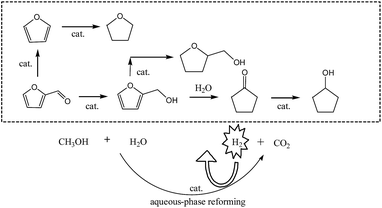
In situ hydrogenation of furfural with additives over a RANEY® Ni catalyst
Ying
Xu
a,
Songbai
Qiu
a,
Jinxing
Long
a,
Chenguang
Wang
a,
Jiamin
Chang
ab,
Jin
Tan
a,
Qiying
Liu
a,
Longlong
Ma
a,
Tiejun
Wang
a and
Qi
Zhang
*a
aKey Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, CAS, Guangzhou 510640, China. E-mail: zhangqi@ms.giec.ac.cn
bFaculty of Environmental Science and Engineering & State Key Lab of International Combustion Engine, Tianjin University, Tianjin 300072, China
First published on 15th October 2015
Abstract
The hydrogenation of furfural was studied over a RANEY® Ni catalyst (RN) under a N2 atmosphere in water. Methanol, as a hydrogen donor, was used for hydrogenation via a reforming reaction. Additives, including acetone, acetic acid and phenol, were deliberately added in the in situ hydrogenation process of furfural to investigate the effect and interaction between two kinds of compounds. The results showed that the conversion of furfural decreased to some extent and the product distribution changed a lot because of second additives. Furfuryl alcohol (FA) was detected in the products in the presence of additives and was not detected when furfural was a single reactant. The selectivity of FA reached its highest degree of 19.01% with the addition of acetic acid. The addition of acetone promoted the decarboxylation reaction of furfural, and the selectivity of tetrahydrofurfuryl alcohol increased from 24.53% to 38.07%. The addition of phenol enhanced the rearrangement reaction of furfural and the selectivity of the five-membered ring increased from 47.99% to 88.76%. Compared with the in situ hydrogenation of the single reactant, the conversion of acetone increased and the conversion of acetic acid and phenol decreased in the presence of furfural. The reaction pathway of the hydrogenation of furfural is also discussed in this paper.
Introduction
Fast pyrolysis is a kind of promising technology for the conversion of biomass into liquid fuels due to its low capital and operating cost advantages.1,2 But the properties of bio-oil, the liquid product of biomass fast pyrolysis, result in multiple significant problems during the utilization and storage of bio-oil because it is chemically and thermally unstable.3 Therefore, the transformations of bio-oil into useful chemicals or stable compounds have attracted much attention.4 Hydrotreatment in the aqueous phase is one of the potential methods to upgrade bio-oil.5,6 Acetone,7 acetic acid,6,8 furfural,9,10 and phenol11 and its derivatives have been chosen as model compounds to investigate the activity of different catalysts and the mechanism for upgrading bio-oil. But the mechanism of the model compounds cannot totally explain the pathway for compound conversion, and the catalysts are less active in raw bio-oil. Investigations on the reasons for this are still scarce.Furfural, as a key platform molecule in biomass conversion, is one of the main components in bio-oil.12,13 The products of furfural from the hydrogenation process are complex in nature. Zhou14 investigated the transformation of furfural to cyclopentanol over Ni/CNT catalysts. Over 30 wt% Ni/CNTs, the conversion of furfural was up to 96.5% with a yield of 83.6% toward cyclopentanol. Guo15 has developed a new catalytic system of CuZnAl for the selective conversion of furfural to cyclopentanone. CuZnAl-500-0.5 was recycled five times and maintained a good activity and stability. Villaverde16 studied the liquid-phase transfer hydrogenation of furfural on Cu-based catalysts. With Cu–Mg–Al, after 6 h at 150 °C, furfural could be converted to furfuryl alcohol completely. Yang9 pointed out that in the conversion of furfural into cyclopentanone over Ni–Cu bimetallic catalysts, furfuryl alcohol, 4-hydroxy-2-cyclopentenone and 2-cyclopentenone were verified to be three key intermediates. The rearrangement of the furan ring was closely related to the attack of a H2O molecule on the 5-position of furfuryl alcohol. In view of high-pressure H2 being used in furfural hydrogenolysis, Paraskevi used alcohols as hydrogen donors in methyl furan production through catalytic transfer hydrogenation of furfural in the liquid phase.17 From the reports on furfural hydrogenation, most of the research performed catalytic hydrogenation to yield different products, but few studies have focussed on the effect of additives on the product distribution during the upgrading process of raw bio-oil.18–21
The present work highlights the in situ hydrogenation of furfural over RN. In addition, the effect of different kinds of compounds on the product distribution in bio-oil is also presented. This study is motivated in part by our recent work.22 The additives included acetone, acetic acid and phenol. The comparison of the hydrogenation vs. in situ hydrogenation, and the hydrogenation pathway of furfural are also discussed in this paper.
Results and discussion
Effect of the reaction conditions on the conversion of furfural and product distribution
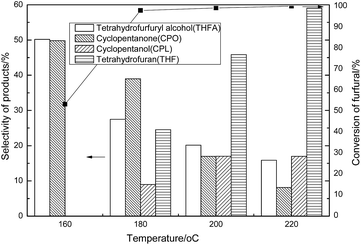
When the reaction temperature was 160 °C, the furfural conversion degree was only 53.11%. But after the temperature reached 180 °C, the conversion of furfural increased to above 90%. With the temperature increasing, the selectivity of tetrahydrofurfuryl alcohol (THFA) and cyclopentanone (CPO) decreased gradually. Correspondingly, cyclopentanol (CPL) and tetrahydrofuran (THF) formation commenced at around 180 °C and the selectivities increased rapidly to 16.96% and 59.03% at 220 °C. More aldehyde groups were cut from furfural molecules when the reaction temperature increased. The falling off of the side chain accounted for the decreasing selectivity of CPO and CPL. On the basis of Fig. 1, it was concluded that the in situ hydrogenation of furfural was favored at 180 °C, leading to high furfural conversion and less carbon loss. In summary, a low temperature was favored for the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, leading to the higher THFA and 5-membered carbon ring compound selectivity, while high temperatures were favored for the cleavage of the aldehyde group from the furan ring, resulting in the higher selectivity of THF.
O, leading to the higher THFA and 5-membered carbon ring compound selectivity, while high temperatures were favored for the cleavage of the aldehyde group from the furan ring, resulting in the higher selectivity of THF.
Furfural conversion increased from 51.04 to 97.79% when the N2 initial pressure increased from 0.1 MPa to 1 MPa. With the initial pressure increasing from 1 MPa to 3 MPa, the conversion of furfural changed little. The product distribution profiles indicated that the THFA and CPO selectivity decreased with increasing N2 initial pressure. In contrast, the CPL selectivity remained low when the initial pressure was below 1 MPa and increased significantly after the pressure increased to 2 MPa. The formation of THF commenced at the initial pressure of 1 MPa and the selectivity increased significantly with increasing initial pressure. The obvious changes might be ascribed to the APR of methanol. From our previous research,22 the conversion of methanol and the selectivity of H2 were affected by the initial pressure. With the initial pressure increasing, the conversion of methanol and the yield of H2 increased. Meanwhile, the solubility of H2 in the solvent also increased, which could have promoted the hydrogenation reaction that happened.
The coupling of APR of methanol and in situ hydrogenation of furfural
In view of hydrogen being produced from the APR of methanol, the coupling of APR of methanol and in situ hydrogenation of furfural was investigated. The conversion of methanol and the product distribution under the optimum conditions with and without furfural are shown in Table 1. From Table 1, the hydrogenation of the substrate could promote the APR of methanol, and the conversion of methanol increased from 18.18% to 25.38%. In the gas product from furfural in situ hydrogenation under the optimum conditions, 2.90% H2 was left over.| C (%) | Content (%) | ||||
|---|---|---|---|---|---|
| H2 | CnHm | CO2 | CO | N2 | |
| a APR of methanol without furfural. b APR of methanol with furfural. c Reaction conditions: 0.5 g of RN, 30 mL of water, 0.2 mol of methanol, 0.04 mol of furfural, temperature of 180 °C, N2 pressure of 1 MPa, and batch reaction time of 4 h. | |||||
| 18.18a | 42.05 | 5.81 | 21.78 | — | 30.36 |
| 25.38b | 2.90 | 9.44 | 29.40 | 0.11 | 58.15 |
Hydrogenation of a mixed feed of furfural and a second additive
Experiments were performed over RN at 180 °C and 1 MPa to investigate the in situ hydrogenation of a mixed feed of furfural and acetone, furfural and acetic acid, and furfural and phenol. For comparison, the reactions with furfural, acetone, acetic acid and phenol as the single reactants were conducted under the same identical reaction conditions.Conversion of the second additives and product distribution
The conversion of the second additives and the product distribution were different from those obtained with a single reactant (Table 2). As a single reactant, acetone showed a conversion of 42.50%, while the conversion degree increased to 72.68% upon hydrogenation with furfural. Isopropanol was the main product whose selectivity was above 96% in both of the in situ hydrogenation processes. The improvement of the conversion of acetone might be attributed to the addition of furfural. In our previous work,22 acetone was not found and isopropanol was detected in the upgraded bio-oil upon in situ hydrogenation. Acetic acid, as a single reactant or as a second additive to furfural, was reacted with methanol via esterification, and was then hydrogenated to ethanol over RN. The ester product was only methyl acetate which was different from the results of one-step hydrogenation–esterification of furfural and acetic acid.23 Because of the abundance of methanol as the solvent and hydrogen donor at the beginning of the reaction, the esterification reaction between acetic acid and methanol was the dominant reaction. As second additives, acetic acid and phenol showed conversions of 40.84% and 36.50%, while, as sole reactants, their conversions were 52.35% and 46.01%. The conversions of acetic acid and phenol were suppressed in the presence of furfural in the mixed feed.24 There was no reaction happening between the second additives and furfural in the in situ hydrogenation system.| Reactant | Con. (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|
| a Single reactant. b As a second additive added to furfural. c Reaction conditions: 0.5 g of RNs, 30 mL of water, 0.2 mol of methanol, 0.04 mol of furfural, 0.02 mol of second additive, temperature of 180 °C, N2 pressure of 1 MPa, and batch reaction time of 4 h. | |||||||
| Acetonea | 42.50 | 98.23 | 1.77 | — | — | — | — |
| Acetoneb | 72.68 | 96.75 | 3.25 | — | — | — | — |
| Acetic acida | 52.35 | — | — | — | — | 83.01 | 16.99 |
| Acetic acidb | 40.84 | — | — | — | — | 89.11 | 10.89 |
| Phenola | 46.01 | — | — | 46.67 | 53.33 | — | — |
| Phenolb | 36.50 | — | — | 82.65 | 17.35 | — | — |
Conversion of furfural and product distribution with second additive
As the single reactant, furfural displayed a conversion as high as 97.79%, while in the mixed feed systems, all of the furfural conversions decreased to some extent (Table 3).| Additive | Con. (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| a Reaction conditions: 0.5 g of RNs, 0.04 mol of furfural, 0.02 mol of second additive, 30 mL of water, 0.2 mol of methanol, temperature of 180 °C, N2 pressure of 1 MPa, and batch reaction time of 4 h. | ||||||
| — | 97.79 | 8.98 | 39.01 | 24.53 | 27.48 | — |
| Acetone | 87.63 | 19.66 | 14.96 | 38.07 | 18.88 | 8.43 |
| Acetic acid | 86.94 | 16.73 | 21.19 | 27.29 | 15.78 | 19.01 |
| Phenol | 94.40 | 79.75 | 9.21 | 0 | 5.63 | 5.41 |
It is interesting to note that, with acetone, acetic acid and phenol addition to furfural, the product distribution changed a lot. FA, which could not be detected in the product from furfural as single reactant, was in the products of furfural in presence of second addition. The selectivity of FA reached the highest point (19.01%) with acetic acid as the coreactant. The CPL selectivity increased to some extent in the presence of acetone and acetic acid. Meanwhile, the rearrangement of the five-membered carbon ring of furfural was significantly improved in the presence of phenol. The total selectivity of CPO and CPL increased from 47.99% to 88.96%, and the selectivity of CPL increased from 8.98% to 79.75%. The THFA selectivity decreased from 27.48% to 18.88% and 15.78% in the presence of acetone and acetic acid, and could barely be detected when the coreactant was phenol.
Comparison of furfural in situ hydrogenation with hydrogenation in a hydrogen atmosphere
The comparison between the in situ hydrogenation and hydrogenation in a H2 atmosphere was conducted under identical reaction conditions (Fig. 3).In the in situ hydrogenation, the conversion of furfural was 97.79%, while in water in a H2 atmosphere, a nearly total conversion (98.51%) was also observed. In water and methanol, the products of the in situ hydrogenation of furfural included CPO, CPL, THFA and THF and there was no THF detected in the H2 atmosphere. Meanwhile, the selectivities of THFA and CPO in the hydrogenation were higher than those in the in situ hydrogenation. The selectivity of THFA decreased from 27.48% to 10.26%. The total selectivity of CPL and CPO in the hydrogenation increased from 47.99% to 89.74% compared with in the in situ hydrogenation, which means that in the absence of methanol, the main reaction pathway is the rearrangement of furfural and furfuryl alcohol to cyclopentanone in water as the solvent under a H2 atmosphere.9 The effect on the rearrangement of the furan ring in the presence of methanol needs further investigation.
In situ hydrogenation of furfural with a recycled RANEY® Ni catalyst
The comparison between the in situ hydrogenation and hydrogenation in a H2 atmosphere was conducted under identical reaction conditions. The RANEY® Ni catalyst can be recycled through filtration and washed with methanol. 70–80% catalyst could be recycled for the next use, but the activity of the catalyst is a little lower than the fresh one.Reaction pathway of the in situ hydrogenation of furfural
For a better understanding of the reaction pathway, the in situ hydrogenation of FA and THFA were conducted under identical reaction conditions (Table 4).| Reactant | Con. (%) | Selectivity (%) | ||
|---|---|---|---|---|
| a 0.5 g of RN, 0.04 mol of FA, 30 mL of water, 0.2 mol of methanol, temperature of 180 °C, N2 pressure of 1 MPa, and batch reaction time of 4 h. | ||||

|
64.18 | 27.07 | 25.57 | 47.36 |

|
0 | — | — | — |
As shown in Table 4, when FA was the reactant, CPO, CPL and THFA were detected in the products but not THF. When THFA was chosen as the reactant, there was no reaction happening over THFA. This suggested that CPO, CPL and THFA were all formed from FA. When the five-membered carbon ring was formed from furfural and FA, a rearrangement reaction was inevitable during the in situ hydrogenation process. That means that FA was one of the key intermediates to form five-membered carbon ring products.10 And also, once the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O happened, leading to C–O, furan was not easy to form, which showed that the formation of FA and furan were parallel reactions. In summary, in the in situ hydrogenation system, there were several parallel and cascade reactions, including decarboxylation, hydrogenation of C
O happened, leading to C–O, furan was not easy to form, which showed that the formation of FA and furan were parallel reactions. In summary, in the in situ hydrogenation system, there were several parallel and cascade reactions, including decarboxylation, hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and ring opening/closing (Fig. 4).
C bonds and ring opening/closing (Fig. 4).
Experimental
Materials
Methanol (≥99.5%, analytical reagent), furfural (≥99.5%, analytical reagent) and furfuryl alcohol (≥99.5%, analytical reagent) were purchased from Beijing Hengzhang Chemical Co Ltd. Acetone (≥99.5%, analytical reagent), acetic acid (≥99.5%, analytical reagent), phenol (≥99.5%, analytical reagent) and tetrahydrofurfuryl alcohol (≥99.5%, analytical reagent) were purchased from Tianjin Fuchen Chemical Reagents Factory.Preparation of catalysts22
The Ni–Al alloy powder, a commercial product supplied by Dalian Toyounger Chemical Co. Ltd, was slowly added to a 20% aqueous solution of NaOH at 50(±2) °C. The solution was precipitated after 1.5 h of magnetic stirring at a temperature of 50 °C. Actually, Al is oxidized and removed from the alloy; then almost pure Ni is obtained. The solid phase was washed with distilled water until the pH reached 8–9 and then with ethanol 6 times. The prepared RN was stored in ethanol.In situ hydrogenation process
In situ hydrogenation of furfural was performed in a 100 mL stainless autoclave in the presence of 0.5 g of RN. The experimental apparatus is similar to the one previously reported.22 In a typical experiment, water (30 mL), methanol (0.2 mol) and furfural (0.04 mol) were loaded into the reactor, which was sealed and purged 3 times with N2 to exclude air. An automatic controller was used to control the temperature and the revolution of the stirrer. The pressure was raised to 0.1–3 MPa and the reaction temperature was set at 160–220 °C for 4 h. After reaction, the autoclave was cooled down to room temperature and then the liquid products were sampled for GC analysis. The second additive (0.02 mol), including acetone, acetic acid and phenol, was added in the in situ hydrogenation process separately.Products analysis
The liquid phase samples were analyzed using a Shimadzu model GC-2014 using a HP-INNOWAX column (30 m × 0.25 mm × 0.25 μm) and a flame ionization detector (FID). The conversion of the reactants (mol%) and the selectivity (mol%) of the main products were calculated according to eqn (1) and (2) using the moles of the reactants and products before and after the in situ hydrogenation, from the GC results. | (1) |
 | (2) |
Conclusions
The conclusions are drawn as follows: (1) over RN, the hydrogenation of furfural could be coupled with APR of methanol. (2) Acetone, acetic acid and phenol had a significant effect on the product distribution from furfural. In the presence of additives, FA was found in the products and FA was not detected when furfural was a single reactant. In the presence of phenol, the main reaction pathway was the rearrangement of furfural to CPL & CPO and the selectivity of five-membered carbon rings was 88.96%. (3) In the in situ hydrogenation of furfural process, there were several parallel and cascade reactions, including decarboxylation, hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and ring opening/closing. (4) In view of the high content of oxygen and water, converting raw bio-oil into stable oxygenated fuel might be one of the potential methods to replace petroleum in the production of fuels for the transportation sector.
C bonds and ring opening/closing. (4) In view of the high content of oxygen and water, converting raw bio-oil into stable oxygenated fuel might be one of the potential methods to replace petroleum in the production of fuels for the transportation sector.
Acknowledgements
This project was supported by the Science and Technology Plan Key Project in Guangdong Province of China (Project No. 2014A010106019), Chinese Academy of Sciences (Project No. KGZD-EW-304-3), Science and Technology Plan Key Project in Dongguan City of China (Project No. 201208101005) and Foundation of Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences (Project No. y307r91001).Notes and references
- A. R. K. Gollakota, M. D. Subramanyam and N. Kishore,
et al., CFD simulations on the effect of catalysts on the hydrodeoxygenation of bio-oil, RSC Adv., 2015, 5(52), 41855–41866 RSC
.
- A. V. Bridgwater, Review of fast pyrolysis of biomass and product upgrading, Biomass Bioenergy, 2012, 38, 68–94 CrossRef CAS PubMed
.
- L. Nie and D. E. Resasco, Kinetics and mechanism of m-cresol hydrodeoxygenation on a Pt/SiO2 catalyst, J. Catal., 2014, 317, 22–29 CrossRef CAS PubMed
.
- Y. S. Fan, Y. X. Cai and X. H. Li,
et al., Catalytic upgrading of pyrolytic vapors from the vacuum pyrolysis of rape straw over nanocrystalline HZSM-5 zeolite in a two-stage fixed-bed reactor, J. Anal. Appl. Pyrolysis, 2014, 108, 185–195 CrossRef CAS PubMed
.
- D. M. Zhang, F. Y. Ye and T. Xue,
et al., Transfer hydrogenation of phenol on supported Pd catalysts using formic acid as an alternative hydrogen source, Catal. Today, 2014, 234, 133–138 CrossRef CAS PubMed
.
- X. B. Li, S. R. Wang and Y. Y. Zhu,
et al., DFT study of bio-oil decomposition mechanism on a Co stepped surface: acetic acid as a model compound, Int. J. Hydrogen Energy, 2015, 40(1), 330–339 CrossRef CAS PubMed
.
- H. Chen, L. Zhao and S. Z. Li,
et al., Effects of water on the hydrogenation of acetone over Ni/MgAlO catalysts, Chin. J. Catal., 2015, 36(3), 380–388 CrossRef CAS
.
- Y. Xu, T. J. Wand and L. L. Ma,
et al., Upgrading of fast pyrolysis liquid fuel from biomass over Ru/Al2O3 catalyst, Energy Convers. Manage., 2012, 55, 172–177 CrossRef PubMed
.
- Y. L. Yang, Z. T. Du and Y. Z. Huang,
et al., Conversion of furfural into cyclopentanone over Ni–Cu bimetallic catalyst, Green Chem., 2013, 15, 1932–1940 RSC
.
- M. H. Zhou, G. M. Xiao and H. Y. Zhu,
et al., Catalytic Hydroprocessing of Furfural to Cyclopentanol Over Ni/CNTs Catalysts: Model Reaction for Upgrading of Bio-oil, Catal. Lett., 2014, 144, 235–241 CrossRef CAS
.
- P. M. Mortensen, J. D. Grunwaldt and P. A. Jensen,
et al., Screening of catalysts for hydrodeoxygenation of phenol as a model compound for bio-oil, ACS Catal., 2013, 3(8), 1774–1785 CrossRef CAS
.
- R. Trane-Restrup and A. D. Jensen, Steam reforming of cyclic model compounds of bio-oil over Ni-based catalysts: product distribution and carbon formation, Appl. Catal., B, 2015, 165, 117–127 CrossRef CAS PubMed
.
- X. L. Li, J. Deng and J. Shi,
et al., Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu–Co catalysts, Green Chem., 2015, 17(2), 1038–1046 RSC
.
- M. H. Zhou, G. M. Xiao and H. Y. Zhu,
et al., Catalytic Hydroprocessing of Furfural to Cyclopentanol Over Ni/CNTs Catalysts: Model Reaction for Upgrading of Bio-oil, Catal. Lett., 2014, 144, 235–241 CrossRef CAS
.
- J. H. Guo, G. Y. Xu and Z. Han, Selective Conversion of Furfural to Cyclopentanone with CuZnAl Catalysts, ACS Sustainable Chem. Eng., 2014, 2(10), 2259–2266 CrossRef CAS
.
- M. M. Villaverde, T. F. Garetto and A. J. Marchi, Liquid-phase transfer hydrogenation of furfural to furfuryl alcohol on Cu–Mg–Al catalysts, Catal. Commun., 2015, 58, 6–10 CrossRef CAS PubMed
.
- P. Paraskevi, M. Nicklas and G. V. Dionisios, Effect of hydrogen donor on liquid phase catalytic transfer hydrogenation of furfural over a Ru/RuO2/C catalyst, J. Mol. Catal. A: Chem., 2014, 392, 223–228 CrossRef PubMed
.
- Q. Q. Yuan, D. M. Zhang and L. van Haandel,
et al., Selective liquid phase hydrogenation of furfural to furfuryl alcohol by Ru/Zr-MOFs, J. Mol. Catal. A: Chem., 2015, 406, 58–64 CrossRef CAS PubMed
.
- M. J. Gilkey, P. Panagiotopoulou and A. V. Mironenko,
et al., Mechanistic Insights into Metal Lewis Acid-Mediated Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran, ACS Catal., 2015, 5(7), 3988–3994 CrossRef CAS
.
- M. Audemar, C. Ciotonea and K. D. Vigier,
et al., Selective Hydrogenation of Furfural to Furfuryl Alcohol in the Presence of a Recyclable Cobalt/SBA-15 Catalyst, ChemSusChem, 2015, 8(11), 1885–1891 CrossRef CAS PubMed
.
- Y. Xu, J. X. Long and Q. Y. Liu,
et al., In situ Hydrogenation of Model Compounds and Raw Bio-Oil over RANEY® Ni Catalyst, Energy Convers. Manage., 2015, 89, 188–196 CrossRef CAS PubMed
.
- W. J. Yu, Y. Tang, L. Y. Mo, P. Chen, H. Lou and X. M. Zheng, One-step hydrogenation-esterification of furfural and acetic acid over bifunctional Pd catalysts for bio-oil upgrading, Bioresour. Technol., 2011, 102(17), 8241–8246 CrossRef CAS PubMed
.
- A. D. Adid, L. Sangheon and C. H. Hyung,
et al., Effects of carbohydrates on the hydrodeoxygenation of lignin-derived phenolic compounds, ACS Catal., 2015, 5(1), 433–437 CrossRef
.
- M. Hronec, Katarina Fulajtarová, Tibor Liptaj. Effect of catalyst and solvent on the furan ring rearrangement to cyclopentanone, Appl. Catal., A, 2012, 437–438, 104–111 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |