Dual energy transfer controlled photoluminescence evolution in Eu and Mn co-activated β-Ca2.7Sr0.3(PO4)2 phosphors for solid-state lighting†
Jin Han‡
a,
Fengjuan Pan‡b,
Wenli Zhou*a,
Zhongxian Qiua,
Miao Tanga,
Jing Wangb and
Shixun Lian*a
aKey Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China), Key Laboratory of Sustainable Resources Processing and Advanced Materials of Hunan Province College, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha, 410081, China. E-mail: chemwlzhou@hunnu.edu.cn; sxlian@hunnu.edu.cn
bSchool of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou, Guangdong 510275, China
First published on 11th November 2015
Abstract
Photoluminescence evolution in single-composition phosphors for solid state lighting can be realized through an energy transfer strategy, which is commonly based on one sensitizer and one activator. In this work, we focus on a dual energy transfer process, that is, energy transfers from the sensitizer Eu2+ at two different cation sites to the activator Mn2+ at another site in whitlockite-type β-Ca2.7Sr0.3(PO4)2. Firstly, we refined the crystal structure of the β-Ca2.7Sr0.3(PO4)2 host by a Rietveld method. Then we studied the phase, morphology and photoluminescence of Eu and Mn co-activated β-Ca2.7Sr0.3(PO4)2 phosphors, which are synthesized by a conventional solid-state reaction. By adjusting the ratio of Eu2+/Mn2+, the emission hue can be controlled from cyan (0.211, 0.281) to white-light (0.332, 0.290) and eventually to red (0.543, 0.276). The dual energy transfers from Eu2+ to Mn2+ are demonstrated to be dipole–dipole and quadrupole–quadrupole mechanisms, respectively. Moreover, white light-emitting diodes (LEDs) were fabricated through the integration of a 375 nm NUV chip and the white-emitting phosphor Ca2.7Sr0.3(PO4)2:0.008Eu2+, 0.03Mn2+ into a single package, which shows a warm white light with color coordinates of (0.40, 0.41) and correlated color temperature of 3731 K.
1. Introduction
White light-emitting diodes (LEDs) are widely used in daily lighting to replace conventional lighting sources. Phosphor-converted (pc-) WLEDs are regarded as a novel lighting source for the next generation.1–3 As one of the key materials, phosphors play a crucial role in determining the optical quality, lifetime, and cost of pc-WLEDs. Currently, pc-WLEDs are fabricated with the combination of blue InGaN LED chip and yellow-emitting YAG:Ce phosphor.4,5 However, the pc-WLEDs based on such combination exhibit poor color rendering index (Ra < 80) and high color temperature (Tc > 7000 K) due to the lack of red emission.6,7 Additionally, it is also possible to produce white light by the combination of tri-color phosphors with ultraviolet (UV) LED chips; while the reabsorption problem commonly causes the poor luminous efficiency.8,9 For these reasons, single-composition white emitting phosphors pumped by UV or near-UV chips, which could exhibit excellent Ra values and color stability, have been attracted much attention for solid-state lighting.10–13Generally, white light emitting from a single-composition phosphor is realized through energy transfer strategy. The strategy mainly depends on co-doped systems,14–17 like Eu2+–Mn2+, Eu2+–Eu3+, Ce3+–Mn2+, and tri-doped systems,18–23 such as Ce3+/Eu2+–Tb3+–Mn2+. These doping ways just adopt Eu2+ or Ce3+ with intense absorption to sensitize other luminescent centers and then produce desirable white light emission. As we know, the white light can be formed by the combinations of yellow and blue, or red, green and blue. In co-doping systems, Mn2+ or Eu3+ centers normally work as the red composition, so the Ce3+ or Eu2+ needs to emit yellow light for the production of white-light, while the yellow phosphors doped with Ce3+ or Eu2+ are very rare. In tri-doped systems, Ce3+ and Eu2+ centers can work as blue composition, Tb3+ and Mn2+ as green and red one, respectively. It is a good strategy for the production of white-light, but forbidden 4f–4f transition of Tb3+ could limit its practical applications due to low luminescence efficiency.24,25 Therefore, exploration of new energy transfer way for single-phase white emitting phosphors is desirable.
Rare-earth ions activated phosphate phosphors have been widely studied because of their low sintering temperature, high luminous efficiency and excellent thermal stability. Huang et al. reported the photoluminescence and energy transfer of Ce3+–Mn2+ and Ce3+–Mn2+ co-activated Ca9Y(PO4)7 phosphors for fluorescent lamp application.13,26 Li et al. reported tunable blue-green emission in Ca3(PO4)2:Eu2+, Tb3+ phosphor by energy transfer.25 Ji et al. reported luminescent and structural property of yellow-emitting Sr1.75Ca1.25(PO4)2:Eu2+ phosphor.27 Recently, they further investigated the cation substitution dependent bimodal photoluminescence in Ca3−xSrx(PO4)2:Eu2+ phosphors.28 Very recently, we proposed two new strategies, empting site and enlarging sites, to tune the photo-luminescence (PL) of the whitlockite phosphor with multiple cation sites.29,30 That work clarified the relation between the sites and PL properties of Eu2+.
Energy transfer from Eu2+ to Mn2+ has been researched in numerous phosphors. While most of work focus on the energy transfer from one Eu2+ site to one Mn2+ site, the spectra can only be tuned between one emission band of Eu2+ and one of Mn2+. This study intents to enrich the strategies of photoluminescence evolution based on a dual energy transfer. The β-Ca2.7Sr0.3(PO4)2 compound is selected herein for the following reasons. (1) Eu2+ doped β-Ca2.7Sr0.3(PO4)2 phosphor can present two emission bands, peaking at 417 and 490 nm, respectively. As our previous research, the two bands should be attributed to two different Eu2+ luminescent centers, three-fold M(4) sites for the former and eight-coordinated M(3) sites for the latter.29,30 (2) Photoluminescence evolution based on a dual energy transfer from two Eu2+ centers to Mn2+ is very rare. (3) Comparison of the dual ET mechanisms in the same phosphor is interesting.
In this work, all the phosphors were successfully synthesized by a high-temperature solid-state technology. The crystal structures, PL evolution, dual energy transfer mechanism, PL thermal stability and performance of WLED package are investigated.
2. Experimental
2.1 Materials and synthesis
A series of TCSP:Eu2+, xMn2+ (x = 0, 0.03, 0.06, 0.12, 0.24) phosphors were synthesized by conventional solid-state reaction. The concentration of Eu2+ in all samples was fixed at 0.008.31 The starting raw materials, CaCO3 (A. R.), SrCO3 (A. R.), NH4H2PO4 (A. R.), MnCO3 (A. R.) and Eu2O3 (99.99%), with stoichiometric molar ratios were thoroughly ground and mixed in an agate mortar. The mixtures were preheated at 1000 °C for 8 h in air and then sintered at 1100 °C for 10 h under a reducing atmosphere of 15% H2/85% N2, and slowly cooled to room temperature.The white-LED package were fabricated by coating a white-emitting phosphor and silicone resin mixture on a NUV LED chip (λ = 375 nm). Air bubbles were removed by vacuum treatment.
2.2 Characterization
The phase purity of all samples was examined by X-ray diffraction (XRD) using a Bruker D8 ADVANCE powder diffractometer with Cu-Kα radiation (λ = 1.54059 Å). High quality XRD data for Rietveld refinement was collected over a 2θ range from 5° to 100° at an interval of 0.02°. The refinement was carried out using the Topas Academic software.32 The morphology and elemental composition were measured using scanning electron microscopy (SEM, FEI Quanta 400). The photoluminescence excitation (PLE) and photoluminescence spectra in the temperature range 3–500 K as well as fluorescence decay curves were recorded on an Edinburgh FSP920 time-resolved and steady-state fluorescence spectrometer, which is equipped with a time-correlated single-photon counting card and a thermoelectric cooled red sensitive photomultiplier tube. A 450 W Xenon lamp is used as the excitation source. The excitation photons for the fluorescence decay curves are provided by a 150 W nF900 flash lamp. The sample temperature is varied by means of a temperature controller (Oxford, CRY TEMP). The electro-luminescence (EL) spectra were measured using an Ocean Optics QE65000 spectrometer.3. Results and discussion
3.1 Phase identification and morphology
We first refined the crystal structure of the TCSP compound by the Rietveld method. Fig. 1a shows the observed and calculated X-ray diffraction patterns of TCSP, as well as difference profile of the Rietveld refinement. The crystal data of β-Ca3(PO4)2 was used as an initial model for the refinement,33 which converged to Rwp = 3.82% and Rb = 3.48%. The refined crystal structural parameters are shown in Table S1 (ESI†). TCSP shows a larger cell volume than β-Ca3(PO4)2 due the partial substitution of Sr for Ca. The refined crystal structure of the compound and coordination polyhedra of all five M cation sites are sketched in Fig. 1b. The refined occupation fractions of Ca and Sr are 0.9443 and 0.0557 at M(1), 0.9802 and 0.0198 at M(2), 0.8079 and 0.1921 at M(3), 0.2529 and 0.2471 at M(4), 1 and 0 at M(5), respectively. The refinement results reveal that Sr atoms preferentially occupy the 3-fold M(4) sites (nearly 50% is replaced), then the 8-coordinated M(3) sites (about 20%). The M(5) sites are too small to be occupied by Sr atoms. Both the enlargement of Ca sites and preferential occupation of Sr on Ca(4) sites caused by the substitution of Sr for Ca could result in the migration of some Eu2+ activators from M(4) to M(3) sites in TCSP:Eu2+, then one can expect that one emission band (violet-blue) will became two bands (violet-blue and cyan).29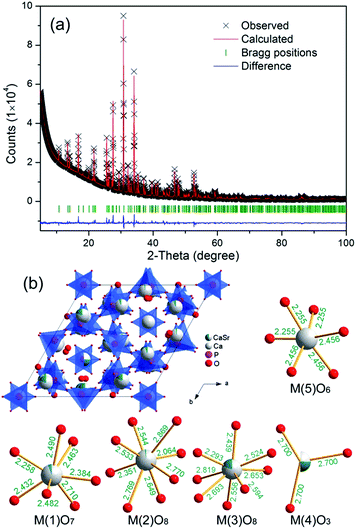 | ||
| Fig. 1 (a) Rietveld refinements of powder XRD profile of β-Ca2.7Sr0.3(PO4)2 sample. (b) Schematic structures of the β-Ca2.7Sr0.3(PO4)2 and Ca/Sr-centered polyhedra. | ||
The composition and phase purity of all Eu2+ and Mn2+ co-doped TCSP samples were also checked by XRD. The XRD patterns are shown in Fig. 2. All the diffraction peaks of the samples can be indexed to rhombohedral structure β-Ca2.7Sr0.3(PO4)2 and do not contain any reflections of foreign phases, indicating that the dopants Eu2+ and Mn2+ are completely dissolved in the TCSP host lattice without inducing significant changes of the crystal structure.
 | ||
| Fig. 2 XRD patterns of Ca2.7−xSr0.3(PO4)2:Eu, xMn samples. The refined TCSP card is got from the Ca2.7−xSr0.3(PO4)2.cif file by diamond software. | ||
The morphology and elemental composition of TCSP:Eu2+, Mn2+ samples were determined using SEM (Fig. 3a). It is found that the particles present smooth morphology and narrow size distribution with diameters of 3–8 micrometers. It is believed that such morphology will be very helpful in the fabrication of white LEDs devices. Energy-dispersive X-ray spectroscopy confirms the existence of the elements Ca, Sr, P, O, Eu and Mn, with atomic ratios of 18.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.16
2.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.15
14.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65.21
65.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.16
0.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13. In addition, the well-resolved crystal lattice planes (104) observed from HRTEM image (Fig. 3c) suggest high crystallization of as-prepared phosphors.
0.13. In addition, the well-resolved crystal lattice planes (104) observed from HRTEM image (Fig. 3c) suggest high crystallization of as-prepared phosphors.
3.2 Photoluminescence properties
Fig. 4a–c show the normalized PLE and PL spectra of TCSP:Eu2+, TCSP:Mn2+ and TCSP:Eu2+, Mn2+ phosphors, respectively. The emission spectrum of TCSP:Eu2+ consists of two bands, peaking at 417 and 490 nm, which are attributed to 5d → 4f transitions of Eu2+ occupying the M(4) and M(3) sites of Ca2.7Sr0.3(PO4)2, respectively.29 For the two Eu2+ centers, here we name them as Eu(I) and Eu(II), respectively. The PLE spectrum (Fig. 4b) of TCSP:Mn2+ contains several bands peaked at 304, 341, 368, 405, and 465 nm, corresponding to the transitions from the 6A1(6S) level to the 4T1(4P), 4E(4D), 4T2(4D), [4A1(4G), 4E(4G)], and 4T1(4G) levels of Mn2+, respectively. The PL spectrum centered at 650 nm is assigned to the 4T1(4G) → 6A1(6S) transition of Mn2+. The red luminescence is very weak due to the spin- and parity-forbidden d–d transition of Mn2+. It is possible to improve the PL of Mn2+ through energy transfer from Eu2+ to Mn2+ due to significant spectral overlap between the emission bands of TCSP:Eu2+ and the excitation bands of TCSP:Mn2+. Fig. 4c presents the PLE and PL of Eu2+ and Mn2+ co-activated phosphor. When monitoring the red emission of Mn2+ ions at 650 nm, the excitation spectrum exhibits the extremely similar profiles with that of Eu2+ ions, indicating the possibility of energy transfer from Eu2+ to Mn2+. | ||
| Fig. 4 PLE and PL spectra of (a) Ca2.7Sr0.3(PO4)2:Eu, (b) Ca2.7Sr0.3(PO4)2:Mn and (c) Ca2.7Sr0.3(PO4)2:Eu, Mn samples. | ||
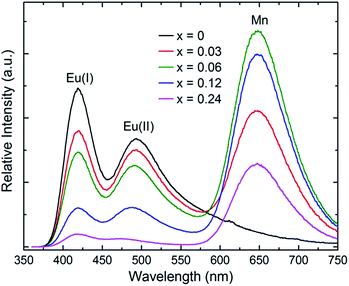
Fig. 5 shows emission spectra of TCSP:Eu2+, xMn2+ under the excitation at 350 nm. The emission intensity of Eu(I) and Eu(II) decreases with increasing x. In contrast, the emission intensity of Mn2+ increases until x = 0.06 and then decreases with further increasing x owing to concentration quenching. The observed evolution of PL further supports the existence of dual energy transfer from Eu(I) and Eu(II) to Mn2+. A schematic graph for the dual energy transfer is shown in Scheme 1.
The CIE chromaticity diagram of as-synthesized phosphors under 350 nm excitation are shown in Fig. 6. On account of the dual energy transfer from Eu2+ to Mn2+, the emission color could be varied from blue-greenish (0.211, 0.281) through white (0.332, 0.290) to red (0.543, 0.276) by tuning the ratio of Eu2+/Mn2+. The CIE color coordinate and calculated correlated color temperature are summarized in Table S3† Above all, we have obtained white light emission in the single-phase TCSP:0.008Eu2+, 0.03Mn2+ phosphor. The insets of Fig. 6 present the photos of the representative phosphors under irradiation of 365 nm UV-lamp.
 | ||
| Fig. 6 CIE chromaticity coordination of the TCSP:Eu, xMn phosphors under 350 nm excitation. The insets show luminescence photos of the corresponding phosphors excited under a 365 nm UV lamp. | ||
3.3 Mechanism of dual energy transfer
The fluorescence decay curves of Eu(I) and Eu(II) as a function of Mn2+ concentration (x) are plotted in Fig. 7. The lifetimes can be calculated by fitting with a second-order exponential function according to the following equations:34
I = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2)
| (1) |
| τ = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (2) |
Generally, the decay lifetime of the donor center decreases due to the energy transfer from the donor to the acceptor. The efficiency (ηT) of energy transfer from Eu2+ to Mn2+ can be expressed as:8,35
| ηT = 1 − τS/τS0 | (3) |
The resonant energy transfer mechanisms from sensitizer to activator mainly include exchange interaction and multipolar interaction according to Dexter theory. The critical distance RC for the energy transfer from Eu2+ to Mn2+ can be estimated using the concentration quenching method.35,36
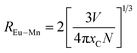 | (4) |
The energy transfer mechanism can be investigated on the basis of Reisfeld's approximation and Dexter's energy transfer expressions of multipolar interaction.28
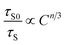 | (5) |
3.4 Thermal stability
The thermal stability is one of the important technological parameters for phosphor to be used in solid-state lighting, especially in high-power WLEDs.37 Fig. 9 shows the temperature dependence of PL intensity for TCSP:0.008Eu2+, 0.03%Mn2+ phosphor excited at 350 nm. Fig. 9a presents a plot of PL intensity versus temperature. The PL intensity decreases with increasing temperature owing to the thermal quenching. Thermal quenching always occurs due to the non-radiative transition from the excited state of luminescent center to the bottom of conduction band in the lattice.38,39 The integrated emission intensity at 100 and 200 °C remains about 80% and 60% in comparison with that at RT. For comparison, the thermal stability of commercial YAG phosphor is also plotted in Fig. 9a (red curve). The results demonstrate that this phosphor has a good thermal stability and might be an excellent candidate for application in pc-WLEDs.3.5 White LED package
Fig. 10 shows the electroluminescence spectrum of the pc-WLED lamp fabricated with the combination of a 375 nm NUV LED chip and a single-phase white-emitting TCSP:0.008Eu2+, 0.03%Mn2+ phosphor driven by a 350 mA forward bias current. The electroluminescence spectrum clearly presents three emission bands at 375, 515, and 645 nm, which belong to the NUV LED chip, Eu2+ emission, and Mn2+ emission, respectively. The optical properties of the pc-WLED lamp give a correlated color temperature of 3731 K and CIE color coordinates of (0.40, 0.41). The inset of Fig. 10 displays the photograph of white light emission from the LED package driven by 350 mA forward bias current. These results indicate that TCSP:0.008Eu2+, 0.03%Mn2+ might have promising applications in NUV white-light LEDs.4. Conclusions
In summary, a series of single-phase Ca2.7Sr0.3(PO4)2:Eu2+, Mn2+ phosphors have been synthesized by conventional solid-state reaction. The refined crystal structure of Ca2.7Sr0.3(PO4)2 shows that Sr preferentially occupy the M(4) and M(3) sites, which causes the sensitizer Eu mainly distribute at the two sites. Activator Mn stays at M(5) site. Photoluminescence evolution from blue-greenish to white to red-light could be realize via the dual energy transfer from Eu2+ to Mn2+. The mechanisms are proposed to be dipole–dipole and quadrupole–quadrupole interaction for Eu(I)–Mn and Eu(II)–Mn, respectively. Moreover, a pc-WLED lamp combined with 375 nm NUV chip and white-emitting TCSP:0.008Eu2+, 0.03Mn2+ phosphor produces a warm white light with color temperature of 3731 K and color coordinates of (0.40, 0.41). These results indicate that TCSP:0.008Eu2+, 0.03Mn2+ is a promising single-composition phosphor for application in white-light NUV LEDs.Acknowledgements
This work is partially supported by the National Natural Science Foundation of China (21501058, 21571059), Hunan Provincial Natural Science Foundation of China (2015JJ2100).Notes and references
- M. Shang, C. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372–1386 RSC.
- W.-T. Chen, H.-S. Sheu, R.-S. Liu and J. P. Attfield, J. Am. Chem. Soc., 2012, 134, 8022–8025 CrossRef CAS PubMed.
- W. B. Im, N. George, J. Kurzman, S. Brinkley, A. Mikhailovsky, J. Hu, B. F. Chmelka, S. P. DenBaars and R. Seshadri, Adv. Mater., 2011, 23, 2300–2305 CrossRef CAS PubMed.
- M. Shang, J. Fan, H. Lian, Y. Zhang, D. Geng and J. Lin, Inorg. Chem., 2014, 53, 7748–7755 CrossRef CAS PubMed.
- J. L. Wu, G. Gundiah and A. Cheetham, Chem. Phys. Lett., 2007, 441, 250–254 CrossRef CAS.
- A. Aboulaich, M. Michalska, R. Schneider, A. Potdevin, J. Deschamps, R. Deloncle, G. Chadeyron and R. Mahiou, ACS Appl. Mater. Interfaces, 2014, 6, 252–258 CAS.
- H. Zhu, C. C. Lin, W. Luo, S. Shu, Z. Liu, Y. Liu, J. Kong, E. Ma, Y. Cao, R.-S. Liu and X. Chen, Nat. Commun., 2014, 5, 4312 CAS.
- N. Guo, C. Jia, J. Li, Y. Zhao, R. Ouyang and W. Lu, J. Am. Ceram. Soc., 2015, 98, 1162–1168 CrossRef CAS.
- A. Kalaji, M. Mikami and A. K. Cheetham, Chem. Mater., 2014, 26, 3966–3975 CrossRef CAS.
- Y. Chen, Y. Li, J. Wang, M. Wu and C. Wang, J. Phys. Chem. C, 2014, 118, 12494–12499 CAS.
- Y. Liu, X. Zhang, Z. Hao, X. Wang and J. Zhang, Chem. Commun., 2011, 47, 10677–10679 RSC.
- C.-H. Huang, P.-J. Wu, J.-F. Lee and T.-M. Chen, J. Mater. Chem., 2011, 21, 10489–10495 RSC.
- C.-H. Huang, T.-W. Kuo and T.-M. Chen, ACS Appl. Mater. Interfaces, 2010, 2, 1395–1399 CAS.
- Y. Zhang, X. Li, K. Li, H. Lian, M. Shang and J. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 2715–2725 CAS.
- Z. Yang, P.-C. Lin, C.-F. Guo and W.-R. Liu, RSC Adv., 2015, 5, 13184–13191 RSC.
- P. Li, Z. Wang, Q. Guo and Z. Yang, J. Am. Ceram. Soc., 2015, 98, 495–500 CrossRef CAS.
- W. B. Dai, J. Mater. Chem. C, 2014, 2, 3951–3959 RSC.
- X. Mi, J. Sun, P. Zhou, H. Zhou, D. Song, K. Li, M. Shang and J. Lin, J. Mater. Chem. C, 2015, 3, 4471–4481 RSC.
- S. Deng, Z. Qiu, M. Zhang, W. Zhou, J. Zhang, C. Li, C. Rong, L. Yu and S. Lian, J. Rare Earths, 2015, 33, 463–468 CrossRef CAS.
- J. Zhang, Y. He, Z. Qiu, W. Zhang, W. Zhou, L. Yu and S. Lian, Dalton Trans., 2014, 43, 18134–18145 RSC.
- Z. Wang, P. Li, Z. Yang, Q. Guo and G. Dong, Ceram. Int., 2014, 40, 15283–15292 CrossRef CAS.
- H. Liu, Y. Luo, Z. Mao, L. Liao and Z. Xia, J. Mater. Chem. C, 2014, 2, 1619–1627 RSC.
- X. Chen, P. Dai, X. Zhang, C. Li, S. Lu, X. Wang, Y. Jia and Y. Liu, Inorg. Chem., 2014, 53, 3441–3448 CrossRef CAS PubMed.
- M. Xie, D. Li, R. Pan, X. Zhou and G. Zhu, RSC Adv., 2015, 5, 22856–22862 RSC.
- K. Li, Y. Zhang, X. Li, M. Shang, H. Lian and J. Lin, Dalton Trans., 2015, 44, 4683–4692 RSC.
- C.-H. Huang, T.-M. Chen, W.-R. Liu, Y.-C. Chiu, Y.-T. Yeh and S.-M. Jang, ACS Appl. Mater. Interfaces, 2010, 2, 259–264 CAS.
- H. Ji, Z. Huang, Z. Xia, M. S. Molokeev, V. V. Atuchin, M. Fang and S. Huang, Inorg. Chem., 2014, 53, 5129–5135 CrossRef CAS PubMed.
- M. Jiao, Y. Jia, W. Lu, W. Lv, Q. Zhao, B. Shao and H. You, J. Mater. Chem. C, 2014, 2, 90–97 RSC.
- J. Han, W. Zhou, Z. Qiu, L. Yu, J. Zhang, Q. Xie, J. Wang and S. Lian, J. Phys. Chem. C, 2015, 119, 16853–16859 CAS.
- J. Han, W. Zhou, M. Tang and S. Lian, Phys. Status Solidi RRL, 2015, 9, 485–488 CrossRef CAS.
- W. Zhou, J. Han, F. Pan, J. Zhang, Q. Xie, S. Lian, L. Yu and J. Wang, J. Am. Ceram. Soc., 2014, 97, 3631–3635 CrossRef CAS.
- A. A. Coelho, J. S. O. Evans, I. R. Evans, A. Kern and S. Parsons, The TOPAS symbolic computation system, Powder Diffr., 2011, 26, S22–S25 CrossRef CAS.
- M. Yashima, A. Sakai, T. Kamiyama and A. Hoshikawa, J. Solid State Chem., 2003, 175, 272–277 CrossRef CAS.
- S. Lian, Y. Qi, C. Rong, L. Yu, A. Zhu, D. Yin and S. Liu, J. Phys. Chem. C, 2010, 114, 7196–7204 CAS.
- W. Zhou, S. Deng, C. Rong, Q. Xie, S. Lian, J. Zhang, C. Li and L. Yu, RSC Adv., 2013, 3, 16781–16787 RSC.
- J. Sun, J. Zeng, Y. Sun and H. Du, J. Lumin., 2013, 138, 72–76 CrossRef CAS.
- S. Pimputkar, J. S. Speck, S. P. DenBaars and S. Nakamura, Nat. Photonics, 2009, 3, 180–182 CrossRef CAS.
- Z. Xia, J. Zhuang and L. Liao, Inorg. Chem., 2012, 51, 7202–7209 CrossRef CAS PubMed.
- Z. Xia, R.-S. Liu, K.-W. Huang and V. Drozd, J. Mater. Chem., 2012, 22, 15183–15189 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: The refined crystal structural parameters of β-Ca2.7Sr0.3(PO4)2 and the lifetimes of Eu2+. See DOI: 10.1039/c5ra17877k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |