Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A hyaluronic acid–pentamidine bioconjugate as a macrophage mediated drug targeting delivery system for the treatment of leishmaniasis†
N.
Micale
*a,
A.
Piperno
b,
N.
Mahfoudh
c,
U.
Schurigt
d,
M.
Schultheis
d,
P. G.
Mineo
 e,
T.
Schirmeister
f,
A.
Scala
b and
G.
Grassi
b
e,
T.
Schirmeister
f,
A.
Scala
b and
G.
Grassi
b
aDepartment of Drug Sciences and Health Products, University of Messina, Viale S.S. Annunziata, 98168 Messina, Italy. E-mail: nmicale@unime.it
bDepartment of Chemical Sciences, University of Messina, Viale F. Stagno D'Alcontres, 98166 Messina, Italy
cDepartment of Medicinal and Organic Chemistry, University of Granada, Faculty of Pharmacy, 18071 Granada, Spain
dInstitute for Molecular Infection Biology, University of Würzburg, Josef-Schneider-Str. 2, Würzburg 97074, Germany
eDepartment of Chemical Sciences, University of Catania, Viale A. Donia 6, 95125 Catania, Italy
fInstitute of Pharmacy and Biochemistry, University of Mainz, staudingerweg 5, D-55128 Mainz, Germany
First published on 2nd November 2015
Abstract
Leishmaniasis is still a serious public health problem worldwide, especially in tropical areas where this infectious disease is endemic. The most severe form of the disease (i.e. visceral) can claim victims if left untreated and the few accessible drugs have several drawbacks including major side effects and parenteral administration. In this context, the investigation of new delivery modalities which might reduce the toxicity and increase the bioavailability of the drugs currently on the market represents a valid strategy to counter these problems. Herein we present the development of a macrophage mediated drug targeting delivery system by conjugating the anti-leishmanial drug pentamidine (Pent) with the biocompatible polymer hyaluronic acid (HA), the latter employed at the same time as a delivery platform and targeting scaffold. Biological assays against Leishmania major amastigote-infected macrophages and primary bone marrow derived macrophages (BMDM) confirmed the validity of our strategy as the resulting bioconjugate HA–Pent increased both the potency and the selectivity index of the drug.
1 Introduction
Leishmaniasis is a vector-borne tropical disease caused by different species of obligate intra-macrophage protozoa of the genus Leishmania transmitted to mammalian hosts during a blood meal of infected female phlebotomine sandflies. This neglected disease is endemic in 88 countries and affects approximately 12 million people worldwide, with 350 million people considered at risk.1The clinical spectrum of Leishmaniasis ranges from a mild and usually self-resolving cutaneous form to a disfiguring mucocutaneous disease and even to a visceral form, which affects several internal organs and is lethal in the absence of chemotherapeutic treatment.2
At present, the development of an effective vaccine is far from being successful and drugs are the only available tool for treatment and control of all Leishmaniasis forms.3 Pentavalent antimony compounds (i.e. sodium stibogluconate and meglumine antimoniate) represent the first-line of intervention. Second-line drugs, such as the polyene amphotericin B (AmB), the aromatic diamidine pentamidine (Pent) and the aminoglycoside paromomycin are important in combination therapy or in cases of antimony treatment failures.4 However, all these drugs have several drawbacks including irreversible toxic effects, high costs, length of treatment, need of adequate medical care, emergence of drug resistance and parenteral administration. As an example, the main factors limiting the widespread use of AmB are the high cost of the drug and the long hospitalization period which makes it difficult to manage the disease within endemic areas strongly linked with poverty and lack of health services. Therefore, several efforts have been made over the past few years to improve the therapeutic index and to reduce the toxicity of this drug by developing lipid-based formulations and other nano- or microstructured delivery systems.5 In this regard, the World Health Organization has recommended the use of liposomal AmB against Leishmaniasis based on its high levels of efficacy and safety.6
In the present work, Pent has been selected as a model drug in virtue of its biological and chemical features. In particular, it possesses a wide range of therapeutic properties including antimicrobial,7 anti-inflammatory8 and anti-cancer activities.9 Pent has been used for more than 50 years in the therapy and prophylaxis of African trypanosomiasis and Pneumocystis carinii pneumonia in AIDS patients, as well as antimony resistant Leishmaniasis,10 but its clinical use is limited by its toxicity, administration by injection and development of resistance.
On the basis of these considerations we focused our interest on the synthesis of a new Pent-bioconjugate using hyaluronic acid (HA) as delivery platform (Fig. 1), with the aim of increasing the activity of the drug and reducing its toxicity. To the best of our knowledge, the preparation of bioconjugates of Pent is to date an unexplored strategy to overcome these issues.
Chemically, Pent is a bifunctional and low-molecular weight compound that can be employed to obtain both mono-linked HA–Pent derivatives and HA–Pent cross-linked frameworks via amide bond formation. The two terminal amidine groups are the sole reactive moieties of the drug, allowing mild synthetic conditions without significant by-products. In addition, one of the two amidine groups could be further exploited for the conjugation with other compounds that might potentiate the activity of the drug and consequently reduce its dosage.
HA is a well-known biocompatible, biodegradable, bioresorbable, non-toxic and non-immunogenic polymer,11 whose chemical versatility can be fruitfully exploited to build polymeric scaffolds able to entrap drugs with different chemo-physical characteristics by means of enzyme-cleavable linkages or cross-linked networks. Additionally, HA emerges in many respects as the ideal substrate for the development of drug targeting delivery systems with sustained release properties for the treatment of macrophage-associated diseases such as Leishmaniasis.12 In fact, macrophages play central roles in mediating a wide range of infectious and inflammatory diseases.13 In particular, as Leishmania parasites are obligatory intracellular pathogens, macrophages are their primary resident cell: they phagocytose and permit parasite proliferation and they are also the major effector cells to eliminate infection.14 Interestingly, HA is an attractive targeting ligand specifically recognized and internalized by macrophages that are known to express HA receptors for endocytosis (HARE/Stab2).15 Targeted drug delivery to the macrophages appears to be a useful proposition to improve therapeutic efficacy of an enclosed drug,16 helping in localized delivery of the drug at the infected site.
On these bases, our unprecedented HA–Pent bioconjugate (Fig. 1) is proposed as drug targeting delivery system for the treatment of Leishmaniasis, exploiting the specific biological recognition of HA by the macrophage. The biological activity of the bioconjugate has been assessed by in vitro assays on Leishmania major amastigote-infected macrophages and primary macrophages.
2 Experimental
2.1 Materials and general methods
HA sodium salt from Streptococcus equi (high molecular weight), Dowex H+ 50W × 8–100 sulfonic resin, pentamidine isethionate salt, 4-methylmorpholine, (NMM), 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) and organic solvents were purchased from Sigma-Aldrich/Fluka. Dialysis membranes (20 kDa Mw cutoff) were purchased from Spectrum Labs. Materials and methods for the biological assays have been described before by the authors.17 AlamarBlue assays for investigation of antileishmanial activities against intracellular Leishmania major amastigotes and cytotoxicity against primary bone marrow derived macrophages (BMDM) were conducted as previously reported.172.2 Synthesis
Entry 1 (Table 1). HA-COOH (76 mg, 0.2 mmol carboxylic acid) was dissolved in 3 mL of deionized water in a 50 mL round-bottomed flask followed by the dropwise addition of 2 mL of acetonitrile while stirring 33 μL (0.3 mmol) of NMM was added to the solution, causing the viscosity to increase temporarily. The solution (pH = 7) was then cooled to 4 °C, and 18 mg (0.1 mmol) of CDMT was added and stirred for 1 h at room temperature. The solution was mixed with 34 mg (0.1 mmol) of Pent (which corresponds to a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio between HA carboxyl groups and Pent amidine groups) and stirred for 68 h at room temperature. The acetonitrile was removed under reduced pressure and the resulting mixture was filtered off to eliminate the unreacted Pent. Extensive cycles of dialysis (20 kDa Mw cutoff) against a mixture of ethanol and ultrapure water (3
1 ratio between HA carboxyl groups and Pent amidine groups) and stirred for 68 h at room temperature. The acetonitrile was removed under reduced pressure and the resulting mixture was filtered off to eliminate the unreacted Pent. Extensive cycles of dialysis (20 kDa Mw cutoff) against a mixture of ethanol and ultrapure water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
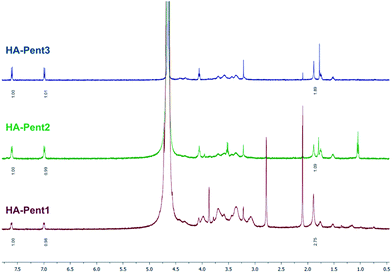
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and finally against ultrapure water were performed to purify the desired product which was recovered by freeze-drying and lyophilization as a cottony white solid and characterized by 1H NMR spectroscopy. The degree of substitution (DS100; defined as the average number of Pent groups per 100 disaccharide repeating unit) was calculated by the ratio between the integral of one aromatic proton of Pent unit (doublets at 6.99 and 7.60 ppm) and the integral of one proton of HA acetyl group (1.90 ppm) and turned out to be 12.2.
1) and finally against ultrapure water were performed to purify the desired product which was recovered by freeze-drying and lyophilization as a cottony white solid and characterized by 1H NMR spectroscopy. The degree of substitution (DS100; defined as the average number of Pent groups per 100 disaccharide repeating unit) was calculated by the ratio between the integral of one aromatic proton of Pent unit (doublets at 6.99 and 7.60 ppm) and the integral of one proton of HA acetyl group (1.90 ppm) and turned out to be 12.2.
Entry 2 (Table 1). The time of ion exchange with resin was reduced to 1 h. The ratio of HA-COOH/Pent was increased to 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 using 0.15 mmol (51 mg) of Pent and the reaction time was maintained to 68 h. DS100 = 54.0.
1.5 using 0.15 mmol (51 mg) of Pent and the reaction time was maintained to 68 h. DS100 = 54.0.
Entry 3 (Table 1). The time of ion exchange with resin was reduced to 1 h. The ratio of HA-COOH/Pent was maintained to 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 using 0.15 mmol (51 mg) of Pent and the reaction time was reduced to 20 h. DS100 = 39.9.
1.5 using 0.15 mmol (51 mg) of Pent and the reaction time was reduced to 20 h. DS100 = 39.9.
2.3 Characterization
Nuclear magnetic resonance (NMR) measurements were performed using a Varian 500 MHz spectrometer. HA-COOH and synthesized derivatives (HA–Pent1, HA–Pent2, HA–Pent3) were dissolved in D2O to concentrations of approximately 10 mg mL−1 and the 1H NMR spectra were recorded with 128–256 scans. The reference signal from HDO was set to δ 4.22 ppm. The 13C NMR spectrum was performed dissolving the HA–Pent3 in D2O/DMSO-d6 (95/5) and the reference signal of DMSO-d6 was set to δ 39.5 ppm. The Fourier Transform Infrared (FT-IR, Perkin Elmer Spectrum 100) spectra were collected, in Attenuated Total Reflectance (ATR) configuration, from 4000 to 450 cm−1.HA–Pent3 IR: (cm−1) 3200–3500, 1712, 1643, 1611, 1492, 1403, 1270, 1033. 1H NMR, selected signals, (D2O; ppm): δ 1.14–1.21 (m, 2H), 1.51–156 (m, 2H), 1.72–180 (m, 2H), 1.90 (s, 3H, N–C(O)CH3), 4.06 (t, 4H, J = 6.4 Hz), 4.30–4.36 (m, 1H, H-1 proton of HA), 4.41–4.44 (m, 1H, H-1 proton of HA), 6.99 (d, 4H, J = 8.8 Hz), 7.60 (d, 4H, J = 8.8 Hz), 8.32 (bs, 1H, N–H). 13C NMR D2O/DMSO-d6 (95/5 ppm): δ 174.8, 173.9, 165.6, 163.1, 129.8, 119.4, 115.2, 103.1, 100.3, 82.7, 79.9, 76.3, 75.3, 73.5, 72.3, 68.3, 60.4, 50.1, 27.6, 22.4, 21.5 (ESI Fig. S2†).
Ultraviolet-visible spectrophotometry (UV-Vis) was used to quantify the amount of conjugated Pent, by dissolving a weighted amount of lyophilized bioconjugate (1 mg) in 2.5 mL of water. On the basis of optical absorbance data and molar extinction coefficient (ε = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1),18 determined by calibration curve at a wavelength of 261 nm, a drug content of 16% was estimated using the following equation: drug content = (drug weight in the conjugate/weight of the conjugate) × 100.
900 M−1 cm−1),18 determined by calibration curve at a wavelength of 261 nm, a drug content of 16% was estimated using the following equation: drug content = (drug weight in the conjugate/weight of the conjugate) × 100.
No significant absorption in the UV-Vis region has been observed for HA-COOH.
Dual detector SEC analysis was performed using a GPC System equipped with a Water 1525 binary HPLC pump and a Water 2410 refractive index detector (RI) (Waters corporation) coupled in series to a miniDAWN Treos (Wyatt Technology) light scattering detector equipped with a WyattQELS DLS Module. This procedure, with in-line refractive index and light scattering detectors, allows the determination of absolute Mw values.19 The analyses were performed using three Progel-TSK columns (Tosoh Bioscience LLC) connected in series (G3000 PWXL, G5000 PWXL and G6000 PWXL, with a separation range up to 50 MDa). The mobile phase was a saline water solution, 0.1 mol L−1 of NaNO3 with 0.02% of NaN3 (higher ionic strength shows similar results), at a flow rate of 1.0 mL min−1. To calculate the average molecular masses, a refractive index increment (dn/dc) of 0.165 mg mL−1 was used. The samples of HA and of its derivatives were prepared with a final concentration of 2 mg mL−1 in the eluant solvent. Acquired data were analyzed by means of ASTRA 6.0.1 software (Wyatt Technology).
2.4 Amastigote drug screening assay and determination of cytotoxicity against macrophages
HA–Pent3 as well HA-COOH and pentamidine isethionate were tested against intracellular amastigote of Leishmania major as recently described using the amastigote drug screening assay.17 In brief a 1801 kbp fragment of the firefly luciferase (LUC)-coding region was cut from pGL4.13 (Promega, Mannheim, Germany) by NcoI/XbaI and subsequently cloned into Leishmania expression vector pLEXSY-hyg2 (Jena Bioscience, Jena, Germany) with a marker gene for selection with hygromycin B (HYG) and cut with NcoI/NheI. After linearization with SwaI, the coding sequences for LUC and HYG were integrated into the 18S rRNA locus of nuclear DNA of L. major. The virulence of LUC-transgenic L. major was maintained by passage in BALB/c mice. Promastigotes were grown in blood agar cultures at 27 °C, 5% CO2, 95% humidity and 50 μg mL−1 hygromycin B. Bone marrow derived macrophages (BMDM) generated as previously described using L929 supernatants17 were seeded (2 × 105 cells per mL, final culture volume: 200 μL per well) into a white 96-well plate (Greiner Bio-One, Frickenhausen, Germany), and incubated for 4 h at 37 °C to promote adhesion. LUC-transgenic promastigotes of L. major were harvested and re-suspended in RPMI-1640 medium, and finally added to each well. The infection rate of macrophages with LUC-transgenic L. major promastigote was adjusted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (3 × 106 promastigotes per mL). These cocultures were incubated for 24 h at 37 °C, 5% CO2, and 95% humidity to ensure infection and differentiation to amastigotes. After washing twice with RPMI-1640 medium, infected macrophages were incubated in the absence or presence of increasing concentrations of test samples (0.8 μM to 100 μM) for further 24 h at 37 °C in duplicate wells (final culture volume: 200 μL per well). Then, 25 μL Britelite™ (PerkinElmer, Waltham, MA, USA) a lysis buffer containing luciferin was added in each well and luminescence was measured with a Victor™ X Light 2030 luminometer (PerkinElmer, Fremont, USA). The intensity of light emission after cell lysis is proportional to the number of intracellular amastigotes in macrophages. The luminescence was reduced after treatment with a leishmanicidal compound compared to the controls without compound (growth control). HA–Pent3 was dissolved in water before testing. HA-COOH and pentamidine isethionate were used as reference compounds and were also dissolved in water. The half-maximal inhibitory concentrations (IC50 values) were calculated by linear interpolation (decreasing concentrations of the samples: 100 μM, 20 μM, 4 μM, 0.8 μM) as previously reported20 and are presented as mean values of two independent experiments against the parasite and primary macrophages.
15 (3 × 106 promastigotes per mL). These cocultures were incubated for 24 h at 37 °C, 5% CO2, and 95% humidity to ensure infection and differentiation to amastigotes. After washing twice with RPMI-1640 medium, infected macrophages were incubated in the absence or presence of increasing concentrations of test samples (0.8 μM to 100 μM) for further 24 h at 37 °C in duplicate wells (final culture volume: 200 μL per well). Then, 25 μL Britelite™ (PerkinElmer, Waltham, MA, USA) a lysis buffer containing luciferin was added in each well and luminescence was measured with a Victor™ X Light 2030 luminometer (PerkinElmer, Fremont, USA). The intensity of light emission after cell lysis is proportional to the number of intracellular amastigotes in macrophages. The luminescence was reduced after treatment with a leishmanicidal compound compared to the controls without compound (growth control). HA–Pent3 was dissolved in water before testing. HA-COOH and pentamidine isethionate were used as reference compounds and were also dissolved in water. The half-maximal inhibitory concentrations (IC50 values) were calculated by linear interpolation (decreasing concentrations of the samples: 100 μM, 20 μM, 4 μM, 0.8 μM) as previously reported20 and are presented as mean values of two independent experiments against the parasite and primary macrophages.
The activity of HA–Pent3 was compared with the free drug activity, by normalizing for the drug content. Cytotoxicities of HA–Pent3, Pent and HA-COOH were tested against uninfected primary BMDM as described above.17
3 Results and discussion
3.1 Chemistry
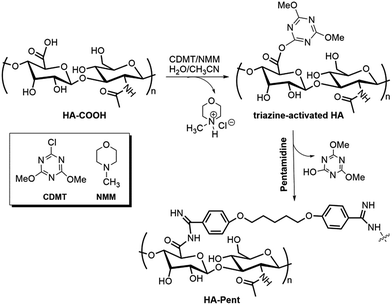
The conjugation of Pent with HA has been achieved by the “triazine-activated amidation” method, a highly versatile synthetic strategy that allows the regiospecific introduction of a wide range of functionalities without any unwanted side reactions,21 under relatively mild conditions (room temperature; neutral pH in aqueous media).22 The strategy entails the use of CDMT as an alternative reagent for coupling amines to HA carboxyl groups. Briefly, HA sodium salt was first converted into HA protonated form by using the appropriate ion-exchange resin. Then, NMM and CDMT were added to a solution of HA to activate the free carboxylic groups. The activation proceeds according to the proposed synthetic route depicted in Scheme 1 and involves the formation of an intermediate triazine-activated ester.23 Finally, pentamidine [as free base (Pent)] was added to the mixture at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratios (Entry 1 and Entry 2 and 3, respectively) with respect to HA carboxyl groups to effect the amide bond formation. Extensive dialysis of the solution and lyophilization afforded the desired amide-modified hyaluronan. Each amidation reaction was performed in a mixture of water and acetonitrile (3
1.5 molar ratios (Entry 1 and Entry 2 and 3, respectively) with respect to HA carboxyl groups to effect the amide bond formation. Extensive dialysis of the solution and lyophilization afforded the desired amide-modified hyaluronan. Each amidation reaction was performed in a mixture of water and acetonitrile (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) which enables the solubilisation of both HA and other organic reagents. The data reported in Table 1 indicate that the molar ratio HA carboxyl groups/Pent (i.e. Entry 1 vs. Entry 2) and the reaction time (i.e. Entry 3 vs. Entry 2) are key factors to increase the DS100. The most notable DS100 was achieved by using 1.5 equivalents of Pent with a reaction time of 68 h; under these conditions, we obtained HA–Pent2 bioconjugate with 54.0 of DS100 (Entry 2, Table 1). Although HA–Pent2 shows the highest degree of drug loading, its low solubility in water (mainly in solution with high ionic strength) prevented the use of it for our biological application. We supposed that the coupling reaction between HA-COOH and our bifunctional drug proceeded towards the formation of a multi-branched (and therefore poorly water-soluble) complex. Thus, to limit the formation of multi-branched structures the reaction time was reduced to 20 h maintaining the 1
2) which enables the solubilisation of both HA and other organic reagents. The data reported in Table 1 indicate that the molar ratio HA carboxyl groups/Pent (i.e. Entry 1 vs. Entry 2) and the reaction time (i.e. Entry 3 vs. Entry 2) are key factors to increase the DS100. The most notable DS100 was achieved by using 1.5 equivalents of Pent with a reaction time of 68 h; under these conditions, we obtained HA–Pent2 bioconjugate with 54.0 of DS100 (Entry 2, Table 1). Although HA–Pent2 shows the highest degree of drug loading, its low solubility in water (mainly in solution with high ionic strength) prevented the use of it for our biological application. We supposed that the coupling reaction between HA-COOH and our bifunctional drug proceeded towards the formation of a multi-branched (and therefore poorly water-soluble) complex. Thus, to limit the formation of multi-branched structures the reaction time was reduced to 20 h maintaining the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 HA carboxyl groups/Pent molar ratio (Table 1, Entry 3). Under these experimental conditions we obtained a bioconjugate (i.e. HA–Pent3) with a good drug loading (although lower than HA–Pent2) and a proper solubility in water. On these bases, HA–Pent3 was advanced to biological screening.
1.5 HA carboxyl groups/Pent molar ratio (Table 1, Entry 3). Under these experimental conditions we obtained a bioconjugate (i.e. HA–Pent3) with a good drug loading (although lower than HA–Pent2) and a proper solubility in water. On these bases, HA–Pent3 was advanced to biological screening.
| Sample | Reaction conditions | DS100b | M wt c (g mol−1) | Drug loadingd |
|---|---|---|---|---|
| a HA carboxylic group/Pent molar ratio. b Number of Pent groups per 100 disaccharide repeating unit. c Theoretical molecular weight at the corresponding DS100. d Loading of Pent in the bioconjugates at the corresponding DS100 (% w/w) calculated considering the formation of a mono-linked derivative. | ||||
| HA–Pent1 | Molar ratioa 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 68 h, (Entry 1) 1, 68 h, (Entry 1) |
12.2 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
9.9 |
| HA–Pent2 | Molar ratioa 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 68 h, (Entry 2) 1.5, 68 h, (Entry 2) |
54.0 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
33.2 |
| HA–Pent3 | Molar ratioa 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 20 h, (Entry 3) 1.5, 20 h, (Entry 3) |
39.9 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
26.7 |
The structure of HA–Pent3 was supported by spectral and analytical data; in particular the FT-IR spectrum shows the broad, strong band of O–H stretching at 3200–3500 cm−1, several bands associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH stretching (1712, 1643 and 1611 cm−1) and the strong band of C–O stretching at 1033 cm−1 (ESI, Fig. S1†).
NH stretching (1712, 1643 and 1611 cm−1) and the strong band of C–O stretching at 1033 cm−1 (ESI, Fig. S1†).
The detection of the doublets of Pent aromatic protons at 6.99 ppm and 7.60 ppm in the 1H NMR spectrum (Fig. 2) indicated the successful achievement of the coupling reaction and their integral values are congruent with the coupling efficiency. Therefore, the DS100 was determined by comparing integrated signals of aromatic protons of Pent with the corresponding methyl signal of the N-acetylglucosamine unit of HA (singlet at 1.90 ppm).
3.2 Determination of Mw by SEC/MALLS
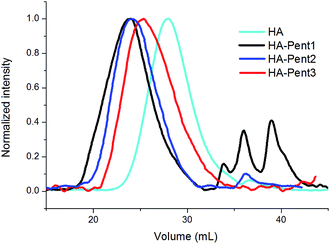
HA is susceptible to degradation through different mechanisms due to the labile nature of its glycoside bonds.24 In our case these fragmentations may occur principally during the ion-exchange with the resin and secondly during the various synthetic steps. Therefore, the average Mw of HA-COOH and its derivatives were measured by SEC-MALLS. The refractive index trace of starting HA-COOH, obtained from HA at high Mw commercially available, shows a distribution with an average Mw of about 30.0 kDa and a dispersity of 1.03 (Table 2). Instead, HA–Pent1, HA–Pent2 and HA–Pent3 peak elution volumes reveal a volume decrease (see Fig. 3) indicating an increase of both hydrodynamic volume and molecular mass. HA–Pent1, obtained by the standard coupling procedure (2 h of treatment with ionic-exchange resin and one equivalent of Pent), showed an increased polydispersity (from 1.03 to 1.48) together with an increased average Mw with respect to HA-COOH. Moreover, its GPC profile showed the presence of different oligomers with low Mw (Fig. 3). The optimization of the reaction conditions (1 h of treatment of HA with ionic-exchange resin and an increased amount of basic reagent Pent as for HA–Pent2 and HA–Pent3) avoided the oligomers formation (Fig. 3). A lower dispersity (i.e. 1.27; HA–Pent3) was obtained by reducing the reaction time from 68 h to 20 h. The molecular mass values obtained from SEC/MALLS are reported in Table 2 for all the samples. These data are in accordance with the qualitative evaluation deduced from the refractive index traces. However, for all HA–Pent derivatives the increase of the molar mass is much higher than expected. Probably, this is due to the bifunctional amidine moiety of Pent that, in addition to the intramolecular reactions (forming internal loop), can react also with others HA-COOH's chains (intermolecular reactions) leading to of multi-branched structures. Because of this, in the case of HA–Pent2, sample with the highest drug loading, the macromolecular species turned out to be only partially water-soluble and exhibited a lower Mw value with respect to HA–Pent1 derivative (case with only about 10% of Pent loading, Table 1). This outcome suggests a lower ability of HA–Pent2 to form network structures with respect to HA–Pent1. However, this deduction is misleading because the increase of Pent leads also to an increase of the bioconjugate branching degree with the consequent formation of an insoluble multi-branched and/or cross-linked polymer fraction. Conversely, the different Mw values observed for HA–Pent2 and HA–Pent3 (both having the same HA/Pent molar ratio) are due to the different reaction times, higher for HA–Pent2 (68 h) than HA–Pent3 (20 h).| Sample | M wt a (g mol−1) | M n b | M w c | Đ d |
|---|---|---|---|---|
| a Theoretical molecular weight at the corresponding DS100. b Number average molecular weight. c Weight average molecular weight. d Dispersity. e Analysis performed on the soluble fraction. | ||||
| HA-COOH | — | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560 |
1.03 |
| HA–Pent1 (Entry 1) | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
283![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.48 |
| HA–Pent2e (Entry 2) | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.32 |
| HA–Pent3 (Entry 3) | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.27 |
The differences between theoretical Mwt and Mn values of bioconjugates suggest that the coupling reaction proceeded towards the formation of linear/branched and/or partly cross-linked bioconjugates in the case of HA–Pent3 (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 vs. 73
800 vs. 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and towards the formation of multi-branched or cross-linked derivatives in the cases of HA–Pent1 and HA–Pent2. Thus, the cross-linking in the coupling reaction depends mainly on DS100 and reaction time parameters.
100) and towards the formation of multi-branched or cross-linked derivatives in the cases of HA–Pent1 and HA–Pent2. Thus, the cross-linking in the coupling reaction depends mainly on DS100 and reaction time parameters.
3.3 Biological evaluation
To validate our drug targeting strategy, HA–Pent3 was employed for the biological tests. In the assay against the intracellular amastigote form of the parasite, HA–Pent3 turned out to be more potent than the reference compound (HA–Pent3, IC50 = 2.7 μM vs. pentamidine isethionate, IC50 = 4.5 μM; Table 3), indicating an efficient internalization of the bioconjugate in the infected macrophages. At the same time HA–Pent3 showed less cytotoxicity than the standard in the assay against primary macrophages (HA–Pent3, IC50 = 61.8 μM vs. pentamidine isethionate, IC50 = 45.2 μM; Table 3). HA did not show any antileishmanial or cytotoxic effect on primary macrophages. Overall, by conjugating Pent with HA, the selectivity index (SI) of the drug was raised over two-fold (Table 3).4 Conclusions
In this work, we reported a straightforward synthetic method to obtain a drug targeting delivery system by using Pent and HA as drug and targeting delivery platform, respectively. The resulting HA–Pent bioconjugate turned out to be effective in the biological assay against L. major amastigote-infected macrophages by increasing nearly two-fold the potency of the drug with no significant cytotoxicity effects towards primary macrophages, showing a potential for the treatment of Leishmaniasis. These results suggest that the drug can be successfully internalized in the macrophages by means of HA receptors and released within the intracellular environment proposing HA as an appealing tool for the development of drug targeting delivery systems for the treatment of macrophage-associate diseases. On the other hand, since Pent is biologically and chemically versatile, our HA–Pent bioconjugate may be exploited for the development of complex/combined systems, i.e. by conjugation with other drugs that may produce synergistic therapeutic effects and/or further reduce the toxicity.Acknowledgements
This work was supported by MIUR [project PRIN 20109Z2XRJ_010]. TS, MS and US gratefully acknowledge financial support by the DFG (Deutsche Forschungsgemeinschaft, SFB 630).Notes and references
- J. Alvar, I. D. Vélez, C. Bern, M. Herrero, P. Desjeux, J. Cano, J. Jannin and M. den Boer, PLoS One, 2012, 7, e35671 CAS.
- C. Posch, J. Walochnik, A. Gschnait, H. Feichtinger and K. Rappersberger, Hautarzt, 2012, 63, 947 CrossRef CAS PubMed.
- J. N. Sangshetti, F. A. Kalam Khan, A. A. Kulkarni, R. Arote and R. H. Patil, RSC Adv., 2015, 5, 32376 RSC.
- K. Seifert, Open Med. Chem. J., 2011, 5, 31 CrossRef CAS PubMed.
- (a) Z. L. Yang, X. R. Li, K. W. Yang and Y. Liu, J. Biomed. Mater. Res., Part A, 2008, 85, 539 CrossRef PubMed; (b) K. Shao, R. Huang, J. Li, L. Han, L. Ye, J. Lou and C. Jiang, J. Controlled Release, 2010, 147, 118 CrossRef CAS PubMed; (c) R. F. Carvalho, I. F. Ribeiro, A. L. Miranda-Vilela, J. Souza-Filho, O. P. Martins, C. S. D. Oliveira, A. C. Tedesco, Z. G. Lacava, Z. N. Báo and R. N. Simpaio, Exp. Parasitol., 2013, 135, 217 CrossRef PubMed; (d) A. E. Silva, G. Barratt, M. Cheŕon and E. S. T. Egito, Int. J. Pharm., 2013, 454, 641 CrossRef CAS PubMed; (e) R. Kumar, G. C. Sahoo, K. Pandey, V. N. R. Das and P. Das, Drug Delivery, 2015, 22, 383 CrossRef CAS PubMed; (f) P. K. Gupta, A. K. Jaiswal, V. Kumar, A. Verma, P. Dwivedi, A. Dube and P. R. Mishra, Mol. Pharm., 2014, 11, 951 CrossRef CAS PubMed; (g) T. G. Ribeiro, J. R. Franca, L. L. Fuscaldi, M. L. Santos, M. C. Duarte, P. S. Lage, V. T. Martins, L. E. Costa, S. O. A. Fernandes, V. N. Cardoso, R. O. Castilho, M. Soto, C. A. P. Tavares, A. A. G. Faraco, E. A. F. Coelho and M. A. Chávez-Fumagalli, Int. J. Nanomed., 2014, 9, 5341 Search PubMed.
- World Health Organization, W. H. O. Tech. Rep. Ser., 2010, 949, 22 Search PubMed.
- (a) M. F. Minnick, L. D. Hicks, J. M. Battisti and R. Raghavan, Int. J. Antimicrob. Agents, 2010, 36, 380 CrossRef CAS PubMed; (b) P. Harris, C. Engler and R. Norton, Int. J. Antimicrob. Agents, 2011, 38, 547 CrossRef PubMed; (c) X. Wang, Z. Fiorini, C. Smith, Y. Zhang, J. Li, L. R. Watkins and H. Yin, PLoS One, 2012, 7, e47703 CAS.
- G. Esposito, E. Capoccia, G. Sarnelli, C. Scuderi, C. Cirillo, R. Cuomo and L. Steardo, J. Neuroinflammation, 2012, 9, 277 CrossRef CAS PubMed.
- (a) M. K. Pathak, D. Dhawan, D. J. Lindner, E. C. Borden, C. Farver and T. Yi, Mol. Cancer Ther., 2002, 1, 1255 CAS; (b) J. Smith, B. J. Stewart, S. Glaysher, K. Peregrin, L. A. Knight, D. J. Weber and I. A. Cree, Anticancer Drugs, 2010, 21, 181 CrossRef CAS PubMed.
- A. Mukherjee, P. K. Padmanabham, M. H. Sahani, M. P. Barrett and R. Madhubala, Mol. Biochem. Parasitol., 2006, 145, 1 CrossRef CAS PubMed.
- (a) B. Chen, R. J. Miller and P. K. Dhal, J. Biomed. Nanotechnol., 2014, 10, 4 CrossRef CAS PubMed; (b) A. Mero and M. Campisi, Polymers, 2014, 6, 346 CrossRef.
- A.-F. Tӑbӑran and C. Catoi, Biotechnol., Mol. Biol. Nanomed., 2014, 2, 17 Search PubMed.
- M. Kamat, K. Boubbou, D. C. Zhu, T. Lansdell, X. Lu, W. Li and X. Huang, Bioconjugate Chem., 2010, 21, 2128 CrossRef CAS PubMed.
- D. Liu and J. E. Uzonna, Front. Cell. Infect. Microbiol., 2012, 2, 83 Search PubMed.
- (a) T. Fernandes Stefanello, A. Szarpak-Jankowska, F. Appaix, B. Louage, L. Hamard, B. G. de Geest, B. van der Sanden, C. V. Nakamura and R. Auzély-Velty, Acta Biomater., 2014, 10, 4750 CrossRef CAS PubMed; (b) S. Y. Park, M. Y. Jung, H. J. Kim, S. J. Lee, S. Y. Kim, B. H. Lee, T. H. Kwon, R. W. Park and I. S. Kim, Cell Death Differ., 2008, 15, 192 CrossRef CAS PubMed.
- N. K. Jain, V. Mishra and N. K. Mehra, Expert Opin. Drug Delivery, 2013, 10, 353 CrossRef CAS PubMed.
- G. Bringmann, K. Thomale, S. Bischof, C. Schneider, M. Schultheis, T. Schwarz, H. Moll and U. Schurigt, Antimicrob. Agents Chemother., 2013, 57, 3003 CrossRef CAS PubMed.
- N. N. Degtyareva, B. D. Wallace, A. R. Bryant, K. M. Loo and J. T. Petty, Biophys. J., 2007, 92, 959 CrossRef CAS PubMed.
- M. H. Ong, K. Jumel, P. F. Tokarczuk, J. M. V. Blanshard and S. E. Harding, Carbohydr. Res., 1994, 260, 99 CrossRef CAS.
- W. Huber and J. C. Koella, Acta Trop., 1993, 55, 257 CrossRef CAS PubMed.
- Z. J. Kamiński, Tetrahedron Lett., 1985, 26, 2901 CrossRef.
- K. Bergman, C. Elvingson, J. Hilborn, G. Svensk and T. Bowden, Biomacromolecules, 2007, 8, 2190 CrossRef CAS PubMed.
- H. L. Rayle and L. Fellmeth, Org. Process Res. Dev., 1999, 3, 172 CrossRef CAS.
- G. Huerta-Angeles, D. Šmejkalová, D. Chladkova, T. Ehlova, R. Buffa and V. Velebny, Carbohydr. Polym., 2011, 84, 1293 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18019h |
| This journal is © The Royal Society of Chemistry 2015 |