Layer-by-layer deposition of CNT− and CNT+ hybrid films for platinum free counters electrodes of dye-sensitized-solar-cells
Abstract
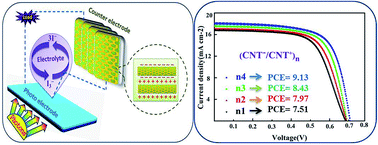
A nanocomposite film of polyaniline blend poly(sodium 4-styrenesulfonate)/multiwall CNT (PANI-PSS/MWCNT) and poly(diallydimethyl ammonium chloride)/multiwall CNT (PDADMAC/MWCNT) has been prepared as a highly-efficient catalytic material for the counter electrode (CE) of a dye-sensitized solar cell (DSSC). The as-prepared CE is constructed by a nanoscale method of layer-by-layer (LbL) assembly based on the electrostatic interactions between negatively (PANI-PSS/MWCNT) and positively (PDADMAC/MWCNT) charged nanoparticles. The (CNT−/CNT+)4 multilayer CE displayed remarkably enhanced electrocatalytic activities toward the reduction reaction and low charge-transfer resistance (Rct) of 0.3 Ω at the CE/electrolyte interface. The DSSC with (CNT−/CNT+)4 multilayer-CE showed the highest fill factor (0.71) and cell efficiency (9.13%) compared to our other electrodes.

 Please wait while we load your content...
Please wait while we load your content...