A domino green method for the rapid synthesis of novel fused isoquinoline derivatives via Knoevenagel/Michael/cyclization reactions on aqueous media and their photophysical properties†
Abstract
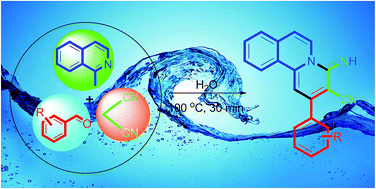
An expedient, eco-friendly and green-protocol has been developed for the synthesis of novel 4-imino-2-aryl-4H-pyrido[2,1-a]isoquinoline-3-carbonitrile derivatives via Knoevenagel/Michael/cyclisation reactions in one pot under catalyst-free conditions on aqueous media. These products exhibited UV-Vis absorption and photoluminescence properties.

 Please wait while we load your content...
Please wait while we load your content...