Melamine trisulfonic acid (MTSA): an efficient and recyclable heterogeneous catalyst in green organic synthesis
Rajesh H. Vekariya
,
Kinjal D. Patel
and
Hitesh D. Patel
*
Department of Chemistry, School of Sciences, Gujarat University, Ahmedabad, Gujarat, India. E-mail: drhiteshpatel1@gmail.com; Fax: +91-079-26308545; Tel: +91-079-26300969
First published on 16th October 2015
Abstract
Melamine trisulfonic acid (MTSA) as a highly efficient heterogeneous solid acid catalyst catalyzes various organic transformations. The great efforts have been made by many scientists to utilize MTSA as a green catalyst for the preparations of N- and O-containing heterocyclic compounds, for the protections of alcohol, phenols, amines and aldehydes and ketones, for the acetylation of alcohols and phenols and for the N-formylation of amines. The superior advantage of this heterogeneous catalyst is that it can be recovered and reused several times without the loss of their efficiency. In this review, preparation and applications of MTSA in organic synthesis are investigated.
1 Introduction
Catalysis has played a significant role in reducing pollution from the chemical processes in our environment. By utilizing catalysts, organic reactions can be more efficient and selective thereby eliminating large amounts of by-products and other waste compounds.1 It is one of the fundamental pillars of green chemistry.2 Homogeneous acid catalysts such as HF, HCl, CH3COOH, HBr, CF3COOH and H2SO4 are widely used in many important industrial processes, but they have some disadvantages in handling, corrosiveness, trouble work-up procedures and production of toxic waste.3 Hence, in recent years, the search for environmentally benign chemical processes or methodologies has received much attention, and the development of heterogeneous catalysts for fine chemical synthesis has become a major area of research. The potential advantages of these materials over homogeneous systems are such as ease of handling, decreased reactor and plant corrosion problems, and more environmentally safe waste disposal procedures.4–8 For this purpose various solid acids such as solid acid zeolite,9 solid super acid sulfated zirconia,10 silica sulfuric acid,5 alumina sulfuric acid,11 tungstate sulphuric acid,12 molybdate sulfuric acid,13 alum,14 ZrOCl2·8H2O,15 MCM-41–SO3H,16 PPA–SiO2,17 SiO2–HClO4,18 SiO2–NaHSO4,19 SiO2–Pr–SO3H,20 Amberlyst-15,21 heteropoly acids22 and montmorillonite K-10 (ref. 23) ware developed by many scientists and utilized in the synthesis of a wide range of organic compounds. Nowadays, the use of SO3H-containing catalysts has received considerable interest in organic synthesis, because of their unique advantages such as efficiency, high reactivity, operational simplicity, environmental compatibility, non-toxicity, low cost, ease of isolation, green nature, easy availability of their starting materials, and ability to promote a wide range of reactions.16,20,24–26 However, cellulose sulfuric acid, starch sulfuric acid and PEG–OSO3H were explored as biodegradable solid acids for the synthesis of various organic compounds, while other SO3H-containing catalysts are metal base catalysts and are non-biodegradable. One of the attractive SO3H-containing catalysts is melamine trisulfonic acid (MTSA), which is also used as a versatile catalyst, which makes organic processes convenient, more economic and environmentally benign. MTSA have been explored as powerful catalysts for the preparations of N- and O-containing heterocyclic compounds, for the protections of alcohol, phenols, amines and aldehydes and ketones, for the acetylation of alcohols and phenols and for the N-formylation of amines. Moreover, this catalyst can be recovered and reused several times without a decrease in activity as well as its efficiency. The present review is intended briefly in recent research progress concerning the various organic transformations catalyzed by MTSA as a heterogeneous and recyclable catalyst.2 Preparation of melamine trisulfonic acid (MTSA)27
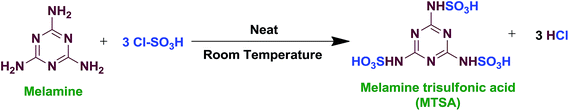
In 250 mL suction flask take 5 mL chlorosulfonic acid (75.2 mmol) was equipped with a gas inlet tube for conducting HCl gas over an adsorbing solution. Melamine (3.16 g, 25.07 mmol) was added in small portions over a period of 30 min at room temperature. HCl gas evolved from the reaction vessel immediately (Fig. 1). After completion of the addition of melamine, the mixture was shaken for 30 min; meanwhile, the residual HCl was removed by suction. MTSA (7.7 g, 85%) was obtained as a white solid, which was stored in a capped bottle. Mp. 142–144 °C. It is hygroscopic in nature and possessing an acidic pH. It is soluble in water. It is insoluble in ethyl acetate, methanol, ethanol, dichloromethane, diethyl ether, n-hexane, chloroform and acetone.3 Melamine trisulfonic acid (MTSA) catalyzed various organic reactions
3.1 Solvent-free organic reactions catalyzed by MTSA
3.1.1.1 Synthesis of 3,4-dihydropyrimidin-2(1H)-one/thione derivatives. 3,4-Dihydropyrimidin-2(1H)-one/thione derivatives possesses a wide range of pharmaceutical activities such as antitumor, antiviral, anti-inflammatory and antibacterial.28 Different catalysts such as MgBr2,29 PPh3,30 ZrCl4,31 Bi(OTf)3,32 CaF2,33 silica triflate,34 FeCl3,35 sulfamic acid,36 Cu(OTf)2 (ref. 37) and Fe(HSO4)3 (ref. 38) have been reported for the preparation of these skeletons. However, high temperatures, expensive catalyst, long reaction times, lower product yields, tedious work-up process and the use of volatile organic solvents are limitations of these protocols. Therefore, Shirini and co-workers represented an environmentally friendly protocol for the preparation of 3,4-dihydropyrimidin-2(1H)-ones/thiones (DHPMs) via three component synthesis of aldehydes, β-ketoesters and urea/thiourea in the presence of melamine trisulfonic acid (MTSA) as green and recyclable catalyst (Scheme 1).39 This synthesis was carried out at the 80 °C under solvent-free conditions. An electronic and steric effect of the nature of the substituents on the aromatic ring does not show any obvious effect on this conversion. In addition, hetero aromatic aldehydes were also reacted efficiently to afford products in excellent yields. Compared to cellulose sulfuric acid (CSA), PEG–OSO3H and starch sulfuric acid (SSA), MTSA shows excellent efficiency for this synthesis in terms of product yield, reaction temperature and catalyst loading.24,25 In addition, CSA catalyzed this reaction was completed within shorter period of time as compared to MTSA and SSA. The added advantage of this method is that the catalyst could be recycled and reused three times without significant loss of their efficiency.
