Microwave absorbing properties based on polyaniline/magnetic nanocomposite powders
Haibo Yang*,
Ting Ye and
Ying Lin
School of Materials Science and Engineering, Shaanxi University of Science and Technology, Xi'an, 710021, P. R. China. E-mail: yanghaibo@sust.edu.cn; Fax: +86-29-86168688; Tel: +86-29-86168688
First published on 27th November 2015
Abstract
Polyaniline/(1 − x)BaFe12O19/CaFe2O4/xCoFe2O4 nanocomposite powders were successfully prepared by an in situ polymerization method. The phase composition, morphology and electromagnetic properties of the nanocomposites were characterized by various instruments. The experimental results show that the polyaniline/(1 − x)BaFe12O19/CaFe2O4/xCoFe2O4 magnetic nanocomposites exhibit obviously enhanced microwave absorption performance in comparison with the single polyaniline/(1 − x)BaFe12O19/CaFe2O4/xCoFe2O4 and PANI/CoFe2O4 nanocomposites and the minimum reflection loss is −39.5 dB at 7.9 GHz with a thickness of 4 mm.
1. Introduction
Microwave absorbing materials have attracted much attention owing to the prospective applications.1,2 The traditional microwave absorbing materials can not satisfy all of the requirements such as thinness, width, lightness and strength. Hence, considerable efforts have been made toward the development of novel microwave absorbing materials.3 It has been reported that microwave absorbing properties can be enhanced via the complementation of dielectric loss and magnetic loss. Recently, considerable research attention has been focused on conducting polymers, such as polyaniline (PANI), polypyrrole (PPY), and so on. PANI has been widely used to optimize the microwave absorbing properties due to its efficient microwave absorbing properties based on the point that it is of lightweight, facile synthesis, good environmental stability, corrosion resistance, controllable electrical conductivity and dielectric loss ability4–7 and shows dramatic changes in its electronic structure and physical properties in the protonated state. PANI as a microwave-absorbing material has only electrical loss, which will not be of any help in improving the microwave absorption property and widening the absorption bandwidth.8 To compensate for these defects, the integration of magnetic materials and conducing polymers has attracted increased interest.9 The composite materials have potential applications in microwave absorption because they are of not only electrical and magnetic properties,10 but also possess special electromagnetic effects generated from a synergistic effect of the components.11–13Spinel ferrites and hexagonal ferrites are well known as traditional microwave absorbers with the advantages of high saturation magnetization and high magnetic loss.14,15 Among the magnetic ferrites, hard magnetic barium hexaferrite BaFe12O19 (BaM) has been widely used due to its low cost, excellent oxidation, corrosion resistance, low density, high electrical resistivity, high saturation magnetization and large coercivity.16,17 As we all know, the spinel ferrites CoFe2O4 (CFO) is a typical soft ferrite which have strong anisotropy, high saturation magnetization and moderate coercivity at room temperature.18,19 Ferrite of calcium CaFe2O4 (CaF) is expected to be more biocompatible since calcium is inherently non-toxic. However, the single ferrite cannot meet the requirement of the current life, the magnetic nanocomposites are of the merits of each phase.
In the previous reports, there are few researches on the combination of three ferrites and the simultaneous application of the dielectric loss fillers and exchange coupling effect. In our work, (1 − x)BaM/CaF/xCFO nanocomposite powders were successfully prepared by the simple physically mixing of the BaM/CaF powders and CFO powders, and then the PANI/(1 − x)BaM/CaF/xCFO nanocomposites were prepared by the in situ polymerization method. The samples can obtain excellent electrical conductivity and electromagnetic properties, which significantly increases the complex permittivity of the nanocomposite and provided the material with interesting microwave absorbing properties.
2. Experimental procedure
2.1. Materials
The analytical grade aniline monomer was distilled under reduced pressure and stored at low temperature. Ammonium peroxydisulfate ((NH4)2S2O8, APS), hydrochloric acid (HCl), ferrite nitrate (Fe(NO3)3·9H2O), barium nitrate (Ba(NO3)2), calcium nitrate (Ca(NO3)2·5H2O) and citric acid (C6H8O7·H2O) were all of analytical reagent grade and used as received. All reagents were purchased from Sinopharm Chemical Reagent Co., Ltd and deionized water was used in all experiments.2.2. Preparation of (1 − x)BaM/CaF/xCFO nanocomposite powders
BaM/CaF powders were synthesized by a one-step sol–gel method with the stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The aqueous solution was prepared by dissolving Ba(NO3)2, Fe(NO3)3·9H2O, Ca(NO3)2·5H2O, and C6H8O7·H2O into distilled water and magnetically stirred at 80 °C. Ammonia was added to the above solution to adjust the pH value to 7. The precursor was obtained by drying the mixture solution at 200 °C for 2 h, which was followed by the annealing treatment in air at 1000 °C for 4 h. The soft ferrite CoFe2O4 nano-powders were purchased from Shanghai Crystal Pure Reagent LTD commercially. (1 − x)BaM/CaF/xCFO nanocomposite powders (with x = 0.1, 0.2, 0.3, 0.4) were obtained by ball-milling the mixtures of BaM/CaF and CFO with different mass ratios and calcining at 400 °C for 3 h.
2. The aqueous solution was prepared by dissolving Ba(NO3)2, Fe(NO3)3·9H2O, Ca(NO3)2·5H2O, and C6H8O7·H2O into distilled water and magnetically stirred at 80 °C. Ammonia was added to the above solution to adjust the pH value to 7. The precursor was obtained by drying the mixture solution at 200 °C for 2 h, which was followed by the annealing treatment in air at 1000 °C for 4 h. The soft ferrite CoFe2O4 nano-powders were purchased from Shanghai Crystal Pure Reagent LTD commercially. (1 − x)BaM/CaF/xCFO nanocomposite powders (with x = 0.1, 0.2, 0.3, 0.4) were obtained by ball-milling the mixtures of BaM/CaF and CFO with different mass ratios and calcining at 400 °C for 3 h.
2.3. Preparation of PANI/(1 − x)BaM/CaF/xCFO nanocomposites
PANI/(1 − x)BaM/CaF/xCFO nanocomposite powders were prepared by the in situ polymerization method. Firstly, a certain amount of (1 − x)BaM/CaF/xCFO ferrite powders were suspended in a 80 ml 1 M HCl solution and stirred for 30 min to get a well-dispersed suspension. 1 ml aniline monomer was then added to the suspension and stirred for 30 min. After that, 2.5 g APS (the molar ratio of APS to aniline was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in a 20 ml 1 M HCl solution was slowly dropped to the suspension mixture under constant stirring and then reacted in a ice-water bath for 10 h. The nanocomposites were obtained by filtering and washing the suspension with deionized water, ethyl alcohol several times, and dried under vacuum at 60 °C for 12 h.
1) in a 20 ml 1 M HCl solution was slowly dropped to the suspension mixture under constant stirring and then reacted in a ice-water bath for 10 h. The nanocomposites were obtained by filtering and washing the suspension with deionized water, ethyl alcohol several times, and dried under vacuum at 60 °C for 12 h.
2.4. Characterization
The phase composition of the samples was detected by an X-ray diffractometer (XRD) with Cu Kα radiation (Rigaku D/MAX-2400, Japan). Fourier transform infrared (FTIR) spectra of the samples were recorded on a FTIR spectrometer (VECTOR-22, Japan) in the range of 4000–500 cm−1 with the KBr pellet technique. The morphology of the nanocomposite powders was analyzed using a scanning electron microscope (SEM) (Hitachi S-4800, Japan) equipped with an energy dispersive X-ray spectroscopy (EDS) and the transmission electron microscopy (TEM, American FEI Tecnai G2 F20 S-TWIN). The magnetic hysteresis loops of the nanocomposite powders were measured by a vibrating sample magnetometer 113 (VSM) (Lake Shore 7410, USA). The microwave absorbing properties of the material was achieved based on the coaxial transmission/reflection method.20 The electromagnetic parameters (ε′, ε′′, μ′, μ′′) of the samples, which were pressed to be toroidal samples with the height about 3 mm according to the mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 of paraffin and composite powders, were measured using the test system composed of the vector network analyzer (VNA) and coaxial fixture.
3 of paraffin and composite powders, were measured using the test system composed of the vector network analyzer (VNA) and coaxial fixture.
3. Results and discussion
3.1. XRD analysis
The XRD patterns of PANI/(1 − x)BaM/CaF/xCFO nanocomposite powders with different mass ratios of CFO and BaM/CaF are shown in Fig. 1. It suggests that the three phases can coexist, the diffraction peak intensities of the each phase are dependent on their relative mass ratios and there are no extra peaks appear in the XRD patterns. Simultaneously, one can notice that the diffraction peak of PANI is not obvious due to its low concentration. Besides, because the polymer is basically amorphous, the strong diffraction peaks of BaM/CaF/CFO may also obscure the diffraction peaks of PANI. Therefore, some other testing instruments were used to analyze the relation between PANI and BaM/CaF/CFO. According to Scherrer's equation D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, one can conclude that the grain sizes of CaF and CFO are around 36 nm and 30 nm, respectively.
θ, one can conclude that the grain sizes of CaF and CFO are around 36 nm and 30 nm, respectively.
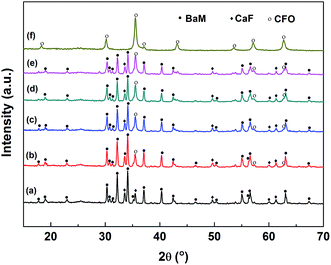 | ||
Fig. 1 XRD patterns of the PANI/BaM/CaF/CFO nanocomposite powders with different mass ratios of BaM/CaF and CFO: (a) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, (b) 9 0, (b) 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 8 1, (c) 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (d) 7 2, (d) 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (e) 6 3, (e) 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, (f) 0 4, (f) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. 10. | ||
3.2. FTIR analysis
FTIR spectra of the representative (a) 0.8BaM/CaF/0.2CFO nanocomposite powder, (b) PANI powder and (c) PANI/0.8BaM/CaF/0.2CFO nanocomposite powder are shown in Fig. 2. The main characteristic bands for the samples are assigned as follows: there is characteristic broad band of BaM/CaF/CFO located at 586 cm−1 and 436 cm−1 in the IR spectrum, which are attributed to stretching vibration between metal ions and oxygen.21 The main characteristic bands for PANI are assigned as follows: the bands at 1583 and 1489 cm−1 can be ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the quinoid (Q) and benzenoid rings, respectively.22 The band at 1296 cm−1 results from the stretching vibration of C–N,23 and the band at 1119 cm−1 is associated with the vibrational mode of N
C stretching vibration of the quinoid (Q) and benzenoid rings, respectively.22 The band at 1296 cm−1 results from the stretching vibration of C–N,23 and the band at 1119 cm−1 is associated with the vibrational mode of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the doped PANI chains, while the band at 800 cm−1 corresponds to the out-of-plane blending of C–H in the substituted benzenoid ring.24 These results indicate that there is an interaction between PANI chains and BaM/CaF/CFO ferrites, and PANI/magnetic nanocomposite is obtained.
N in the doped PANI chains, while the band at 800 cm−1 corresponds to the out-of-plane blending of C–H in the substituted benzenoid ring.24 These results indicate that there is an interaction between PANI chains and BaM/CaF/CFO ferrites, and PANI/magnetic nanocomposite is obtained.
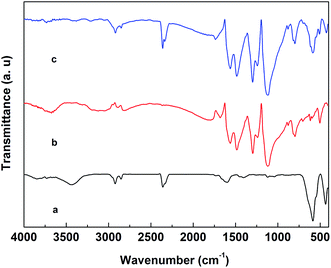 | ||
| Fig. 2 FTIR spectra of the representative (a) 0.8BaM/CaF/0.2CFO nanocomposite powder, (b) PANI powder and (c) PANI/0.8BaM/CaF/0.2CFO nanocomposite powder. | ||
3.3. Morphology
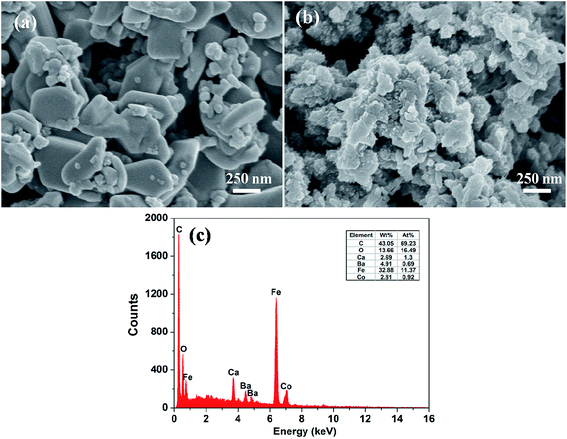
Fig. 3 shows the SEM micrographs of (a) the 0.8BaM/CaF/0.2CFO nanocomposite powder, (b) the PANI/0.8BaM/CaF/0.2CFO nanocomposite powder, and (c) the EDS mapping analysis result of the representative PANI/0.8BaM/CaF/0.2CFO nanocomposite powder. It can be found that after compositing with PANI, a continuous overlayer of conducting polymers is produced on the ferrite particle surface. One can notice from the EDS mapping that the sample contains C, Ba, Ca, Fe, O and Co four elements. The atomic ratio of Ca to Ba is 0.55, which is approximately equal to the nominal value of 0.5. The abundant element C may originate from the PANI. From the SEM micrograph of 0.8BaM/CaF/0.2CFO nanocomposite powders, it can be concluded that the granular grains with small size are of cubic CFO and CaF phases while the plate-like grains with big size are of hexagonal BaM phase. It can also be found that the three phases are uniformly distributed. The grain sizes of CaF and CFO are around 30–40 nm and the grain size of BaM is around 200 nm, which is consistent with the above XRD results.The TEM and HRTEM images of the representative PANI/0.8BaM/CaF/0.2CFO nanocomposite powder are shown in Fig. 4. According to the TEM images, the ferrite nanocomposites distributed in PANI matrixes can be seen clearly from Fig. 4(a) and the grain sizes are in the range of 30–200 nm. The HRTEM image (Fig. 4(b)) of the samples indicates that the ferrite particles are highly crystalline and it shows sets of lattice fringes of BaM phase (200), CaF phase (302) and CFO phase (311). This confirms that the BaM phase, CaF phase and CFO phase can coexist in the nanocomposite powders, which is corresponding with the above XRD results.
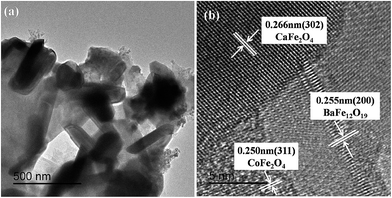 | ||
| Fig. 4 (a) TEM image and (b) HRTEM image of the representative PANI/0.8BaM/CaF/0.2CFO nanocomposite powder. | ||
3.4. Magnetic property
Fig. 5 shows the magnetic hysteresis (M–H) loops of the PANI/(1 − x)BaM/CaF/xCFO nanocomposite powders with different mass ratios of CFO and BaM/CaF. It evidently presents that the saturation magnetizations of nanocomposite powders are mostly higher than those of the single PANI/BaM/CaF and PANI/CFO composites. The magnetic parameters observed from Fig. 5 are listed in Table 1. As expected, with increasing the mass ratio of CFO, the coercivity (Hc) decreases due to the fact the Hc of CFO ferrite is smaller than that of BaM ferrite. When the mass ratio of CFO is 0.2, 0.3 and 0.4, the saturation magnetization (Ms) of the PANI/BaM/CaF/CFO nanocomposite powders is enhanced compared with those of PANI/BaM/CaF and PANI/CFO composites.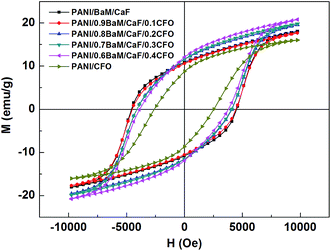 | ||
| Fig. 5 Magnetic hysteresis (M–H) loops of the PANI/BaM/CaF/CFO nanocomposite powders with different mass ratios of BaM/CaF and CFO. | ||
| Composition | Coercivity (Oe) (emu g−1) | Saturation magnetization (emu g−1) |
|---|---|---|
| PANI/BaM/CaF | 4568.97 | 18.05 |
| PANI/0.9BaM/CaF/0.1CFO | 4506.27 | 17.65 |
| PANI/0.8BaM/CaF/0.2CFO | 4114.42 | 19.57 |
| PANI/0.7BaM/CaF/0.3CFO | 4098.75 | 19.95 |
| PANI/0.6BaM/CaF/0.4CFO | 3963.64 | 20.78 |
| PANI/CFO | 2599.27 | 16.07 |
3.5. Electromagnetic property analyses
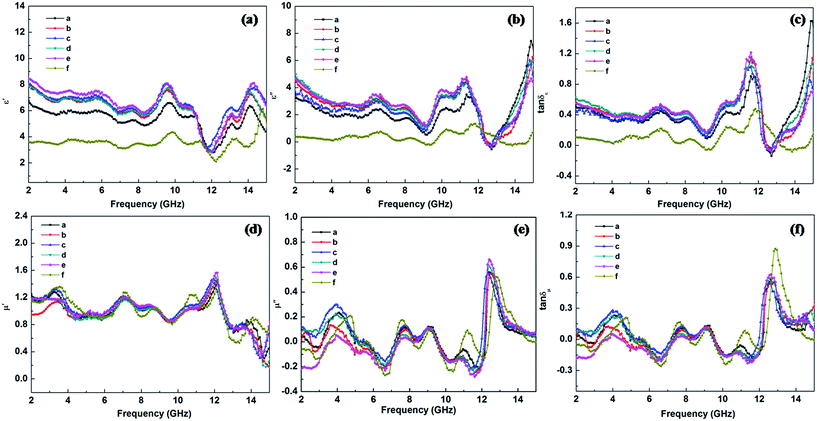
Fig. 6 shows frequency dependence of the complex permittivity, complex permeability and loss tangent of dielectric/magnetic of the samples between 2 GHz and 15 GHz. As is well known, the real parts (ε′, μ′) of complex permittivity and permeability represent the storage of the electric and magnetic energy, respectively. While the imaginary parts (ε′′, μ′′) of complex permittivity and permeability symbolize the loss of the electric and magnetic energy,25 and all the values of the PANI/BaM/CaF/CFO magnetic nanocomposites is between those of PANI/BaM/CaF and PANI/CFO. With increasing the mass ratio of CFO, the complex permittivities of all the samples are gradually enhanced. This is due to the fact that the increasing of CFO concentration enlarges the interface of PANI/BaM/CaF/CFO nanocomposites, the dipole and interfacial polarization of the nanocomposites are enhanced due to the synergistic effect of two kinds of materials which makes the dielectric constant of material increase and have larger dielectric loss and is advantageous to optimize the microwave absorbing properties. Moreover, the dielectric performance of an absorber can be explained by considering the various polarization mechanism (ionic, electronic, dipole, and space charge polarization) occurring in an absorber under the treatment of microwave. The space charge polarization is induced by the difference in dielectric constants and electrical conductivities among the component of the composites and the heterogeneity exists at the interface between the components of the composites. The dipole polarization happens due to the presence of bound charges (dipoles) of the absorber. The above-mentioned polarizations are important polarization processes and associated relaxation will give rise to dielectric loss and the oscillations as well.To further evaluate the microwave absorbing performance, the reflection loss [RL(dB)] of the specimens was calculated according to the following equations:26,27
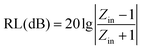 | (1) |
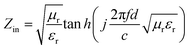 | (2) |
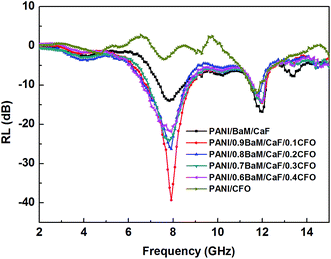
The calculated reflection loss curves in 2–15 GHz for the PANI/BaM/CaF/CFO nanocomposites with different mass ratios and the specimen thickness of 3 mm are shown in Fig. 7. It can be seen that the PANI/BaM/CaF/CFO magnetic nanocomposites have more obvious effect on microwave absorbing properties than the PANI/BaM/CaF and PANI/CFO composites, and the value of reflection loss is effectively enhanced when the mass ratio of BaM/CaF and CFO is 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The excellent microwave absorbing property exhibits that there is an effective absorption band with the bandwidth at 5.3–9.6 GHz and the minimum reflection loss is −39.5 dB at 7.9 GHz. While the reflection loss of the PANI/BaM/CaF and the PANI/CFO composites are only −14 dB and −12 dB, respectively. The possible reason for this is that the strong attenuation characteristics of microwave absorbing materials must satisfy a proper matching of the magnetic loss and dielectric loss,28 the combination of BaM/CaF and CFO can be of the more appropriate complementation of dielectric loss and magnetic loss, which is consistent with Fig. 6.
1. The excellent microwave absorbing property exhibits that there is an effective absorption band with the bandwidth at 5.3–9.6 GHz and the minimum reflection loss is −39.5 dB at 7.9 GHz. While the reflection loss of the PANI/BaM/CaF and the PANI/CFO composites are only −14 dB and −12 dB, respectively. The possible reason for this is that the strong attenuation characteristics of microwave absorbing materials must satisfy a proper matching of the magnetic loss and dielectric loss,28 the combination of BaM/CaF and CFO can be of the more appropriate complementation of dielectric loss and magnetic loss, which is consistent with Fig. 6.
Fig. 8 shows the calculated microwave reflection loss in 2–15 GHz for the PANI/BaM/CaF/CFO nanocomposites with the mass ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at different specimen thicknesses. It is found that the peak value shifts to a lower frequency with increasing the specimen thickness. When the specimen is 4 mm thick, the minimum RL is about −39.5 dB at 7.9 GHz, from 5.3 to 9.6 GHz. The reflection loss values exceeding −10 dB can be obtained by tuning the specimen thickness between 3 and 5 mm.
1 at different specimen thicknesses. It is found that the peak value shifts to a lower frequency with increasing the specimen thickness. When the specimen is 4 mm thick, the minimum RL is about −39.5 dB at 7.9 GHz, from 5.3 to 9.6 GHz. The reflection loss values exceeding −10 dB can be obtained by tuning the specimen thickness between 3 and 5 mm.
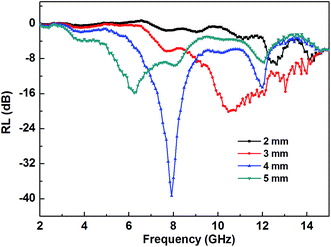 | ||
| Fig. 8 Reflection loss (RL) in 2–15 GHz for the PANI/0.9BaM/CaF/0.1CFO nanocomposite with different specimen thicknesses. | ||
4. Conclusions
In this article, the PANI/BaM/CaF/CFO magnetic nanocomposite powders have been successfully synthesized by the in situ polymerization method. The PANI/BaM/CaF/CFO magnetic nanocomposites have excellent microwave absorbing properties compared with the pure PANI/BaM/CaF and PANI/CFO composites. Its excellent microwave absorption performance could be attributed to the appropriate complementarities between dielectric and magnetic losses of the magnetic nanocomposites and the minimum reflection loss of the nanocomposite is −39.5 dB at 7.9 GHz. Therefore, the prepared nanocomposites have potential applications in microwave absorbing area.Acknowledgements
This work is supported by the National Natural Science Foundation of China (grant no. 51572159), the Science and Technology Foundation of Shaanxi Province (grant no. 2013KJXX79, 2013JQ6004) and the Special Foundation of the Ministry of Shaanxi Province (grant no. 2013JK0937).References
- C. L. Zhu, M. L. Zhang, Y. J. Qiao, G. Xiao, F. Zhang and Y. J. Chen, Fe3O4/TiO2 core/shell nanotubes: synthesis and magnetic and electromagnetic wave absorption characteristics, J. Phys. Chem. C, 2010, 114, 16229–16235 CAS.
- H. L. Yu, T. S. Wang, B. Wen, M. M. Lu, Z. Xu, C. L. Zhu, Y. J. Chen, X. Y. Xue, C. W. Sun and M. S. Cao, Graphene/polyaniline nanorod arrays: synthesis and excellent electromagnetic absorption properties, J. Mater. Chem., 2012, 22, 21679–21685 RSC.
- X. H. Guo, Y. H. Deng, D. Gu, R. C. Che and D. Y. Zhao, Synthesis and microwave absorption of uniform hematite nanoparticles and their core–shell mesoporous silica nanocomposites, J. Mater. Chem., 2009, 19, 6706–6712 RSC.
- T. H. Ting, R. P. Yu and Y. N. Jau, Synthesis and microwave absorption characteristics of polyaniline/NiZn ferrite composites in 2–40 GHz, Mater. Chem. Phys., 2011, 126, 364–368 CrossRef CAS.
- S. H. Hosseini, S. H. Mohseni, A. Asadnia and H. Kerdari, Synthesis and microwave absorbing properties of polyaniline/MnFe2O4 nanocomposite, J. Alloys Compd., 2011, 509, 4682–4687 CrossRef CAS.
- H. B. Yang, Y. Y. Yang, Y. Lin and M. Liu, Preparation and electromagnetic properties of in situ Ba0.8Sr0.2TiO3/YFeO3 nanocomposites, Ceram. Int., 2013, 39, 7235–7239 CrossRef CAS.
- J. Jiang, C. C. Chen, L. H. Ai, L. C. Li and H. Liu, Synthesis and characterization of novel ferromagnetic PPy-based nanocomposite, Mater. Lett., 2009, 63, 560–562 CrossRef CAS.
- A. Pud, N. Ogurtsov, A. Korzhenko and G. Shapoval, Some aspects of preparation methods and properties of polyaniline blends and composites with organic polymers, Prog. Polym. Sci., 2003, 28, 1701–1753 CrossRef CAS.
- H. Zengin, H. Zengin, W. Zhou, J. Jin, R. Czerw, D. W. Smith Jr, L. Echegoyen, D. L. Carroll, S. H. Foulger and J. Ballato, Carbon nanotube doped polyaniline, Adv. Mater., 2002, 14, 1480–1483 CrossRef CAS.
- H. B. Yang, T. Ye, Y. Lin, M. Liu, G. Zhang and P. Kang, Giant enhancement of (BH)max in BaFe12O19/Y3Fe5O12 nanocomposite powders, Mater. Lett., 2015, 145, 19–22 CrossRef CAS.
- Y. F. Zhu, Q. Q. Ni, Y. Q. Fu and T. Natsuki, Synthesis and microwave absorption properties of electromagnetic functionalized Fe3O4–polyaniline hollow sphere nanocomposites produced by electrostatic self-assembly, J. Nanopart. Res., 2013, 15, 1988 CrossRef PubMed.
- S. W. Phang, M. Tadokoro, J. Watanabe and N. Kuramoto, Effect of Fe3O4 and TiO2 addition on the microwave absorption property of polyaniline micro/nanocomposites, Polym. Adv. Technol., 2009, 20, 550–557 CrossRef CAS.
- O. Akman, Z. Durmus, H. Kavas, B. Aktas, U. Kurtan, A. Baykal and H. Sözeri, Effect of conducting polymer layer on microwave absorption properties of BaFe12O19–TiO2 nanocomposite, Phys. Status Solidi A, 2013, 210, 395–402 CrossRef CAS.
- H. B. Yang, T. Ye, Y. Lin, M. Liu, G. Zhang and P. Kang, Preparation and magnetic properties of CoFe2O4/Y3Fe5O12 nanocomposite powders, J. Mater. Sci.: Mater. Electron., 2015, 26, 1827–1831 CrossRef CAS.
- H. B. Yang, M. Liu, Y. Lin and Y. Y. Yang, Simultaneous enhancements of remanence and (BH)max in BaFe12O19/CoFe2O4 nanocomposite powders, J. Alloys Compd., 2015, 631, 335–339 CrossRef CAS.
- Y. Lin, H. B. Yang, J. F. Zhu and F. Wang, Y3Fe5O12/BaFe12O19 composite with giant dielectric constant and high magnetization, Mater. Lett., 2013, 93, 230–232 CrossRef CAS.
- Y. Lin, P. Kang, H. B. Yang and M. Liu, Preparation and characterization of BaFe12O19/Y3Fe5O12 composites, J. Alloys Compd., 2015, 641, 223–227 CrossRef CAS.
- H. B. Yang, Y. Lin, J. F. Zhu and F. Wang, Ba0.6Sr0.4TiO3/CoFe2O4 nanocomposite with giant dielectric constant and high saturation magnetization, Mater. Manuf. Processes, 2012, 27, 910–913 CrossRef CAS.
- Y. Lin, P. Kang, H. B. Yang and M. Liu, Preparation and characterization of La0.1Bi0.9FeO3/CoFe2O4 nanocomposite powders, Ceram. Int., 2015, 41, 6079–6083 CrossRef CAS.
- K. C. Yaw, Measurement of dielectric material properties, Application Note, Rohde & Schwarz, 2012, pp. 1–35 Search PubMed.
- T. H. Ting and K. H. Wu, Synthesis, characterization of polyaniline/BaFe12O19 composites with microwave-absorbing properties, J. Magn. Magn. Mater., 2010, 322, 2160–2166 CrossRef CAS.
- G. C. Sun, K. L. Yao, H. X. Liao, Z. C. Niu and Z. L. Liu, Microwave absorption characteristics of chiral materials with Fe3O4–polyaniline nanocomposite matrix, Int. J. Electron., 2000, 87, 735–740 CrossRef CAS.
- B. Lesiak, A. Jablonski, J. Zemek, M. Trchova and J. Stejskal, Determination of the inelastic mean free path of electrons in different polyaniline samples, Langmuir, 2000, 16, 1415–1423 CrossRef CAS.
- Y. S. Yang and M. X. Wan, Chiral nanotubes of polyaniline synthesized by a template-free method, J. Mater. Chem., 2002, 12, 897–901 RSC.
- S. Tyagi, H. B. Baskey, R. C. Agarwala, V. Agarwala and T. C. Shami, Development of hard/soft ferrite nanocomposite for enhanced microwave absorption, Ceram. Int., 2011, 3, 2631–2641 CrossRef.
- H. B. Yang, T. Ye, Y. Lin and M. Liu, Excellent microwave absorption property of ternary composite: Polyaniline-BaFe12O19-CoFe2O4 powders, J. Alloys Compd., 2015, 653, 135–139 CrossRef CAS.
- H. B. Yang, T. Ye, Y. Lin and M. Liu, Preparation and microwave absorption property of graphene/BaFe12O19/CoFe2O4 nanocomposite, Appl. Surf. Sci., 2015, 357, 1289–1293 CrossRef.
- P. Xu, X. J. Han, C. Wang, D. H. Zhou, Z. S. Lv, A. H. Wen, X. H. Wang and B. Zhang, Synthesis of electromagnetic functionalized nickel/polypyrrole core/shell composites, J. Phys. Chem. B, 2008, 112, 10443–10448 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |