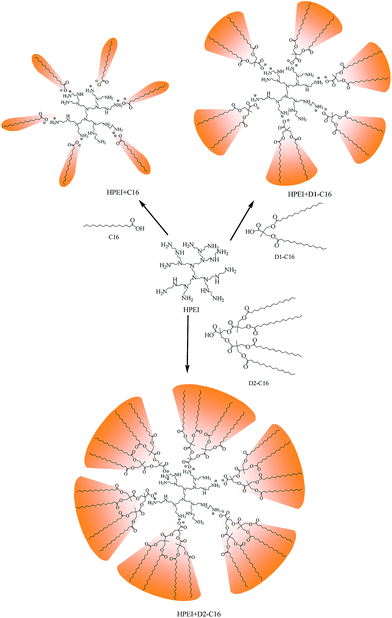
Co-assembly of two types of complementary dendritic units into amphiphilic supramolecular complexes capable of hosting guest molecules†
Yi
Liu
a,
Ting
Xiao
b,
Li
Xu
a and
Xun-Yong
Liu
*a
aSchool of Chemistry and Materials Science, Ludong University, 264025 Yantai, Shandong Province, People’s Republic of China
bDepartment of Physics & Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, 200062 Shanghai, People’s Republic of China
First published on 9th October 2015
Abstract
A new supramolecular complex, which can be used as a nanocarrier, was formed conveniently by the co-assembly of hyperbranched polyethylenimine (HPEI) with a novel carboxylic acid which has one or two generations of dendrites. The obtained supramolecular complexes consisting of two different dendritic polymeric segments were verified by FTIR, 1H NMR and DLS. After extracting with water or solutions of dyes, the supramolecules can form large aggregates as verified by DLS analysis. These supramolecular aggregates could be used as nanocarriers to efficiently encapsulate polar dyes such as the anionic dyes methyl orange (MO), eosin Y (EY) and fluorescein sodium (FS). This encapsulation behavior of the complexes based on an aggregation mechanism is different from that of the simple inverted micelles and unimolecular transporter systems reported. And the encapsulation capacities of the aggregates could reach a maximal value as the HPEI concentration and the density of the carbon chains increased to a certain value. Besides, the carrier with a dendritic shell could accommodate more guests than the corresponding carriers with only a linear shell, which shows the superiority of the dendritic shell. Meanwhile, an increase of the length of the outer carbon chain in the complex could distinctly improve the encapsulation ability. Therefore, supermolecules with a dendritic shell not only simplify the synthesis approach of covalent polymers, but also maintain a high encapsulation ability for guests, which are attributes worth popularizing.
1. Introduction
Recently, host–guest encapsulation has been the focus of attention, due to its wide application in many different fields, such as catalysis, drug delivery, phase transfer, environmental purification, reactors, and so on.1–5 A great deal of amphiphilic hyperbranched polymers,6–17 dendronized polymers18,19 and dendrimers20–23 with core–shell architecture are especially attractive for the loading of guest molecules, which is attributed to their favorable structures and superior capability of guest encapsulation to their corresponding linear analogues.6–8 The connections between the core and shell of these nanocarriers are divided into two types: covalent bonds and non covalent bonds. Surprisingly, nearly all reported amphiphilic dendritic or hyperbranched polymers for encapsulating guest molecules are formed by covalently connecting the shell to the core,6–17,20–23 followed by the protracted steps of synthesis, separation and purification. And these covalently bonded transport systems based on amphiphilic dendrimers or hyperbranched polymers can be divided into two classes: (1) unimolecular micelles and (2) aggregate architectures. The most reported encapsulation mechanisms for loading guests in amphiphilic core–shell nanocarriers pertain to the unimolecular micelles.6–15,20–23 The aggregation encapsulation of covalent polymers is scarce.16,17 Fleige et al. demonstrated a core–multishell nanoparticle of methoxypoly(ethylene glycol) (mPEG), octadecandioic acid and hyperbranched polyglycerolamine, which could load solvatochromic dyes Coumarin 153 and Nile red by an aggregation mechanism.17However, supramolecular nanocarriers based on a simple noncovalent bond between core and shell are seldom reported.24–27 The self assembly complexes possess some advantages over the covalent ones. They are not only prepared facilely, but are also apt to be separated with the core bonded to the shell just through a simple change of environment, and the encapsulated guest could be released synchronously which could realize the potential for recycling. Thirdly, it is also easy to adjust the contents of the shell and core. For example, Chechik et al. introduced a noncovalent means for the creation of toluene-soluble inverted micelles that were obtained through the formation of ion pairs between terminal amine groups of amine-terminated poly(amidoamine) (PAMAM) dendrimers