Iron–silver oxide nanoadsorbent synthesized by co-precipitation process for fluoride removal from aqueous solution and its adsorption mechanism
Abstract
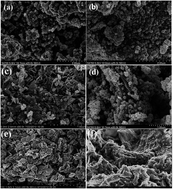
Fe–Ag magnetic binary oxide nanoparticles (Fe–Ag MBON) are prepared with co-precipitation of ferric and ferrous chloride solutions, and used for the adsorption of fluoride from aqueous solution. The surface morphology of the adsorbent was characterized by XRD, SEM, TEM, FTIR, XPS, EDX, BET, DLS and VSM techniques. Batch method was followed to optimize the conditions for the removal of fluoride. The results showed maximum removal occurred at pH 3.0 and adsorption equilibrium was achieved within 20 min. Chemical kinetics of the adsorption were well fitted by pseudo-second order models (R2 > 0.968) and the adsorption process followed the Langmuir isotherm model well (R2 > 0.976). The fluoride adsorption capacity of Fe–Ag MBON was 22.883 mg g−1, and decreased with increasing the temperature. Thermodynamic values revealed that the fluoride adsorption process was spontaneous and exothermic. Regeneration experiments were carried out for six cycles and the results indicate a removal efficiency loss of <22%.

 Please wait while we load your content...
Please wait while we load your content...