3.1.1.2 Synthesis of 1,4-dihydropyridines (1,4-DHPs) derivatives. 1,4-Dihydropyridines possesses various biological activities such as glycoprotein inhibitors,40 anticancer,41 anticoagulant,42 anti-inflammatory and anti-microbial agents43 and neurotropic.44 Mansoor and co-workers have reported a green method for the synthesis of 1,4-dihydropyridines (1,4-DHPs) through three component condensation reaction of ethyl acetoacetate or methyl acetoacetate, aldehydes and ammonium acetate catalysed by melamine trisulfonic acid (MTSA) as an efficient and eco-friendly catalyst (Scheme 2).45 This synthesis was performed under solvent-free condition at the 60 °C. Moreover, the catalyst could be recyclable and reused three times without significant loss of their efficiency. In this synthesis the authors have screened various solvents like methanol, THF, acetonitrile, ethanol, dichloromethane, cyclohexane, benzene as well as solvent-free conditions. However, the best result was obtained under solvent-free conditions in terms of product yields and reaction times. All the electron-rich and electron deficient aldehydes worked well leading to excellent yields of the products. The authors have demonstrated various Lewis acid catalysts such as AlCl3, ZnCl2, FeCl3, BiBr3, BiCl3 and Bi(OTf)3 for the comparative results with MTSA in this transformation. Although, the results presented that MTSA is a more efficient catalyst with respect to catalyst load, reaction temperature, product yield and reaction time than other catalysts. However, for this synthesis the CSA shows higher efficiency in terms of reaction times, while MTSA shows greater efficiency in terms of product yield.24
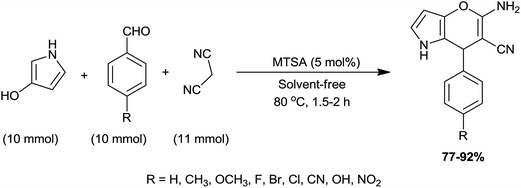
3.1.1.3 Synthesis of 5-amino-7-aryl-6-cyano-4H-pyrano[3,2-b]pyrroles derivatives. 4H-Pyrans possess potent biological activities like antitumor,46 antibacterial,47,48 antiviral,49 spasmolytic50 and antianaphylactic.51 An environmentally friendly preparation of 5-amino-7-aryl-6-cyano-4H-pyrano[3,2-b]pyrroles via three component synthesis of malononitrile, 3-hydroxypyrrole and several aromatic aldehydes in the presence of melamine trisulfonic acid (MTSA), which was discovered by the Mansoor's group (Scheme 3).52 The authors have screened various solvents such as ethanol, benzene, acetonitrile, methanol, cyclohexane, toluene, dichloroethane as well as solvent-free conditions in this synthesis. However, solvent-free condition was proved to be the best for this transformation. Only, 5 mol% MTSA was sufficient to achieving products in excellent yield within shorter period of time. To find out the optimize reaction condition; the authors have established reaction at various temperatures from 50 °C to 100 °C. Finally, they have achieved 80 °C temperature as the optimum temperature for this transformation. The catalyst was tested for four runs. It was seen that the catalyst exhibited very good reusability efficiency.
3.1.1.4 Synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinolines. Aswin and associates have reported a green protocol for the synthesis of synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinolines via three component synthesis of dimedone, aldehydes, malononitrile and ammonium acetate under solvent-free conditions (Scheme 4).53 The authors have examined various solvents like 1,4-dioxane, methanol, ethanol, acetonitrile, cyclohexane and toluene as well as solvent-free conditions in this synthesis. Although, the best result was obtained by using 5 mol% MTSA under solvent-free condition at 60 °C. Both an electron-withdrawing and an electron-donating substituents on aldehydes were reacted efficiently to afford corresponding products in high to excellent yield within a short period of times. The catalyst could be reused four times without substantial damage of its activity.
3.1.1.5 Synthesis of 10-aryl-6,8-dimethyl-6,10-dihydro-5-oxa-6,8-diazaanthra[2,3-d][1,3]dioxole-7,9-dione derivatives. Li and co-workers have reported utilization of melamine trisulfonic acid (MTSA) as an efficient and recyclable catalyst for the one-pot synthesis of 10-aryl-6,8-dimethyl-6,10-dihydro-5-oxa-6,8-diazaanthra[2,3-d][1,3]dioxole-7,9-dione derivatives by condensation of 3,4-methylene dioxyphenol, aromatic aldehydes and 1,3-dimethylbarbituric acid under solvent-free conditions at the 110 °C (Scheme 5).54 Maximum yield was obtained with 5 mol% of the catalyst. Different types of aromatic aldehydes bearing electron releasing as well as electron accepting groups were used in the reaction and in all cases the products were obtained in good to excellent yields. An aliphatic aldehydes, such as phenyl acetaldehyde, propionaldehyde, n-butyl aldehyde and n-heptaldehyde, were not reacting efficiently with this protocol and the desired products were not found and obtained successfully. The catalyst could be successfully recovered and recycled at least for three runs without significant loss in activity. A tentative mechanism for this transformation was also proposed by the authors. The reaction likely proceeds via the initial formation of oxonium species, which then undergo dehydration to give olefin. Subsequent Michael-type addition of 3,4-methylenedioxyphenol to the olefin followed by cyclization and dehydration to afford the corresponding products in excellent yield.
