Chiral electrochemical recognition of tryptophan enantiomers at a multi-walled carbon nanotube–chitosan composite modified glassy carbon electrode
Abstract
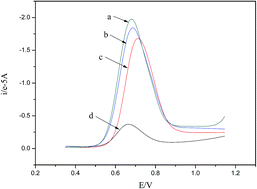
A multi-walled carbon nanotube–chitosan composite modified glassy carbon electrode (MWCNT–CS/GCE) was prepared and used for the chiral recognition of tryptophan (Trp) enantiomers. Cyclic voltammetry (CV) was employed to characterize the electrical conductivity of the modified electrode. Differential-pulse voltammetry (DPV) was employed to observe the oxidation peak potentials (Ep) of L- and D-Trp at the modified electrode. Different Ep of L- and D-Trp at the modified GCE were observed in the solutions containing only L- or D-Trp enantiomers. As for a mixed aqueous solution containing both L- and D-Trp, only one Ep peak would appear. However, the Ep of the peak was found to shift positively and linearly with an increasing percentage of L-isomer in the racemic Trp mixture solution, making it possible to determine the percentage of L- and D-Trp enantiomers in a racemic Trp mixture.

 Please wait while we load your content...
Please wait while we load your content...