; (b) S. J. Pastine, K. M. McQuaid and D. Sames, J. Am. Chem. Soc., 2005, 127, 12180–12181 CrossRef CAS PubMed.
- (a) M. A. Rahman and A. A. Siddiqui, Int. J. Pharm. Sci. Drug Res., 2010, 2, 165–175 CAS; (b) S. Sharma, P. K. Sharma, N. Kumar and R. Dudhe, Biomed. Pharmacother., 2011, 65, 244–251 CrossRef CAS PubMed; (c) J. Wang, Z.-Y. Bu, G.-H. Li, Y.-Z. Li and T.-G. Ren, Huaxue Yanjiu, 2013, 24, 326–330 CAS; (d) Y.-Q. Yu and X.-L. Xu, Zhejiang Huagong, 2013, 44, 8–18 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1422567. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra18471a |
| This journal is © The Royal Society of Chemistry 2015 |