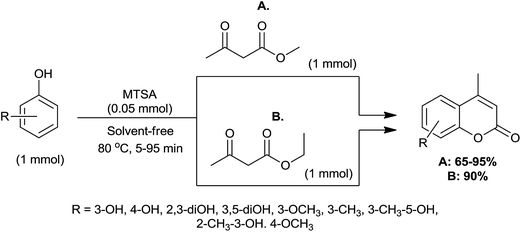
3.1.1.6 Synthesis of coumarins via Pechmann condensation. Coumarins and their derivatives are important classes of heterocyclic compounds, whose synthesis has been the focus of attention of many organic and medicinal chemists.55 Generally, the Pechmann reaction is carried out using various catalysts such as H2SO4,56 CF3COOH,57 InCl3,58 Bi(NO3)3·5H2O,59 silica triflate,60 ZrCl4 (ref. 61) and TiCl4.62 The main disadvantages of the processes are, long reaction times, low yields, harsh reaction conditions, non-reusability of the catalyst and use of excess amounts of the reagent. Thus, Shirini et al. have discovered green synthesis of coumarins via Pechmann condensation of phenols and ethyl acetoacetate or methyl acetoacetate by utilizing melamine trisulfonic acid (MTSA) as a bio-degradable catalyst under solvent-free conditions at the 80 °C (Scheme 6).63 Only, 0.05 mmol of catalyst was sufficient for this transformation. Phenols with electron-donating groups are easily converted to their corresponding coumarins, while electron-withdrawing groups were not reacting under these conditions and starting materials remained intact even after prolonged heating. The catalyst could be reused several times without a considerable change in the reaction times and yields. Compared to MTSA, PEG–OSO3H catalysed this synthesis afforded products within shorter period of time, while higher product yield was observed in case of MTSA.25
3.1.1.7 Preparation of triazolo[1,2-a]indazole-1,3,8-trione and 2H-indazolo[2,1-b]phthalazine-1,6,11-trione derivatives. Khazaei and associates discussed a sustainable synthetic method for the preparation of triazolo[1,2-a]indazole-1,3,8-trione and 2H-indazolo[2,1-b]phthalazine-1,6,11-trione derivatives by the three-component condensation reaction of urazoles or phthalhydrazide, dimedone or 1,3-cyclohexanedione and aldehydes using melamine trisulfonic acid (MTSA) as an efficient heterogeneous catalyst under solvent-free conditions (Scheme 7a–c).64 Only, 15 mol% was sufficient for the excellent yields of the products. The influence of electron-withdrawing and electron-donating substituents on the aromatic ring of aldehydes have no significant effect on the reaction yields. In the case of aliphatic aldehydes longer reaction time was observed for the completion of the reaction. Influence of different catalysts such as p-TSA,65 silica sulfuric acid,66 sulfuric acid67 and N-halosulfonamides68 on the reaction of benzaldehyde, dimedone and phthalhydrazide was also studied by the authors. However, they were not efficient as compared to MTSA in terms of product yields and reaction times. In this synthesis, PEG–OSO3H shows greater activity in terms of product yield and reaction time as compared to MTSA.25
3.1.1.8 Preparation of 7-alkyl-6H,7H-naphtho-[10,20:5,6]pyrano[3,2-c]chromen-6-ones. Chromene derivatives possessing a wide range of pharmaceutical activities such as antirhinovirus,69 antioxidant,70 antimicrobial,71 anticancer72 and antihypertensive.73 An efficient preparation of 7-alkyl-6H,7H-naphtho-[10,20:5,6]pyrano[3,2-c]chromen-6-one derivatives by three-component condensation reaction of β-naphthol, aromatic aldehydes and 4-hydroxycoumarin catalyzed by melamine trisulfonic acid (MTSA) as a green and reusable catalyst under solvent-free conditions, which was developed by Ma's group (Scheme 8).74 At 120 °C, the reaction proceeded smoothly to afforded excellent product yields within shorter period of time. In addition, maximum yield was obtained with 2 mol% of the MTSA. The authors have also suggested reaction mechanism, according to them the reaction likely proceeds via the initial formation of ortho-quinone methide. The oxonium species are then formed by reaction with 4-hydroxycoumarin, which then undergoes dehydration to afford the desired product in excellent yield. A variety of aryl aldehydes including those bearing electron-withdrawing and electron-donating groups reacted smoothly with this protocol to afford efficient product yields. For comparison the effect of different catalysts such as sulfuric acid, NaHSO3, NaHSO4, AlCl3, I2 and MTSA on this reaction was also examined by the authors. However, MTSA showed better results as compared to others.
3.1.1.9 Synthesis of 14-aryl-14H-dibenzo[a,j]xanthene derivatives. Zare et al. have also reported catalytic application of a melamine trisulfonic acid (MTSA) in the synthesis of 14-aryl-14H-dibenzo[a,j]xanthene derivatives via condensation of 2-naphthol with arylaldehydes under solvent-free conditions (Scheme 9).75 This reaction was performed under conventional thermal (110 °C) or microwave irradiation (540 W). Only, 2.5 mol% of the catalyst was sufficient to promote the reaction efficiently under thermal conditions, while under microwave irradiation 0.5 mol% of the catalyst was sufficient to promote the reaction in forward direction. In addition, lower catalyst loading and shorter reaction time was required in case of MTSA and CSA as compared to PEG–OSO3H, while higher product yield was obtained in the case of MTSA as compared to PEG–OSO3H and CSA.24,25 Moreover, the reaction under microwave irradiation was completed within shorter period of time as compared to the conventional thermal conditions. Both electro-releasing and electron-withdrawing substituents on aromatic aldehydes were efficiently converted into the products.
