A Pd-catalyzed direct entry to 11-substituted 6H-isoindolo[2,1-a]indol-6-one derivatives as potential anticancer agents†
Suresh Babu Nallapatiab,
Raju Adepua,
Mohd Ashraf Ashfaqa,
B. Yogi Sreenivasa,
K. Mukkantib and
Manojit Pal*a
aDr Reddy's Institute of Life Sciences, University of Hyderabad Campus, Gachibowli, Hyderabad-500046, India. E-mail: manojitpal@rediffmail.com; Tel: +91 40 6657 1500
bInstitute of Science and Technology, JNT University Hyderabad, Hyderabad-500085, India
First published on 13th October 2015
Abstract
We describe Pd-mediated one-step synthesis of 11-substituted 6H-isoindolo[2,1-a]indol-6-ones via a sequential intramolecular Heck reaction of the corresponding dihalo N-allyl substituted N-arylbenzamide derivatives. Several of these compounds showed promising antiproliferative properties when tested against a number of cancer cell lines in vitro.
Cascade reactions that involve multiple bond formations in a single step are of particular interest in organic synthesis as these reactions improve the greenness of the overall process by avoiding multiple work-up and purification procedures thereby enhancing the synthetic efficiency substantially. Thus the development of new synthetic strategies based on cascade reactions is of great demand both in academia as well as industrial organizations.
Indole based fused heterocycles are widespread in various bioactive molecules and drugs. This is exemplified by a pyrazino[1,2-a]indole derivative as 5-hydroxytryptamine 2c (5-HT2c) agonist,1 a β-carboline as imidazoline receptor ligand,2 benzo[g]pyrido[4,3-b]indoles as DNA intercalator,3 pyrazino[1,2-a]indole-1,4-diones (simple analogues of gliotoxin) as selective inhibitors of geranylgeranyl transferase I,4 ellipticine as an antineoplastic agent,5 etc. Among various indole based fused heterocycles, 6H-isoindolo[2,1-a]indol-6-one A (Fig. 1) attracted our particular attention because compounds based on these framework have been identified as melatonin MT3 ligands6a (e.g. B, Fig. 1), agents having serotonin receptor affinity,6b and more importantly as potential anti-tumor agents7 (e.g. C, Fig. 1). Indeed, compound C showed antiproliferative properties against L1210 leukemia cells with IC50 = 4.4 μM. These reports and our continuing interest in indole based fused heterocycles prompted us to explore a 6H-isoindolo[2,1-a]indol-6-one based framework D for the design and identification of new anticancer agents. While various groups and substituents were introduced into the core structure of D in order to create diversity around this framework, our particular interest was on C-11 substituted analogs as except for one patent6b no pharmacological studies have been reported for these compounds.
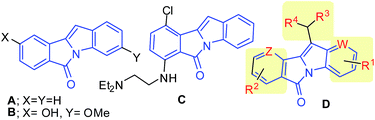 | ||
| Fig. 1 6H-Isoindolo[2,1-a]indol-6-one A, its bioactive derivatives B and C and newly designed framework D. | ||
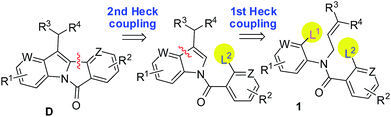
While a number of efficient and elegant methods (most of them being Pd-mediated protocols) are known for the synthesis of 6H-isoindolo[2,1-a]indol-6-one derivatives8–13 only few have been reported for the synthesis of their C-11 substituted analogs.10g,12g,12l,13–16 Moreover, a general and single approach for the synthesis of diversity based complex or functionalized 6H-isoindolo[2,1-a]indol-6-ones as represented by D has not been explored. In the recent past, double and multiple Heck reactions17 have found considerable applications in organic synthesis. Indeed, molecules possessing fair degree of structural complexities have been synthesized by using this single-step method. The strategy based on double or sequential Heck reaction attracted our attention due to the desired and inherent efficiency of this process. Moreover, a retro synthetic analysis of D (Fig. 2) revealed that this strategy might serve the purpose. It is evident from Fig. 2 that our strategy relies on intramolecular sequential Heck coupling18 based on the reactivity differences of the two leaving groups i.e. halides of the starting amide 1.
Based on the known reactivity order of aromatic halides as ArI > ArBr ≫ ArCl we have generally chosen iodo group as L1 and bromo as L2 (Fig. 2). However, this reactivity sequence is known to be altered when the halide is attached to an azomethine carbon (e.g. X–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–; X = halogen)19 and our choice of L1 and L2 was also changed accordingly (vide infra). Herein we report a Pd-mediated one-step synthesis of 11-substituted 6H-isoindolo[2,1-a]indol-6-ones (2 or D) via a sequential Heck reaction of the corresponding dihalo N-allyl substituted N-arylbenzamide derivatives (1) (Scheme 1).
N–; X = halogen)19 and our choice of L1 and L2 was also changed accordingly (vide infra). Herein we report a Pd-mediated one-step synthesis of 11-substituted 6H-isoindolo[2,1-a]indol-6-ones (2 or D) via a sequential Heck reaction of the corresponding dihalo N-allyl substituted N-arylbenzamide derivatives (1) (Scheme 1).
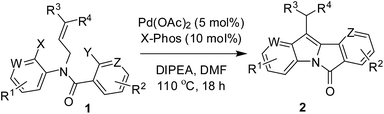
The key starting material 1 was prepared via a 2-step method (Scheme 2) involving the reaction of (hetero)aryl amine (3) with acid chloride (4) followed by allylation of the resulting amide (S-1). Initially, the feasibility of sequential intramolecular Heck reaction was examined with N-allyl-2-bromo-N-(4-chloro-2-iodophenyl)benzamide (1a) using a range of catalysts, bases and solvents (Table 1). The reaction afforded the compound 5 as a result of single intramolecular Heck reaction of 1a when performed in the presence of 5 mol% Pd(OAc)2 and 3.0 equivalent of DIPEA in DMF (entry 1, Table 1). However, in the presence of a ligand e.g. PPh3 the reaction afforded the desired product 2a albeit in poor yield (entry 2, Table 1).20 Moreover, the change of ligand to X-Phos improved the product yield dramatically (entry 3, Table 1). This observation prompted us to explore the reaction conditions further if 2a could be obtained in higher yield. The use of other Pd-catalysts e.g. Pd2(dba)3, Pd(PPh3)2Cl2 and Pd(PPh3)4 did not improve the yield of 2a (entries 4–6, Table 1). The use of Et3N in place of DIPEA too did not increase the yield (entry 7, Table 1) whereas change of solvent to DMA or 1,4-dioxane decreased the yield (entries 8–9, Table 1). The requirement of Pd-catalysts was also examined. For example, the use of a lower quantity of Pd-catalyst decreased the product yield (entry 10, Table 1) and the reaction did not proceed in the absence of Pd(OAc)2 (entry 11, Table 1). Overall, the combination of Pd(OAc)2, X-Phos and DIPEA in DMF was found to be optimum and was used for further study.
| Entry | Catalyst/ligand | Base/solvent | % Yieldb | |
|---|---|---|---|---|
| 2a | 5 | |||
| a Reactions were carried out using 1a (1 mmol), catalyst, ligand and base (3 mmol) in a solvent (2 mL) at 110 °C for 18 h.b Isolated yield.c 2.5 mol% catalyst and 5 mol% of ligand was used.d No catalyst/ligand used. | ||||
| 1 | Pd(OAc)2 | DIPEA/DMF | — | 72 |
| 2 | Pd(OAc)2/PPh3 | DIPEA/DMF | 12 | 68 |
| 3 | Pd(OAc)2/X-Phos | DIPEA/DMF | 71 | — |
| 4 | Pd2(dba)3 | DIPEA/DMF | 21 | 50 |
| 5 | Pd(PPh3)2Cl2 | DIPEA/DMF | 12 | 59 |
| 6 | Pd(PPh3)4 | DIPEA/DMF | 13 | 60 |
| 7 | Pd(OAc)2/X-Phos | Et3N/DMF | 67 | 9 |
| 8 | Pd(OAc)2/X-Phos | DIPEA/DMA | 69 | — |
| 9 | Pd(OAc)2/X-Phos | DIPEA/1,4-dioxane | 10 | 54 |
| 10c | Pd(OAc)2/X-Phos | DIPEA/DMF | 50 | 26 |
| 11d | — | DIPEA/DMF | — | — |
Having successfully established the appropriate reaction conditions for the sequential intramolecular Heck coupling leading to a 11-substituted 6H-isoindolo[2,1-a]indol-6-one (2a) we then examined the scope and generality of this method. Thus, a range of dihalo N-allyl substituted N-arylbenzamide derivatives (1) were employed under the optimized reaction conditions (Table 2, see also Table S-1 in ESI†). The reaction proceeded well in all these cases affording the desired products in good to acceptable yields. The substituents like F, Cl, and Me on the iodoarene ring of 1 (entry 1–6, Table 2) were well tolerated. Notably, like iodoarene of 1a–f, the 2-chloropyridine moiety of 1g also participated well in this cascade reaction (entry 7, Table 2). However, it seemed that the iodoarene moiety was preferred over 2-chloropyridine in the initial Heck coupling step when both were present in the same molecule e.g. 1h–j (entries 8–10, Table 2). The presence of substituents like Me, Ph or ester on the allyl moiety of 1 was also tested and found to be tolerated (entries 12–17, Table 2). Finally, the presence of electron withdrawing or releasing groups such as NO2 (e.g. 1k) or OMe (e.g. 1r) on the aroyl moiety was found to be equally effective (entry 11 and 18, Table 2). Altogether 18 compounds were prepared by using this methodology among which except 2d10g all are new. It is worthy to mention that the reactivity difference of halides of 1 towards the Pd-catalyst was the key for the success of this cascade reaction. Moreover, it was necessary to choose 1 possessing higher reactive halide present on the aniline ring rather than on the aroyl moiety. This was supported by the fact that the sequential intramolecular Heck reaction of N-allyl-2-iodo-N-(2-iodophenyl)benzamide was not successful as the reaction afford an unidentified product instead of the desired product.
| Entry | N-Allyl-N-arylbenzamide (1) R1, R2, R3, R4, W, X, Y, Z | Product (2) | Yieldb (%) |
|---|---|---|---|
| a All the reactions were carried out using 1 (1 mmol), 5 mol% Pd(OAc)2, 10 mol% X-Phos and DIPEA (3 mmol) in DMF (2 mL) at 110 °C for 18 h.b Isolated yield. | |||
| 1 | 4-Cl, H, H, H, CH, I, Br, CH 1a | 2a | 71 |
| 2 | 4-Me, H, H, H, CH, I, Br, CH 1b | 2b | 72 |
| 3 | 4-F, H, H, H, CH, I, Br, CH 1c | 2c | 67 |
| 4 | H, H, H, H, CH, I, Br, CH 1d | 2d | 70 |
| 5 | 2,4-DiMe, H, H, H, CH, I, Br, CH 1e | 2e | 62 |
| 6 | 3-Me, H, H, CH, I, Br, CH 1f | 2f | 67 |
| 7 | H, H, H, H, N, Cl, Br, CH 1g | 2g | 64 |
| 8 | 4-F, H, H, H, CH, I, Cl, N 1h | 2h | 62 |
| 9 | 4-Me, H, H, H, CH, I, Cl, N 1i | 2i | 66 |
| 10 | 2,4-DiMe, H, H, H, CH, I, Cl, N 1j | 2j | 60 |
| 11 | 4-Me, 5-NO2, H, H, CH, I, Cl, CH 1k | 2k | 64 |
| 12 | 4-Me, H, Me, H, CH, I, Br, CH 1l | 2l | 66 |
| 13 | H, H, Me, H, CH, I, Br, CH 1m | 2m | 64 |
| 14 | H, H, Me, Me, CH, I, Br, CH 1n | 2n | 61 |
| 15 | 4-Me, H, CO2 Me, H, CH, I, Br, CH 1o | 2o | 62 |
| 16 | 4-Me, H, Ph, H, CH, I, Br, CH 1p | 2p | 60 |
| 17 | H, H, Ph, H, CH, I, Br, CH 1q | 2q | 59 |
| 18 | H, 3,4-diOMe, H, H, CH, I, Br, CH 1r | 2r | 64 |
Based on results presented in Table 1 a plausible reaction mechanism is proposed for the present cascade reaction (Scheme 3). Thus the reaction pathway involved (i) chemoselective oxidative addition of Pd(0) to the higher reactive Ar–I bond of 1 to give E-1 (ii) 5-exo-trig cyclization of E-1 to give E-2 (iii) β-hydride elimination followed by olefinic double bond isomerisation to provide the indole intermediate E-4 via E-3 and then (iv) a second exo-trig mode Heck cyclization of E-4 to 2 via E-5 and E-6. Notably, the β-hydride elimination of E-6 leading to 2 may proceed either via pathway a or b. Pathway b would be energetically less favorable due to the anti orientation of the β-hydrogen (shown in red at ring juncture carbon) with respect to Pd-species. Thus the side chain hydrogen (shown in blue) was involved in the β-hydride elimination process of E-6 to give E-7 possessing an exocyclic double bond which on isomerisation afforded the desired product 2. The intermediacy of E-4 in the present reaction was further supported by isolation and conversion of compound 5 (Table 1) to 2a.21 A similar mechanism can be proposed for other substrates containing chloro or bromoarene moiety.
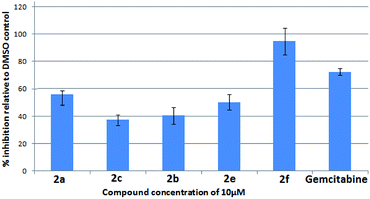
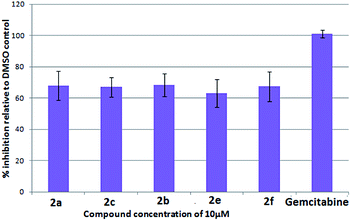
To evaluate their potential in inhibiting the growth of cancer cells, some compounds were tested at 10 μM against five cancer cell lines e.g. A549 (lung), Cal27 (oral), Hep G2 (liver), MCF-7 (breast) and MDA-MB-231 (breast) using the sulphorhodamine B (SRB) assay22a,b with gemcitabine22c as a reference compound. Effects of compounds on these 5 cancer cells are presented in Fig. 3–7. Thus compounds 2a and 2f (70–80% inhibition vs. gemcitabine's 100% inhibition) against lung cancer cells (Fig. 3), 2f (>90% inhibition vs. gemcitabine's > 70% inhibition) against oral cancer cells (Fig. 4), 2a, 2c, 2b and 2f (>80% inhibition vs. gemcitabine's > 80% inhibition) against hepatocellular liver carcinoma cells (Fig. 5), 2f (>50% inhibition vs. gemcitabine's < 50% inhibition) against breast adenocarcinoma cells (Fig. 6) and 2a, 2e, 2c, 2b, 2e and 2f (>60% inhibition vs. gemcitabine's 100% inhibition) against breast cancer cells (Fig. 7) were found to be active and of further interest. While the detailed pharmacological studies of these and rest of the compounds are in progress the present class of 11-substituted 6H-isoindolo[2,1-a]indol-6-one derivatives appeared to have potential medicinal value.
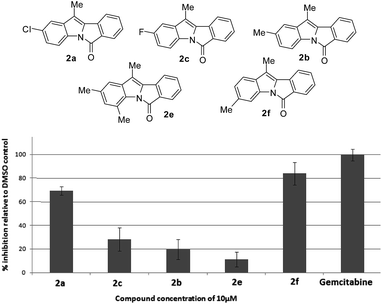 | ||
| Fig. 3 % Inhibition of lung cancer cells (A549) by compounds after 72 h of compound treatment at 10 μM. | ||
 | ||
| Fig. 5 % Inhibition of hepatocellular liver carcinoma cells (Hep G2) after 72 h of compound treatment at 10 μM. | ||
In conclusion, a Pd-mediated straightforward and one-step method has been developed for the synthesis of various 11-substituted 6H-isoindolo[2,1-a]indol-6-one derivatives. The methodology involved a sequential intramolecular Heck reaction of the corresponding dihalo N-allyl substituted N-arylbenzamide derivatives in the same pot. The reactivity differences of the two leaving groups i.e. halides of the starting amide is the key point for the success of this reaction. Some of these compounds showed promising antiproliferative properties when tested against a number of cancer cell lines in vitro indicating usefulness of this class of compounds for potential medicinal applications. Thus, the present general and one-pot approach towards diversity based complex or functionalized 6H-isoindolo[2,1-a]indol-6-ones could be of interest to researchers working in the area of organic/medicinal/pharmaceutical chemistry.
Acknowledgements
The authors thank management of DRILS and CSIR [Grant 02(0127)/13/EMR-II] for support.Notes and references
- M. Bos, F. Jenck, J. R. Martin, J. L. Moreau, V. Mutel, A. J. Sleight and U. Widmer, Eur. J. Med. Chem., 1997, 32, 253 CrossRef CAS.
- A. L. Hudson, R. Price, R. J. Tyacke, M. D. Lalies, C. A. Parker and D. J. Nutt, Br. J. Pharmacol., 1999, 126, 2P Search PubMed.
- C. Escude, C.-H. Nguyen, J.-L. Mergny, J.-S. Sun, E. Bisagni, T. Garestier and C. Helene, J. Am. Chem. Soc., 1995, 117, 10212 CrossRef CAS.
- J. R. Johnson, W. F. Bruce and J. D. Dutcher, J. Am. Chem. Soc., 1943, 65, 2005 CrossRef CAS.
- (a) M. Stiborova, C. A. Bieler, M. Wiessler and E. Frei, Biochem. Pharmacol., 2001, 62, 1675 CrossRef CAS PubMed; (b) C. Auclair, Arch. Biochem. Biophys., 1987, 259, 1 CrossRef CAS PubMed.
- (a) M. F. Boussard, S. Truche, A. Rousseau-Rojas, S. Briss, S. Descamps, M. Droual, M. Wierzbicki, G. Ferry, V. Audinot, P. Delagrange and J. A. Boutin, Eur. J. Med. Chem., 2006, 41, 306 CrossRef CAS PubMed; (b) V. Jasti, V. S. N. Ramakrishna, R. S. Kambhampati, S. R. Battula and V. S. V. V. Rao, World Patent Application No. WO 2004/000205A2, 31 Dec 2003.
- (a) J. Guillaumel, S. Leonce, A. Pierre, P. Renard, B. Pfeiffer, P. B. Arimondo and C. Monneret, Eur. J. Med. Chem., 2006, 41, 379 CrossRef CAS PubMed; (b) J. Guillaumel, L. Pierre, P. Renard, B. Pfeiffer, L. Peruchon, P. Arimondo and C. Monneret, Oncol. Res., 2003, 13, 537 CrossRef PubMed.
- From 2-aryl indole, see: (a) K. Hayashi, T. Choshi, K. Chikaraishi, A. Oda, R. Yoshinaga, N. Hatae, M. Ishikura and S. Hibino, Tetrahedron, 2012, 68, 4274 CrossRef CAS; (b) F. J. Reboredo, M. Treus, J. C. Estevez, L. Castedo and R. J. Estevez, Synlett, 2003, 1603 CAS; (c) L. A. Crawford, N. C. Clemence, H. McNab and R. G. Tyas, Org. Biomol. Chem., 2008, 6, 2334 RSC.
- Via the reaction of 2-iodo anilide with a propiolic acid derivative, see: T. Ponpandian and S. Muthusubramanian, Tetrahedron Lett., 2012, 53, 4248 CrossRef CAS.
- From N-benzoyl indole, see: (a) J. K. Laha, N. Dayal, K. P. Jethava and D. V. Prajapati, Org. Lett., 2015, 17, 1296 CrossRef CAS PubMed; (b) B. Liegault, D. Lee, M. P. Huestis, D. R. Stuart and K. Fagnou, J. Org. Chem., 2008, 73, 5022 CrossRef CAS PubMed; (c) S. R. Kandukuri and M. Oestreich, J. Org. Chem., 2012, 77, 8750 CrossRef CAS PubMed; (d) T. A. Dwight, N. R. Rue, D. Charyk, R. Josselyn and B. DeBoef, Org. Lett., 2007, 9, 3137 CrossRef CAS PubMed; (e) J. I. Ambrus, M. J. Kelso, J. B. Bremner, A. R. Ball, G. Casadei and K. Lewis, Bioorg. Med. Chem. Lett., 2008, 18, 4294 CrossRef CAS PubMed; (f) A. P. Kozikowski and D. Ma, Tetrahedron Lett., 1991, 32, 3317 CrossRef CAS; (g) R. Grigg, V. Sridharan, P. Stevenson, S. Sukirthalingam and T. Worakun, Tetrahedron, 1990, 46, 4003 CrossRef CAS.
- For intramolecular Heck-type reaction of 2-(2-iodophenyl)-3-methyleneisoindolin-1-one, see: G. Kim, J. H. Kim, W.-J. Kim and Y. A. Kim, Tetrahedron Lett., 2003, 44, 8207 CrossRef CAS.
- For other methods, see: (a) P. Duncanson, Y.-K. Cheong, M. Motevalli and D. V. Griffiths, Org. Biomol. Chem., 2012, 10, 4266 RSC; (b) T. Tang, X. Jiang, J.-M. Wang, Y.-X. Sun and Y.-M. Zhu, Tetrahedron, 2014, 70, 2999 CrossRef CAS; (c) C. Dai, A. B. Draganov and B. Wang, Heterocycl. Commun., 2010, 16, 245 CrossRef CAS; (d) H. K. Kadam and S. G. Tilve, Eur. J. Org. Chem., 2013, 4280 CrossRef CAS; (e) T. Itahara, Heterocycles, 1986, 24, 2557 CrossRef CAS; (f) M. D. Crenshaw and H. Zimmer, J. Heterocycl. Chem., 1984, 21, 623 CrossRef CAS; (g) L. A. Crawford, N. C. Clemence, H. McNab and R. G. Tyas, Org. Biomol. Chem., 2008, 6, 2334 RSC; (h) M. Hooper and S. H. Imam, J. Chem. Soc., Perkin Trans. 1, 1985, 1583 RSC; (i) B. W. Disanayaka and A. C. Weedon, Can. J. Chem., 1987, 65, 245 CrossRef CAS; (j) T. Itahara, Synthesis, 1979, 151 CrossRef CAS; (k) T. Itahara and T. Sakakibara, Heterocycles, 1979, 12, 148 CrossRef; (l) Y. Kanaoka and K. Koyama, Tetrahedron Lett., 1972, 13, 4517 CrossRef; (m) W. Carruthers and N. Evans, J. Chem. Soc., Perkin Trans. 1, 1974, 1523 RSC.
- For via Pd-mediated cascade reaction of N-benzoyl o-gem-dibromovinyl aniline, see: H.-F. He, S. Dong, Y. Chen, Y. Yang, Y. Le and W. Bao, Tetrahedron, 2012, 68, 3112 CrossRef CAS.
- O. Tsuge, T. Hatta and H. Tsuchiyama, Chem. Lett., 1998, 155 CrossRef CAS.
- A. M. Signori, E. Latocheski, B. L. Albuquerque, D. Faggion, T. B. Bisol, L. Meier and J. B. Domingos, New J. Chem., 2015, 39, 1574 RSC.
- K. Dev and R. Maurya, RSC Adv., 2015, 5, 13102 RSC.
- S. Bräse and A. de Meijere, Intermolecular Heck Reaction: Double and Multiple Heck Reactions, in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E.-I. Negishi, John Wiley & Sons, Inc., New York, USA, 2002; DOI:10.1002/0471212466.ch47.
- For our earlier efforts on intramolecular Heck type reactions, see: (a) R. Adepu, B. Prasad, M. A. Ashfaq, N. Z. Ehtesham and M. Pal, RSC Adv., 2014, 4, 49324 RSC; (b) B. Prasad, B. Y. Sreenivas, A. Sushma, S. Yellanki, R. Medisetti, P. Kulkarni and M. Pal, Org. Biomol. Chem., 2014, 12, 2864 RSC; (c) P. V. Babu, S. Mukherjee, G. S. Deora, K. S. Chennubhotla, R. Medisetti, S. Yellanki, P. Kulkarni, S. Sripelly, K. V. L. Parsa, K. Chatti, K. Mukkanti and M. Pal, Org. Biomol. Chem., 2013, 11, 6680 RSC; (d) B. Prasad, B. Y. Sreenivas, G. R. Krishna, R. Kapavarapu and M. Pal, Chem. Commun., 2013, 49, 6716 RSC.
- See for example: M. Pal, V. R. Batchu, N. K. Swamy and S. Padakanti, Tetrahedron Lett., 2006, 47, 3923 CrossRef CAS.
- Both the compound 2a and 5 was characterized by spectral data, [e.g. compound 2a: yellow solid, mp: 166–168 °C; Rf = 0.45 (10% EtOAc/n-hexane); 1H NMR (400 MHz, CDCl3) δ: 7.78–7.75 (m, 2H), 7.59–7.51 (m, 2H), 7.37–7.30 (m, 2H), 7.24–7.22 (dd, J = 7.8, 1.8 Hz, 1H), 2.41 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.9, 137.0, 134.6, 133.6, 133.2, 131.7, 129.1, 128.7, 127.6, 126.3, 125.3, 124.5, 120.0, 118.3, 113.9, 9.5; MS (ES mass): 268.1 (M + 1); compound 5: white solid; mp: 115–117 °C; Rf = 0.40 (10% EtOAc/n-hexane); 1H NMR (400 MHz, CDCl3) δ: 8.48 (bs, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.49–7.47 (m, 3H), 7.44–7.40 (m, 1H), 7.35 (d, J = 7.1 Hz, 1H), 6.70 (bs, 1H), 2.19 (s, 3H); MS (ES mass): 268.1 (M + 1); 348.1]; the Me group appeared at 2.40 δ and 9.5 ppm in the 1H and 13C NMR spectra of 2a whereas at 2.19 δ in the 1HNMR spectra of 5.
- Treatment of compound 5 (1 mmol) with 5 mol% Pd(OAc)2, 10 mol% X-Phos and DIPEA (3 mmol) in DMF (2 mL) at 110 °C for 10 h afforded the product 2a in 53% yield.
- (a) L. V. Rubinstein, R. H. Shoemaker, K. D. Paull, R. M. Simon, S. Tosini, P. Skehan, D. A. Scudiero, A. Monks and M. R. Boyd, J. Natl. Cancer Inst., 1990, 82, 1113 CrossRef CAS PubMed; (b) P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney and M. R. Boyd, J. Natl. Cancer Inst., 1990, 82, 1107 CrossRef CAS PubMed; (c) E. Chu and V. T. DeVita, Physicians' Cancer Chemotherapy Drug Manual, Jones & Bartlett, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data for all new compounds, and copies of spectra. See DOI: 10.1039/c5ra17908d |
| This journal is © The Royal Society of Chemistry 2015 |