and fatty acids.24 Chen et al. described the simple self-assembly of hyperbranched polyethylenimine (HPEI) and fatty acids, and these supramolecular nanocapsules could encapsulate water-soluble guests into CHCl3.25 Chun et al. reported a supramolecular complex self-assembled by poly(ethylene glycol) and a generation 4 poly(amidoamine) (G4-PAMAM) dendrimer, which could load Cu2+ ions.26 All of these studies had some common points: firstly, most of the studies which reported self-assembly encapsulation only highlighted the function of loading guests; but the mechanisms of loading which involve aggregates or covalent unimolecules are seldom studied. Secondly, the structures of the complexes were all composed of a dendritic core and a linear shell; and every chain of the shell was capped by one carboxylic acid as a single terminus. To the best of our knowledge, however, a complex consisting of only two different dendritic polymeric segments has not been reported. In our previous study,28 the participation of a dendritic structure as an inner shell in covalent polymers could enhance the efficiency of guest encapsulation. So compared with a solitary linear shell, whether branching units in the shell would also improve the loading ability of the core–shell self-assembly complex deserves to be studied. For this purpose, we prepared a novel self-assembly of a complex composed of a hyperbranched core and a novel one or two generation dendritic shell; the formation and guest transport system of the complexes are also discussed.
2. Experimental
2.1 Materials
Hyperbranched polyethylenimine (HPEI) with an average molecular weight of 104 g mol−1 and a polydispersity of 2.5 was obtained from Aldrich and dried in a vacuum oven at 50 °C before use. 2,2-Bis(hydroxymethyl)propionic acid (BHP) was obtained from Beijing Ouhe Technology Co. Oxalyl dichloride, pyridine and triethylamine (TEA) were purchased from Tianjin Kewei Chemical Company. Palmitic acid and octanoic acid were obtained from Alfa Aesar. The syntheses of a generation 1 BHP dendron with two palmitate tails (D1-C16) and a generation 2 BHP dendron with four palmitate tails (D2-C16) have been published elsewhere.28 The syntheses of D1-C8 was the same as D1-C16, and the pure products were obtained under reduced pressure before removing the solid residue from a solution of ethyl acetate. Methyl orange (MO, MW = 327.3 g mol−1), eosin Y (EY, MW = 691.85 g mol−1) and fluorescein sodium salt (FS, MW = 376.27 g mol−1) were obtained from Tianjin Damao Reagent Factory. De-ionized water was double-distilled before use.2.2 Nomenclature
C16 represents the palmitic acid, and C8 represents the n-octoic acid. D1-C16/C1–C8 represents the 2,2-bis(hydroxymethyl)propionic acid (BHP)-based generation 1 dendron with two palmitate tails or octanoic tails. D2-C16 represents the 2,2-bis(hydroxymethyl)propionic acid (BHP)-based generation 2 dendron with four palmitate tails. HPEI + C16/D1-C16/D2-C16/C8/D1-C8-x represents supra-molecular complexes with hyper-branched polyethylenimine (HPEI) and C16/D1-C16/D2-C16/C8/D1-C8, and the x represents the ratio between the carboxyl groups of the acid tails and the amidogen of HPEI, namely, the degree of substitution ([COOH]/[N] ratio).2.3 Characterization
The 1H NMR spectra of the D1-C8, D1-C16, D2-C16 and corresponding composites with HPEI were obtained on a Varian INOVA 500 MHz spectrometer. UV-vis spectra were obtained on a T6 UV/Vis Spectrophotometer (Purkinje General, China). FT-IR measurements were performed on a Nicolet 5DXC FTIR spectrometer using KBr pellets. Dynamic light scattering (DLS) measurements were performed on a Malvern Nano-ZS90 instrument at 25 °C.2.4 Synthesis of a BHP-based generation 1 dendron with two octanoate tails (D1-C8)
Under the protection of nitrogen, caprylyl chloride (30.3 g, 0.186 mol) was slowly added to a solution of BHP (10.0 g, 74.6 mmol) in 40 mL of DMF–pyridine (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) solvent at 25 °C with vigorous stirring. Subsequently, the reaction was continued at 40 °C for 6 h. After the reaction finished, the reaction mixture was dissolved in 40 mL of ethyl acetate. Then the co-product pyridine hydrochloride was removed by a decompress filter, and the excess caprylyl chloride was removed by reduced pressure distillation. Yield: 96%; 1H NMR (CDCl3, δ ppm): 0.89 (t, 6H); 1.30 (br, 16H), 1.23 (s, 3H), 1.62 (m, 4H), 2.33 (t, 4H), 4.25 (m, 4H).
2) solvent at 25 °C with vigorous stirring. Subsequently, the reaction was continued at 40 °C for 6 h. After the reaction finished, the reaction mixture was dissolved in 40 mL of ethyl acetate. Then the co-product pyridine hydrochloride was removed by a decompress filter, and the excess caprylyl chloride was removed by reduced pressure distillation. Yield: 96%; 1H NMR (CDCl3, δ ppm): 0.89 (t, 6H); 1.30 (br, 16H), 1.23 (s, 3H), 1.62 (m, 4H), 2.33 (t, 4H), 4.25 (m, 4H).
D1-C16 and D2-C16 were synthesized according to previously published routes.28
2.5 Formation of supramolecular complexes
The formation of a supramolecular complex was exemplified by the HPEI + D1-C16 complex. The supramolecular complex HPEI + D1-C16 was spontaneously formed by simply mixing together the solutions of D1-C16 and HPEI in chloroform according to the target ratio of carboxylic acid to amino groups (from 0.27 to 1).2.6 Methodology for encapsulating anionic dyes using the supramolecular complex
This was exemplified using the HPEI + D1-C16 complex. A solution of HPEI + D1-C16 in chloroform ([HPEI] = 2 × 10−6 to 1 × 10−5 M) with different ratios of carboxylic acid to amino groups was added to the same volume of aqueous solution of the anionic dyes MO, EY, FS, (2.14 × 10−4 M). The solution was shaken for 10 min and kept in a dark chamber at room temperature for 3 days prior to measurment. The UV/Vis spectra of the chloroform phase were recorded. The ε values of MO, EY and FS in water and chloroform were determined according to the method reported previously28 and are shown in Table S1.† The concentration of the dyes in chloroform can be calculated according to the Beer–Lambert law. Then, the amount of the dyes transferred into the CHCl3 phase could be obtained.3. Results and discussion
3.1 Synthesis of the supermolecular nanocapsules
Amphiphilic supramolecular complexes with a core–shell structure were prepared through the assembly of carboxyl molecules onto the commercially available hyperbranched polyethylenimine polymer in chloroform solvent. In the formed supramolecular complexes, the HPEI possesses plenty of primary, secondary and tertiary amine functional groups and the fatty acid has a high carbon alkyl group, acting as the hydrophilic core and the hydrophobic shell, respectively. Herein, we chose three kinds of molecules containing carboxyl end groups to form complexes with HPEI. One kind comprised the traditional linear palmitic acid (C16) and octanoic acid (C8). The second kind comprised D1-C16 (or D1-C8) possessing two palmitate tails (or two caprylate tails) and one carboxyl group in one molecule, which has attracted much attention due to its special dendritic structures and superior properties in application compared to the traditional linear molecules. The third comprised D2-C16 which consists of four long carbon chains and one carboxyl per molecule. The neutralization reaction between the carboxyl groups of the surfactants and the amino groups of HPEI resulted in the generation of the supramolecular complexes HPEI + C16 (or C8), HPEI + D1-C16 (or D1-C8), and HPEI + D2-C16 (Scheme 1).3.2 Morphology of the supermolecular nanocapsules
FT-IR analyses of the complexes and their precursors are shown in Fig. 1. Obviously, in comparison to palmitic acid, D1-C16 and D2-C16, the corresponding complexes exhibit a new asymmetric carboxylate peak at 1569 cm−1, the peak of an ammonium radical at 1400 and 798 cm−1 along with the disappearance of an intense carboxyl absorption peak located at 1705 cm−1. Furthermore, once the carboxyl molecules are mixed with the HPEI, the amidogen peak in HPEI (3302 cm−1) disappears, and the shapes of the HPEI peaks around 3400, 2810, 1593 and 1448 cm−1 also vary due to the formation of the complex. Meanwhile, the peak at 1746 cm−1 corresponding to the ester group of D1-C16 and D2-C16 remained in HPEI + D1-C16 and HPEI + D2-C16. In addition, the complexes (HPEI + C8, HPEI + D1-C8) were also characterized by FT-IR (Fig. S1†), which demonstrates similar peak-to-peak variations with HPEI + C16 and HPEI + D1-C16. Therefore, it can be deduced from the above results that the complexes were successfully prepared through the neutralization reaction between the carboxylic acid and HPEI.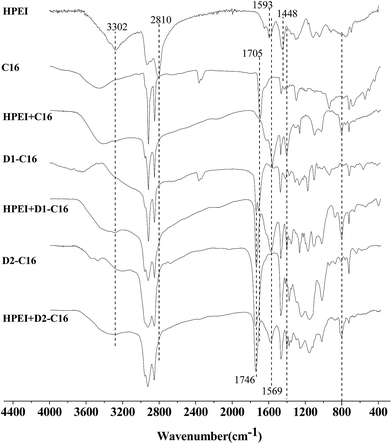 | ||
| Fig. 1 FT-IR spectra of HPEI, palmitic acid (C16), the HPEI + C16-0.54 complex, D1-C16, the HPEI + D1-C16-0.54 complex, D2-C16 and the HPEI + D2-C16-0.54 complex, 0.54 is the ratio of [COOH]/[N]. | ||
The successful formation of the supramolecular complexes is also verified by the 1H NMR spectra (Fig. 2). The HPEI signals at 1.5 ppm disappear and the ones at 2.1–2.6 ppm shift downfield after HPEI compounding with D1-C16 (or C16), owing to the influence of the formation of the electron-donating carboxylate anions. Furthermore, when D1-C16 (or C16) is mixed with HPEI, all the signals of D1-C16 (or C16) become deformed, which is ascribed to the limited rotational and diffusional mobility of the protons of D1-C16 (or C16) after the complex formation. And the complexes (HPEI + C8, HPEI + D1-C8) were also characterized by 1H NMR (Fig. S2†), which demonstrated similar phenomena with HPEI + C16 and HPEI + D1-C16. Therefore, all the above 1H NMR features additionally prove that the ionic interaction between the carboxylic acid groups of D1-C16 (or C16) and the amino groups of HPEI results in the formation of these complexes.
 | ||
| Fig. 2 1H NMR spectra of HPEI, palmitic acid (C16), the HPEI + C16-0.54 complex, D1-C16, and the HPEI + D1-C16-0.73 complex, 0.73 is the ratio of [COOH]/[N]. | ||
To further confirm the successful formation of the supramolecular complexes, we designed an experiment such that pure HPEI, HPEI + C16, HPEI + D1-C16 and HPEI + D2-C16 complexes dissolved in chloroform solutions were extracted by distilled water, and the corresponding water phase was tested by 1H NMR (Fig. S3†). It can be found that only the water phase mixed with the pure HPEI chloroform solution exhibits the characteristic peak of HPEI in the 1H NMR spectrum, which indicates that C16, D1-C16 and D2-C16 can catch hold of all the hydrophilic HPEI in the chloroform by ionic bonding. Therefore, this can also further prove the generation of the complexes.
To determine the morphology of the supermolecular nanocarriers, the average diameters of the complexes and their precursors in chloroform solution were measured by DLS. As shown in Fig. S4,† the average diameters of HPEI, C16, D1-C16, D2-C16, C8 and D1-C8 in chloroform ranged from 30 to 310 nm, which indicates that these precursors are in aggregate states. For HPEI, the formation of the supramolecular aggregates was due to hydrogen bonds among the amidogens. For C16, D1-C16, D2-C16, C8 and D1-C8, their corresponding aggregates are the result of the association among the carboxylic groups of the molecules as exemplified by D1-C16 in Scheme 2A.
However, after HPEI and carboxylic acid were mixed in chloroform, the average sizes of their corresponding complexes all unexpectedly decrease. For example, the dominant sizes of the complexes in a mixture of HPEI and D1-C16 decrease to approximately 10 nm which is far smaller than those found in pure HPEI or D1-C16 in chloroform, indicating the interaction between the HPEI and these carboxyl molecules is surely done by co-assembly. And the size of complex HPEI + D1-C16 was also tested through TEM which illustrated a coincident result with the DLS (Fig. S5A†). This shows that unimolecules are most likely formed, which once again indicates that the precursors assemble successfully onto HPEI, forming the supramolecular complexes. The amino groups of HPEI and the carboxyl group on the end of the precursor interact closely with each other and form a strong ionic linkage. Then, the previous aggregate forms of the carboxyl molecules are destroyed by the ionic bonds so that the molecules are scattered over the chloroform solution. Finally, the unimolecular base complex with two types of complementary dendritic units was formed through the ionic interaction shown in Scheme 2B. To throw further light on this phenomenon, we conducted dynamic experiments with HPEI + D1-C16 and HPEI + C16 (Fig. S6†), which showed the evolution from aggregates to this kind of unimolecule, and this further validates the above discussions. We also tested the sizes of HPEI + C16, HPEI + D1-C16 and HPEI + D2-C16 with different HPEI concentrations and [COOH]/[N] ratios. From the test results shown in Table S2,† it can be seen that HPEI + C16, HPEI + D1-C16 and HPEI + D2-C16 all show similar changes. Thus, the amphiphilic complexes with the hydrophobic aliphatic shell and hydrophilic HPEI core are obtained successfully, and are expected to display an inverted micellar behavior.
Surprisingly, when the complex chloroform solution interacts fully with pure water or MO aqueous solution by extraction technology, it is detected that the average diameter of these complexes in the organic phase undergoes a large increase to more than 1000 nm (Fig. 3c and d), which indicates that the unimolecular base complexes obviously turn into large aggregates. And the morphology of the HPEI + D1-C16 aggregates was also tested by TEM which indicated a coincident result with the DLS (Fig. S5B and S5C†). As shown in Fig. 3e, the diameter of the complex extracted by an MO solid is 362 nm. So it indicates that the cause of complex aggregation is not only solid dyes17 but also water or dye solutions, and the latter could initiate much larger aggregates which are expected to load more guests and are our main object of study in this paper. Furthermore, after contact with water or MO solutions, the diameters of HPEI + C16, HPEI + D1-C16 and HPEI + D2-C16 with different HPEI concentrations and [COOH]/[N] ratios are all obviously increased, too (Table S2†). Why do the unimolecular base complexes turn into aggregates after the extraction with water or MO aqueous solution? We tentatively explain this change as follows: when the complex chloroform solution is extracted with water, the hydrophilic amidogen groups of the HPEI core would contact with water molecules, and they are linked together by hydrogen bonds. So it forces the configuration of the complex to change and the HPEI core is exposed with more chances for amidogen interaction with water.
In addition, the following experimental results concerning dye encapsulation capacity proved again the correctness of our above analysis. Compared with the complexes in this paper, our previous work has found that the corresponding covalent core–shell amphiphilic compounds PD2-1, PD1-1, PD1-2, PD1-3, PL-1, PL-2 and PL-3 display a unimolecular morphology,28 and can be used as nanocapsules whose dye encapsulation capacity is unaffected by the concentration of polymers (Fig. S7B†). In addition, it has been reported that if the plot of dye concentration in organic solution versus polymer concentration is linear and passes through the origin (Fig. S7A†), the nanocarrier would be of a unimolecular morphology.29 However, in HPEI + C16, HPEI + D1-C16 or HPEI + D2-C16 nanocarrier systems, the amount of MO transferred to the organic phase increased nonlinearly when the concentration of HPEI increased from 2 × 10−6 mol L−1 to 1 × 10−5 mol L−1 with the [COOH]/[N] ratio in chloroform fixed at 0.54 (Fig. 4A). The encapsulation capacity of the hyperbranched complex, i.e. the number of MO molecules encapsulated by each HPEI molecule of the nanocarrier, increased firstly and then decreased as the concentration of the HPEI increased (Fig. 4B). And these phenomena also appeared in a similar way when the ratio of [COOH]/[N] was 0.27, 0.73 and 1. Therefore, this experimental rule is different to the previous unimolecular encapsulation, which could offer another perspective on the aggregation encapsulation. Consequently, this adequately implies that after contacting with aqueous solution, all the nanocarriers exist as aggregates but not as unimolecular micelles within the experimental concentration range.28,29
3.3 The study of encapsulation performance
The aggregates of the supramolecular complexes with a hydrophilic core and hydrophobic shell are expected to display the behavior of inverted micelle aggregates, which are able to accommodate hydrophilic guest molecules in the HPEI core due to their similar polarity, leading to the hydrophilic guest molecules being dissolved in their poor solvents. Therefore, the classic and water soluble anionic dyes methyl orange (MO), eosin Y (EY) and fluorescein sodium (FS) were used as the guests to investigate the encapsulation behaviours of the supramolecular complexes by the water–chloroform biphasic extraction technique (Fig. S8†). After contact with the mixture of HPEI and C16, D1-C16 or D2-C16, the MO, EY and FS dye molecules could be efficiently transferred into the chloroform phase. The UV/Vis spectra show that the surface plasmon resonance peaks of MO, EY and FS undergo a blue shift to 420, 536 and 502 nm in the chloroform phase compared with 465, 512 and 487 nm in water (Fig. S9†), indicating that a new environment for the MO, EY and FS guests is created in the supramolecular nanocarriers. It is noted additionally, that neither HPEI nor C16, D1-C16 or D2-C16 alone present similar phase-transfer behavior. Therefore, the guest encapsulation behaviors of these amphiphilic complexes with different shell branching as nanocarriers were comparatively studied.For accurately measuring the guest encapsulation capacity of the obtained complexes, the following factors were considered. Firstly, for all the encapsulation experiments, the two phase mixtures were equilibrated at room temperature for at least one day before the UV-vis spectrometry measurement. Secondly, for an effective comparison of the different nanocarriers, the concentrations of dyes used were high enough to make sure that the encapsulation capacity of the different nanocarriers was close to the maximal value.
The primary reason for this phenomenon can be explained as follows. Firstly, with the increased HPEI concentration, the aggregates formed and grew larger and larger and their ability for encapsulation became higher and higher (Scheme S1A–C†) which corresponds to the first portion. Secondly, when the HPEI concentration reached a certain level (e.g. 5 × 10−6 mol L−1 of HPEI + D1-C16-0.54), the sizes of most aggregates grew to their maximum which were unaffected by the addition of more complexes (Scheme S1-D†) corresponding to the second portion. This changing law has been proved by the size testing results of the complex of organic solutions after contact with the MO solution (Fig. 5): when the HPEI concentration of the HPEI + D1-C16-0.54 complex is less than 5 × 10−6 mol L−1, the main diameters of the aggregates gradually increase as the concentration increases; and when higher than 5 × 10−6 mol L−1, the corresponding diameters stay nearly unchanged. Therefore, the concentration of HPEI, at which the size of the aggregate grows to maximum, is called the saturated concentration. And 5 × 10−6 mol L−1 is the saturated concentration of HPEI + D1-C16-0.54.
It is reported that large self-assembly is conducive to dye loading,17 so in the first portion of Fig. 4B, the complex capability for dye loading increased with the increase in size of the aggregates, when the concentration of HPEI was increased. As the HPEI reaches a saturated concentration, the diameter of the aggregates reaches a stable maximum and the number of MO molecules encapsulated by each HPEI molecule of the nanocarrier is maximised under this condition. In the second portion of Fig. 4B, the number of dye molecules encapsulated by each HPEI molecule of the complex decreased when the HPEI concentration was higher than its saturated concentration (e.g. 5 × 10−6 mol L−1 of the HPEI + D1-C16-0.54). This is because more complexes could no longer form larger aggregates but only some other small aggregates or unimolecules at these high concentrations. As the experimental results in Fig. 5 show, an additional small peak appeared in the DLS measurement when the concentration was higher than the saturated concentration. Therefore, the average number of dye molecules encapsulated by the complexes definitely decreased due to the system containing some small aggregates or unimolecules with a weak encapsulation ability. So this further proved the influence of HPEI concentration on the aggregation encapsulation. The consistency between the dye loading test and the measurement of complex diameter has commendably validated our conclusion.
In addition, we performed the encapsulation experiments and DLS measurements not only for HPEI + D1-C16-0.54, but also for HPEI + C16, HPEI + D1-C16, HPEI + D2-C16 with different ratios of [COOH]/[N] i.e. 0.27, 0.54, 0.73 and 1.0 (e.g.Fig. 4 and S10, Table S2†), and similar results were obtained. It was found that the different complexes with different [COOH]/[N] ratios all show a saturated concentration (Table S3†) which is a particular property of super-molecule complexes which differs from covalent polymers.28
We also tested the encapsulation ability and size distribution of HPEI + C16-0.54, HPEI + D1-C16-0.54 and HPEI + D2-C16-0.54 with different concentrations of HPEI after the complex solutions were equilibrated for 60 days, and they produced roughly the same results as the complexes that were equilibrated for 3 days (Fig. S11, S12† and 5). The results show that all these supramolecular aggregates have high stabilities because the hydrogen bonds of HPEI allow the creation and stabilization of the aggregates in solution. Besides, the presence of long chain fatty acids also plays an important role for the stabilization of aggregates.
 | ||
| Fig. 6 Effect of [COOH]/[N] ratio on the encapsulation capacity under a fixed concentration, where the concentration of HPEI is 5 × 10−6 M. | ||
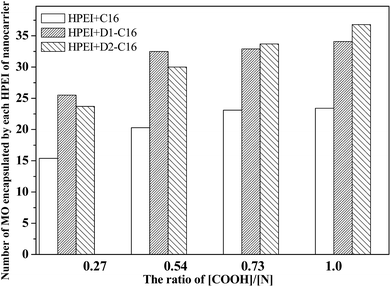 | ||
| Fig. 8 Number of MO molecules encapsulated by each HPEI molecule of the HPEI + C16, HPEI + D1-C16 and HPEI + D2-C16 complexes at their respective saturated concentrations. | ||
Moreover, the branched shells influence not only the loading capability of dyes, but also the resistance to emulsification. Fig. S13† shows the emulsification of different complexes under the same conditions. It can be seen that the anti-emulsifying effect of the complexes with a dendritic shell is obviously better, which can be explained tentatively as follows: the carbon chain density of complexes with branched shells is larger and the configuration of the complex is hard to invert, decreasing the chance of interaction with water. It once again shows the advantages of complexes with dendritic shells, relative to the ones with linear shells.
4. Conclusion
In this work, these new supramolecular complexes were successfully prepared through the noncovalent interaction between hyperbranched PEI and dendritic D1-C16, D1-C8 or D2-C16. Interestingly, after extraction with water or MO solution, the diameter of the complex unexpectedly increased and the complexes formed large aggregates which could encapsulate polar dyes including MO, EY and FS. The guests are accommodated in the hydrophilic HPEI core and stabilized in the non-polar solution by the long carbon chains of hydrophobic D1-C16, D1-C8 or D2-C16. Importantly, the aggregate morphology and encapsulation behavior of these complexes are fundamentally different from the simple inverted micelles and unimolecular transporter systems reported.7,10,13,21,28 The encapsulation capacities of the aggregates reached a maximal value with an increase in HPEI concentration and at a certain density of carbon chains (i.e. at saturated concentration and saturated ratio, respectively). Furthermore, the comparative experiments of HPEI + D2-C16, HPEI + D1-C16 and HPEI + C16 indicate that the complexes with dendritic shells are superior over the complex with linear shells in the matter of aggregating encapsulation capacity and the anti-emulsifying effect. And it only required a smaller dosage of the complexes to achieve a higher encapsulation ability, which is meaningful for practical applications. Besides, when comparing HPEI + D1-C16 and HPEI + D1-C8, the increase of the length of the shell could distinctly improve the encapsulation ability because of the stronger hydrophobic properties and flexibility of the sixteen carbon chains. Consequently, this new supramolecular carrier system which is conveniently prepared can realize guest transport from the water phase to the organic phase, which has great potential for environmental purification, biomedical applications and so on.Acknowledgements
This work was financially supported by the National Science Foundation of China (21304043, 51403097), the Natural Science Foundation of Shandong Province (ZR2012BQ024, 2014ZRB019WZ) and Natural Science Foundation of Ludong University (LY2012003, LY2013010).References
- L. J. Twyman, A. S. H. King and I. K. Martin, Chem. Soc. Rev., 2002, 31, 69–82 RSC.
- R. Haag, Angew. Chem., Int. Ed., 2004, 43, 278–282 CrossRef CAS PubMed.
- J. L. Chavez, J. L. Wong, A. V. Jovanovic, E. K. Sinner and R. S. Duran, IEE Proc.: Nanobiotechnol., 2005, 152, 73–84 CrossRef CAS.
- M. Irfan and M. Seiler, Ind. Eng. Chem. Res., 2010, 49, 1169–1196 CrossRef CAS.
- G. Barratt, Cell. Mol. Life Sci., 2003, 60, 21–37 CrossRef CAS.
- K. R. Kumar and D. E. Brooks, Macromol. Rapid Commun., 2005, 26, 155–159 CrossRef CAS PubMed.
- Y. Chen, Z. Shen, L. Pastor-Pérez, H. Frey and S.-E. Stiriba, Macromolecules, 2005, 38, 227–229 CrossRef CAS.
- H. Liu, Y. Chen, D. Zhu, Z. Shen and S.-E. Stiriba, React. Funct. Polym., 2007, 67, 383–395 CrossRef CAS PubMed.
- R. K. Kainthan and D. E. Brooks, Bioconjugate Chem., 2008, 19, 2231–2238 CrossRef CAS PubMed.
- M. Krämer, J. F. Stumbé, H. Türk, S. Krause, A. Komp, L. Delineau, S. Prokhorova, H. Kautz and R. Haag, Angew. Chem., Int. Ed., 2002, 41, 4252–4256 CrossRef.
- D. Wan, H. Pu and X. Cai, Macromolecules, 2008, 41, 7787–7789 CrossRef CAS.
- S. E. Stiriba, H. Kautz and H. Frey, J. Am. Chem. Soc., 2002, 124, 9698–9699 CrossRef CAS PubMed.
- D. Wan, J. Yuan and H. Pu, Macromolecules, 2009, 42, 1533–1540 CrossRef CAS.
- D. Wan, G. Wang, H. Pu and M. Jin, Macromolecules, 2009, 42, 6448–6456 CrossRef CAS.
- D. Wan, H. Pu, M. Jin, H. Pan and Z. Chang, React. Funct. Polym., 2010, 70, 916–922 CrossRef CAS PubMed.
- M. Radowski, A. Shukla, H. Berlepsch, C. Bottcher, G. Pickaert, H. Rehage and R. Haag, Angew. Chem., Int. Ed., 2007, 46, 1265–1269 CrossRef CAS PubMed.
- E. Fleige, B. Ziem, M. Grabolle, R. Haag and U. Resch-Genger, Macromolecules, 2012, 45, 9452–9459 CrossRef CAS.
- X. Yan, B. Jiang, T. R. Cook, Y. Zhang, J. Li, Y. Yu, F. Huang, H.-B. Yang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 16813–16816 CrossRef CAS PubMed.
- X. Yan, S. Li, T. R. Cook, X. Ji, Y. Yao, J. B. Pollock, Y. Shi, G. Yu, J. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 14036–14039 CrossRef CAS PubMed.
- L. A. Paquette, K. D. Combrink, S. W. Elmore and R. D. Rogers, J. Am. Chem. Soc., 1991, 113, 1335–1344 CrossRef CAS.
- S. Stevelmans, J. C. M. van Hest, J. F. G. A. Jansen, D. A. F. J. van Boxtel, E. M. M. de Brabander-van den Berg and E. W. Meijer, J. Am. Chem. Soc., 1996, 118, 7398–7399 CrossRef CAS.
- M. W. P. L. Baars, E. W. Meijer and P. E. Froehling, Chem. Commun., 1997, 1959–1960 RSC.
- A. I. Cooper, J. D. Londono, G. Wignall, J. B. McClain, E. T. Samulski, J. S. Lin, A. Dobrynin, M. Rubinstein, A. L. C. Burke, J. M. J. Frechet and J. M. DeSimone, Nature, 1997, 389, 368–371 CrossRef CAS.
- V. Chechik, M. Zhao and R. M. Crooks, J. Am. Chem. Soc., 1999, 121, 4910–4911 CrossRef CAS.
- Y. Chen, Z. Shen and H. Frey, Chem. Commun., 2005, 38, 755–757 RSC.
- D. Chun, F. Wudl and A. Nelson, Macromolecules, 2007, 40, 1782–1785 CrossRef CAS.
- X. L. Lou, F. Cheng, P. F. Cao, Q. Tang, H. J. Liu and Y. Chen, Chem.–Eur. J., 2009, 15, 11566–11572 CrossRef CAS PubMed.
- Y. Liu, Y. Fan, x.-y. Liu, s.-z. Jiang, Y. Yuan, Y. Chen, F. Cheng and s.-c. Jiang, Soft Matter, 2012, 8, 8361–8369 RSC.
- D. Wan, F. Chen, T. Kakuchi and T. Satoh, Polymer, 2011, 52, 3405–3412 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17836c |
| This journal is © The Royal Society of Chemistry 2015 |