3.1.1.10 Synthesis of polyhydroquinolines. Quinolines and their derivatives have attracted much attention, because a large number of natural products and drugs possess this heterocyclic scaffold.76 Zare and co-workers have reported an environmentally friendly and clean method for the synthesis of polyhydroquinolines via one-pot multi-component condensation reaction between aryl aldehydes, dimedone (5,5-dimethylcyclohexane-1,3-dione), β-ketoesters and ammonium acetate in the presence of melamine trisulfonic acid (MTSA) under solvent-free conditions at 60 °C (Scheme 10).77 Only, 2.5 mol% of the catalyst was sufficient to promote the reaction in forward direction efficiently. An electronic and steric effect of the nature of the substituents on the aromatic ring does not show any obvious effect on this conversion. The authors have suggested that at first dimedone is converted to its enol form using MTSA. On the other hand, the activated β-ketoester (by the catalyst) and ammonia (resulted from ammonium acetate) gives enamine. Afterward, the enol and enamine react with the activated aldehyde (by MTSA) to afford intermediate, which was tautomerized followed by an intramolecular nucleophilic attack of the NH2 group to the activated carbonyl group and again tautomerized to form polyhydroquinonine in good yields. After completion of the reaction, the catalyst could be recovered and reused several times without loss in their activity.
3.1.1.11 Synthesis of 9-aryl-1,8-dioxo-octahydroxanthene derivatives. Xanthene derivatives are essential unit of a large number of naturally occurring as well as synthesized biological compounds.78,79 Zare et al. have disclosed an efficient synthesis of 9-aryl-1,8-dioxo-octahydroxanthene derivatives from 1,3-cyclohexanediones and aryl aldehydes in the presence of catalytic amount of melamine trisulfonic acid (MSTA), which afforded products in excellent yields (Scheme 11).80 This reaction was performed under thermal (solvent-free/80 °C), microwave (solvent-free/180 W/90 °C) and ultrasound (solvent/34–37 kHz/350 W/60 °C) conditions. A wide range of electro releasing as well as electron withdrawing groups bearing aldehydes was efficiently converted into the products makes this protocol have wide synthetic utility. Best results were obtained under microwave irradiation (MWI) as compared to thermal and ultrasound conditions in terms of products yield and reaction time. In addition, in ultrasound condition more time was needed to complete the reaction as compared to thermal and MWI.
3.2 Water mediated organic reactions catalyzed by MTSA
3.2.7.1 Synthesis of 2-substituted benzothiazoles. The Mansoor's group has utilized melamine trisulfonic acid (MTSA) as an efficient and recyclable catalyst for the synthesis of 2-substituted benzothiazoles by condensation reaction of aromatic aldehydes and o-aminothiophenol in water at the 70 °C (Scheme 25).146 Various oxidative reagents and catalysts, such as CTAB, FeCl3/montmorillonite K-10, ZnO-beta zeolite, KAl(SO4)2·12H2O, silica sulfuric acid, iodine, H2O2/CAN and H2O2/HCl have all been used in the reaction.147 However, they do not give efficient results as compared to MTSA. The authors have examined various solvents such as methanol, ethanol, dichloromethane, 1,4-dioxane, THF, acetonitrile, DMF, ethyl acetate, chloroform and water. Although, the best conversion was obtained, when the reaction was performed in water. Only, 0.05 mmol of MTSA was sufficient for this conversion. The catalyst could be recycled and reused four times without any additional treatment or appreciable reduction in catalytic activity.
3.2.7.2 Preparation of 7,8-dihydro-10-aryl-5H-indeno[1,2-b]quinoline-9,11-diones. Shirini and co-workers have demonstrated a green protocol for the preparation of 7,8-dihydro-10-aryl-5H-indeno[1,2-b]quinoline-9,11-diones via a one-pot four component condensation of aromatic aldehydes, dimedone, 1,3-indandione and ammonium acetate using melamine trisulfonic acid (MTSA) as a recyclable catalyst in ethanol (Scheme 26).148 A 50 mg loading of MTSA was found to be sufficient to promote the reaction in forward direction. Good yields were obtained using aromatic aldehydes with electron-donating or electron-withdrawing substituents. Aromatic aldehydes with electron-withdrawing groups yielded the products in a short time rather than electron-donating ones. The authors have also discussed this synthesis with mechanistic point of view, according to them in the first step Knoevenagel product was formed via condensation of aldehydes and active methylene compounds. The second key intermediate is enamine, produced by the condensation of dimedone with ammonia. Condensation of these two fragments gives the acyclic Michael adduct, which undergoes intermolecular cyclization with the participation of the amino function and one of the indanedione carbonyl groups to form the dihydroindenoquinoline in good yield. The catalyst could be recycled and reused three times without significant loss of activity.
3.2.7.3 Synthesis of spiro[pyrazolo[3,4-b]pyridine-4,3-indoline] derivatives. Yang et al. have discussed an efficient protocol for the synthesis of spiro[pyrazolo[3,4-b]pyridine-4,3-indoline] derivatives by the three component reaction of isatins, 3-methyl-1-phenyl-1H-pyrazol-5-amine and Meldrum's acid in the presence of a catalytic amount of melamine trisulfonic acid (MTSA) in water under reflux conditions (Scheme 27).149 The authors have demonstrated comparison study of various catalysts such as sulfuric acid, p-TSA, sulfamic acid and FeCl3 with MTSA. Use of an inexpensive, non-toxic, reusable and an easily available catalyst makes this protocol green and clean.
3.2.7.4 Synthesis of 4,4′-(arylmethylene)-bis-(1H-pyrazol-5-ols). Iravani and co-workers discussed catalytic application of a melamine trisulfunic acid (MTSA) as a recyclable catalyst for the condensation reaction of aromatic aldehydes with 3-methyl-l-phenyl-2-pyrazolin-5-one in ethanol under refluxing conditions, which afforded products in excellent yields (Scheme 28).150 Only, 15 mol% of catalyst was sufficient for this conversion. Different benzaldehydes with electron-donating or electron-withdrawing groups were smoothly reacted with this protocol to afforded products in excellent yields. The recycled catalyst could be reused five times without significant loss of their efficiency. As compared to CSA, MTSA shows good efficiency for this synthesis in terms of product yield and reaction time.24
4 Conclusion
In summary, we gave an overview on application of, melamine trisulfunic acid (MTSA) as an efficient, recyclable and heterogeneous catalyst in organic reactions. This catalyst with mild acidity, have shown excellent activity in various chemical reactions especially organic synthesis. Excellent accessibility, biodegradability, thermal stability and inexpensiveness are significant properties of this catalyst. The superior advantage of this heterogeneous catalyst is that it can be recovered and reused several times without the loss of their efficiency. A wide range of the reactions catalyzed by MTSA were carried out under solvent-free conditions, which makes those protocols green and clean. Here, MTSA proved to be a recyclable, green, and highly effective solid acid catalyst for a wide range of organic transformation and no doubt we will see large number of organic transformation catalyzed by MTSA.Acknowledgements
The authors are thankful to the Department of Chemistry, Gujarat University, Ahmedabad, for providing the necessary facilities. UGC-Info net & INFLIBNET Gujarat University are acknowledged for providing the e-source facilities. R. H. V. is thankful to UGC-BSR (F.7-74/2007 (BSR)) for financial assistance.References
- M. Lancaster, Green chemistry: an introductory text, Royal Society of Chemistry, Cambridge, 2002 Search PubMed.
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686–694 CrossRef CAS PubMed.
- B. Karimi and M. Khalkhali, J. Mol. Catal. A: Chem., 2005, 232, 113–117 CrossRef CAS.
- B. Karimi and D. Zareyee, Org. Lett., 2008, 10, 3989–3992 CrossRef CAS PubMed.
- P. Salehi, M. Ali Zolfigol, F. Shirini and M. Baghbanzadeh, Curr. Org. Chem., 2006, 10, 2171–2189 CrossRef CAS.
- K. Niknam and M. Zolfigol, J. Iran. Chem. Soc., 2006, 3, 59–63 CrossRef CAS.
- K. Niknam, B. Karami and M. A. Zolfigol, Catal. Commun., 2007, 8, 1427–1430 CrossRef CAS.
- K. Niknam, M. A. Zolfigol, G. Chehardoli and M. Dehghanian, Chin. J. Catal., 2008, 29, 901–906 CrossRef CAS.
- A. Corma and H. Garcia, Chem. Rev., 2002, 102, 3837–3892 CrossRef CAS PubMed.
- P. B. Venuto, Microporous Mater., 1994, 2, 297–411 CrossRef CAS.
- S. Besoluk, M. Kucukislamoglu, M. Nebioglu, M. Zengin and M. Arslan, J. Iran. Chem. Soc., 2008, 5, 62–66 CrossRef CAS.
- B. Karami, M. Montazerozohori and M. H. Habibi, Phosphorus, Sulfur Silicon Relat. Elem., 2006, 181, 2825–2831 CrossRef CAS.
- B. Karami, S. Khodabakhshi and M. Nikrooz, Polycyclic Aromat. Compd., 2011, 31, 97–109 CrossRef CAS.
- M. Dabiri, P. Salehi, S. Otokesh, M. Baghbanzadeh, G. Kozehgary and A. A. Mohammadi, Tetrahedron Lett., 2005, 46, 6123–6126 CrossRef CAS.
- H. Firouzabadi, N. Iranpoor, M. Jafarpour and A. Ghaderi, J. Mol. Catal. A: Chem., 2006, 253, 249–251 CrossRef CAS.
- S. Rostamizadeh, A. Amirahmadi, N. Shadjou and A. M. Amani, J. Heterocycl. Chem., 2012, 49, 111 CrossRef CAS.
- H. R. Shaterian, A. Hosseinian and M. Ghashang, ARKIVOC, 2009, 2, 59–67 Search PubMed.
- P. K. Mandal and A. K. Misra, Lett. Org. Chem., 2006, 3, 848–853 CrossRef CAS.
- C. Ramesh, J. Banerjee, R. Pal and B. Das, Adv. Synth. Catal., 2003, 345, 557–559 CrossRef CAS.
- R. H. Vekariya and H. D. Patel, Synth. Commun., 2014, 45, 1031–1054 CrossRef.
- S. Ko and C.-F. Yao, Tetrahedron Lett., 2006, 47, 8827–8829 CrossRef CAS.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171–198 CrossRef CAS PubMed.
- A.-X. Li, T.-S. Li and T.-H. Ding, Chem. Commun., 1997, 1389–1390 RSC.
- R. H. Vekariya and H. D. Patel, ARKIVOC, 2015, 1, 136–159 Search PubMed.
- R. H. Vekariya and H. D. Patel, RSC Adv., 2015, 5, 49006–49030 RSC.
- R. H. Vekariya and H. D. Patel, ARKIVOC, 2015, 1, 70–96 Search PubMed.
- F. Shirini and J. Albadi, Bull. Korean Chem. Soc., 2010, 31, 1119–1120 CrossRef CAS.
- C. O. Kappe, Tetrahedron, 1993, 49, 6937–6963 CrossRef CAS.
- H. Salehi and Q. X. Guo, Synth. Commun., 2004, 34, 171–179 CrossRef CAS.
- A. Debache, M. Amimour, A. Belfaitah, S. Rhouati and B. Carboni, Tetrahedron Lett., 2008, 49, 6119–6121 CrossRef CAS.
- C. V. Reddy, M. Mahesh, P. Raju, T. R. Babu and V. N. Reddy, Tetrahedron Lett., 2002, 43, 2657–2659 CrossRef CAS.
- R. Varala, M. Mujahid Alam and S. R. Adapa, Synlett, 2003, 67–70 CAS.
- S. Chitra and K. Pandiarajan, Tetrahedron Lett., 2009, 50, 2222–2224 CrossRef CAS.
- F. Shirini, K. Marjani and H. T. Nahzomi, ARKIVOC, 2007, 51–57 CAS.
- J. Lu and Y. Bai, Synthesis, 2002, 466–470 CrossRef CAS.
- P. M. Pihko, Lett. Org. Chem., 2005, 2, 398–403 CrossRef CAS.
- A. Paraskar, G. Dewkar and A. Sudalai, Tetrahedron Lett., 2003, 44, 3305–3308 CrossRef CAS.
- F. Shirini, M. Zolfigol and A.-R. Abri, J. Iran. Chem. Soc., 2008, 5, 96–99 CrossRef CAS.
- F. Shirini, M. A. Zolfigol and J. Albadi, Chin. Chem. Lett., 2011, 22, 318–321 CrossRef CAS.
- X.-f. Zhou, L. Zhang, E. Tseng, E. Scott-Ramsay, J. J. Schentag, R. A. Coburn and M. E. Morris, Drug Metab. Dispos., 2005, 33, 321–328 CrossRef CAS PubMed.
- T. Tsuruo, H. Iida, M. Nojiri, S. Tsukagoshi and Y. Sakurai, Cancer Res., 1983, 43, 2905–2910 CAS.
- R. S. Kumar, A. Idhayadhulla, A. J. A. Nasser and J. Selvin, Eur. J. Med. Chem., 2011, 46, 804–810 CrossRef CAS PubMed.
- S. R. Kumar, A. Idhayadhulla, A. J. A. Nasser and J. Selvin, J. Serb. Chem. Soc., 2011, 76, 1–11 CrossRef.
- A. Krauze, S. Ģērmane, O. Eberlin, I. Šturms, V. Klusā and G. Duburs, Eur. J. Med. Chem., 1999, 34, 301–310 CrossRef CAS.
- S. S. Mansoor, K. Aswin, K. Logaiya and S. Sudhan, J. King Saud Univ., Sci., 2013, 25, 191–199 CrossRef.
- J.-L. Wang, D. Liu, Z.-J. Zhang, S. Shan, X. Han, S. M. Srinivasula, C. M. Croce, E. S. Alnemri and Z. Huang, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 7124–7129 CrossRef CAS.
- D. Kumar, V. B. Reddy, S. Sharad, U. Dube and S. Kapur, Eur. J. Med. Chem., 2009, 44, 3805–3809 CrossRef CAS PubMed.
- N. J. Thumar and M. P. Patel, ARKIVOC, 2009, 13, 363–380 Search PubMed.
- A. Martinez-Grau and J. Marco, Bioorg. Med. Chem. Lett., 1997, 7, 3165–3170 CrossRef CAS.
- H. Valizadeh and A. Azimi, J. Iran. Chem. Soc., 2011, 8, 123–130 CrossRef CAS.
- L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517–520 CrossRef CAS.
- S. S. Mansoor, K. Aswin, K. Logaiya, S. Sudhan and H. Ramadoss, J. Saudi Chem. Soc., 2013 DOI:10.1016/j.jscs.2012.12.010.
- K. Aswin, K. Logaiya, P. N. Sudhan and S. S. Mansoor, J. Taibah Univ. Sci., 2012, 6, 1–9 CrossRef.
- W. Li and Q. Luo, Eur. J. Chem., 2011, 8, 1972–1978 CAS.
- R. H. Vekariya and H. D. Patel, Synth. Commun., 2014, 44, 2756–2788 CrossRef CAS.
- H. Von Pechmann and C. Duisberg, Chem. Ber., 1884, 17, 929–936 CrossRef.
- L. L. Woods and J. Sapp, J. Org. Chem., 1962, 27, 3703–3705 CrossRef CAS.
- D. S. Bose, A. Rudradas and M. H. Babu, Tetrahedron Lett., 2002, 43, 9195–9197 CrossRef.
- V. M. Alexander, R. P. Bhat and S. D. Samant, Tetrahedron Lett., 2005, 46, 6957–6959 CrossRef CAS.
- F. Shirini, K. Marjani, H. T. Nahzomi and M. A. Zolfigol, Chin. Chem. Lett., 2007, 18, 909–911 CrossRef CAS.
- G. Sharma, J. J. Reddy, P. S. Lakshmi and P. R. Krishna, Tetrahedron Lett., 2005, 46, 6119–6121 CrossRef CAS.
- H. Valizadeh and A. Shockravi, Tetrahedron Lett., 2005, 46, 3501–3503 CrossRef CAS.
- F. Shirini, M. Zolfigol and J. Albadi, J. Iran. Chem. Soc., 2010, 7, 895–899 CrossRef CAS.
- A. Khazaei, M. A. Zolfigol, T. Faal Rastegar, G. Chehardoli and S. Mallakpour, Iran. J. Catal., 2013, 3, 211–220 CAS.
- M. Sayyafi, M. Seyyedhamzeh, H. R. Khavasi and A. Bazgir, Tetrahedron, 2008, 64, 2375–2378 CrossRef CAS.
- H. R. Shaterian, M. Ghashang and M. Feyzi, Appl. Catal., A, 2008, 345, 128–133 CrossRef CAS.
- J. M. Khurana and D. Magoo, Tetrahedron Lett., 2009, 50, 7300–7303 CrossRef CAS.
- R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri and M. Ghavidel, Tetrahedron, 2011, 67, 1930–1937 CrossRef CAS.
- C. Conti and N. Desideri, Bioorg. Med. Chem., 2010, 18, 6480–6488 CrossRef CAS PubMed.
- A. Foroumadi, G. Dehghan, A. Samzadeh-Kermani, F. Arabsorkhi, M. Sorkhi, A. Shafiee and M. Abdollahi, Asian J. Chem., 2007, 19, 1391–1396 CAS.
- M. S. El-Gaby, M. A. Zahran, M. M. Ismail and Y. A. Ammar, Il Farmaco, 2000, 55, 227–232 CrossRef CAS PubMed.
- H. Gourdeau, L. Leblond, B. Hamelin, C. Desputeau, K. Dong, I. Kianicka, D. Custeau, C. Boudreau, L. Geerts and S.-X. Cai, Mol. Cancer Ther., 2004, 3, 1375–1384 CAS.
- F. Cassidy, J. M. Evans, M. S. Hadley, A. H. Haladij, P. E. Leach and G. Stemp, J. Med. Chem., 1992, 35, 1623–1627 CrossRef CAS PubMed.
- W. Ma, X. Wang, F. Yan, L. Wu and Y. Wang, Monatsh. Chem., 2011, 142, 163–167 CrossRef CAS.
- A. Zare and F. Monfared, Iran. Chem. Commun., 2013, 2, 1–17 Search PubMed.
- S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 4, 24463–24476 RSC.
- A. Zare, M. Dashtizadeh and M. Merajoddin, Iran. Chem. Commun., 2015, 3, 208–217 Search PubMed.
- M. A. Pasha and V. P. Jayashankara, Bioorg. Med. Chem. Lett., 2007, 17, 621–623 CrossRef CAS PubMed.
- G. W. Rewcastle, G. J. Atwell, L. Zhuang, B. C. Baguley and W. A. Denny, J. Med. Chem., 1991, 34, 217–222 CrossRef CAS PubMed.
- A. Moosavi-Zare, M. Rezaei, M. Merajoddin, H. Hamidian, A. Zare and M. Kazem-Rostami, Sci. Iran., 2014, 21, 2049 Search PubMed.
- R. H. Vekariya and H. D. Patel, Synth. Commun., 2014, 44, 2313–2335 CrossRef CAS.
- J. Albadi, F. Shirini, B. Ghabezi and T. Seiadatnasab, Arabian J. Chem., 2012 DOI:10.1016/j.arabjc.2012.1010.1011.
- P. G. Wuts and T. W. Greene, Greene's protective groups in organic synthesis, John Wiley & Sons, New York, 2007 Search PubMed.
- M. Fallah-Mehrjardi and K. Ghanemi, Jordan J. Chem., 2012, 7, 393–399 CAS.
- D. J. Kalita, R. Borah and J. C. Sarma, Tetrahedron Lett., 1998, 39, 4573–4574 CrossRef CAS.
- B. Wang, Y. Gu, G. Song, T. Yang, L. Yang and J. Suo, J. Mol. Catal. A: Chem., 2005, 233, 121–126 CrossRef CAS.
- N. Srivastava, S. K. Dasgupta and B. K. Banik, Tetrahedron Lett., 2003, 44, 1191–1193 CrossRef CAS.
- P. G. Wuts and T. W. Greene, Greene's protective groups in organic synthesis, John Wiley & Sons, New York, 2006 Search PubMed.
- L. Wu, P. Sun and F. Yan, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 2055–2060 CrossRef CAS.
- H. R. Shaterian and M. Ghashang, Phosphorus, Sulfur Silicon Relat. Elem., 2007, 183, 194–204 CrossRef.
- J. Yadav, B. Reddy, A. Basak, G. Baishya and A. Venkat Narsaiah, Synthesis, 2006, 3831–3834 CrossRef CAS.
- A. V. Narsaiah, J. Organomet. Chem., 2007, 692, 3614–3618 CrossRef CAS.
- F. Shirini, M. A. Zolfigol and A.-R. Abri, Monatsh. Chem., 2008, 139, 17–20 CrossRef CAS.
- F. Shirini and E. Mollarazi, Catal. Commun., 2007, 8, 1393–1396 CrossRef CAS.
- H. R. Shaterian, R. Doostmohammadi and M. Ghashang, Chin. J. Chem., 2008, 26, 1709–1714 CrossRef CAS.
- B. Akhlaghinia and S. Tavakoli, Synthesis, 2005, 1775–1778 CrossRef CAS.
- I. Saxena, N. Deka, J. C. Sarma and S. Tsuboi, Synth. Commun., 2003, 33, 4185–4191 CrossRef.
- W.-S. Cho and C.-H. Lee, Bull. Korean Chem. Soc., 1998, 19, 314–318 CAS.
- F. Chevalier, G. R. Geier III and J. S. Lindsey, J. Porphyrins Phthalocyanines, 2002, 6, 186–197 CrossRef CAS.
- L. Wu, Y. Yan and F. Yan, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 149–154 CrossRef CAS.
- N. Iranpoor and F. Kazemi, Tetrahedron, 1998, 54, 9475–9480 CrossRef CAS.
- W. Bao and Y. Zhang, Chin. J. Org. Chem., 1998, 18, 272–274 CAS.
- J.-T. Li, W.-Z. Yang, G.-F. Chen and T.-S. Li, Synth. Commun., 2003, 33, 2619–2625 CrossRef CAS.
- X. Zhang, X. Fan, H. Niu and J. Wang, Green Chem., 2003, 5, 267–269 RSC.
- G. Deng and T. Ren, Synth. Commun., 2003, 33, 2995–3001 CrossRef CAS.
- M. Abaee, M. Mojtahedi, R. Sharifi, M. Zahedi, H. Abbasi and K. Tabar-Heidar, J. Iran. Chem. Soc., 2006, 3, 293–296 CrossRef CAS.
- B. Das, P. Thirupathi, I. Mahender and K. R. Reddy, J. Mol. Catal. A: Chem., 2006, 247, 182–185 CrossRef CAS.
- L. Wang, J. Sheng, H. Tian, J. Han, Z. Fan and C. Qian, Synthesis, 2004, 3060–3064 CrossRef CAS.
- P. Salehi, M. M. Khodaei, M. A. Zolfigol and A. Keyvan, Monatsh. Chem., 2002, 133, 1291–1295 CrossRef CAS.
- S. Bhagat, R. Sharma and A. K. Chakraborti, J. Mol. Catal. A: Chem., 2006, 260, 235–240 CrossRef CAS.
- F. Shirini, S. Sarvi Beigbaghlou and S. V. Atghia, Iran. J. Catal., 2012, 2, 157–163 Search PubMed.
- A. R. Kiasat and M. Fallah-Mehrjardi, Synth. Commun., 2010, 40, 1551–1558 CrossRef CAS.
- M. R. M. Bhojegowd, A. Nizam and M. A. Pasha, Chin. J. Catal., 2010, 31, 518–520 CrossRef CAS.
- M. Lei, L. Ma and L. Hu, Tetrahedron Lett., 2010, 51, 4186–4188 CrossRef CAS.
- B. Das, M. Krishnaiah, P. Balasubramanyam, B. Veeranjaneyulu and D. N. Kumar, Tetrahedron Lett., 2008, 49, 2225–2227 CrossRef CAS.
- M. Hosseini-Sarvari and H. Sharghi, J. Org. Chem., 2006, 71, 6652–6654 CrossRef CAS PubMed.
- X. J. Yang and Y. S. Zhang, Res. Chem. Intermed., 2013, 39, 2843–2848 CrossRef CAS.
- N. Iravania, M. Keshavarzb, M. Mousavia and M. Baghernejadc, Iran. J. Catal., 2015, 5, 65–71 Search PubMed.
- D. Enders, M. Moser, G. Geibel and M. C. Laufer, Synthesis, 2004, 2040–2046 CrossRef CAS.
- B. Das, M. Krishnaiah, K. Laxminarayana and K. R. Reddy, J. Mol. Catal. A: Chem., 2007, 270, 284–288 CrossRef CAS.
- I. N. Rao, E. Prabhakaran, S. K. Das and J. Iqbal, J. Org. Chem., 2003, 68, 4079–4082 CrossRef CAS PubMed.
- A. K. Tiwari, R. M. Kumbhare, S. B. Agawane, A. Z. Ali and K. V. Kumar, Bioorg. Med. Chem. Lett., 2008, 18, 4130–4132 CrossRef CAS PubMed.
- A. Zare, Eur. J. Chem., 2012, 9, 2322–2331 CAS.
- A. R. Momeni and M. Sadeghi, Appl. Catal., A, 2009, 357, 100–105 CrossRef CAS.
- M. T. Maghsoodlou, A. Hassankhani, H. R. Shaterian, S. M. Habibi-Khorasani and E. Mosaddegh, Tetrahedron Lett., 2007, 48, 1729–1734 CrossRef CAS.
- R. Ghosh, S. Maiti, A. Chakraborty, S. Chakraborty and A. K. Mukherjee, Tetrahedron, 2006, 62, 4059–4064 CrossRef CAS.
- A. T. Khan, L. H. Choudhury, T. Parvin and M. A. Ali, Tetrahedron Lett., 2006, 47, 8137–8141 CrossRef CAS.
- F. Shirini, M. A. Zolfigol, J. Albadi and T. F. Rastegar, Iran. J. Catal., 2011, 1, 11–17 CAS.
- F. Shirini, M. A. Zolfigol and J. Albadi, Synth. Commun., 2010, 40, 910–914 CrossRef CAS.
- R. Dalpozzo, A. De Nino, L. Maiuolo, A. Procopio, M. Nardi, G. Bartoli and R. Romeo, Tetrahedron Lett., 2003, 44, 5621–5624 CrossRef CAS.
- F. Shirini, M. Zolfigol and A. Safari, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2005, 44, 201 Search PubMed.
- B. Das and P. Thirupathi, J. Mol. Catal. A: Chem., 2007, 269, 12–16 CrossRef CAS.
- I. Mohammadpoor-Baltork, H. Aliyan and A. R. Khosropour, Tetrahedron, 2001, 57, 5851–5854 CrossRef CAS.
- F. Shirini, M. A. Zolfigol and K. Mohammadi, Bull. Korean Chem. Soc., 2004, 25, 325–327 CrossRef CAS.
- R. Alleti, M. Perambuduru, S. Samantha and V. P. Reddy, J. Mol. Catal. A: Chem., 2005, 226, 57–59 CrossRef CAS.
- A. Kamal, M. N. A. Khan, K. S. Reddy, Y. Srikanth and T. Krishnaji, Tetrahedron Lett., 2007, 48, 3813–3818 CrossRef CAS.
- F. Shirini, M. A. Zolfigol and M. Abedini, Monatsh. Chem., 2004, 135, 279–282 CrossRef CAS.
- F. Shirini, M. A. Zolfigol, A.-R. Aliakbar and J. Albadi, Synth. Commun., 2010, 40, 1022–1028 CrossRef CAS.
- F. Shirini and J. Albadi, Bull. Korean Chem. Soc., 2010, 31, 1119–1120 CrossRef CAS.
- J. W. Ralls, R. Dodson and B. Riegel, J. Am. Chem. Soc., 1949, 71, 3320–3325 CrossRef CAS.
- C. Djerassi and M. Gorman, J. Am. Chem. Soc., 1953, 75, 3704–3708 CrossRef CAS.
- B. Karimi and H. Seradj, Synlett, 2000, 805–806 CAS.
- A. Kamal, G. Chouhan and K. Ahmed, Tetrahedron Lett., 2002, 43, 6947–6951 CrossRef CAS.
- B. C. Ranu and A. Das, Aust. J. Chem., 2004, 57, 605–608 CrossRef CAS.
- H. R. Shaterian, A. Hosseinian and M. Ghashang, Synth. Commun., 2008, 38, 4097–4106 CrossRef CAS.
- S. S. Mansoor, K. Logaiya, S. Sudhan, K. Aswin, N. R. Ahmed and A. M. Hussain, J. Indian Chem. Soc., 2014, 91, 1–7 Search PubMed.
- N. P. Prajapati, R. H. Vekariya, M. A. Borad and H. D. Patel, RSC Adv., 2014, 4, 60176–60208 RSC.
- F. Shirini, S. S. Beigbaghlou, S. V. Atghia and S. A.-R. Mousazadeh, Dyes Pigm., 2013, 97, 19–25 CrossRef CAS.
- L. M. Yang, P. L. Sun and L. Q. Wu, J. Chin. Chem. Soc., 2012, 59, 1500–1503 CrossRef CAS.
- N. Iravani, J. Albadi, H. Momtazan and M. Baghernejad, J. Chin. Chem. Soc., 2013, 60, 418–424 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |