Controlled protein separation based on pressure–voltage (P–V) coupling effects in a nanopore based device
Abstract
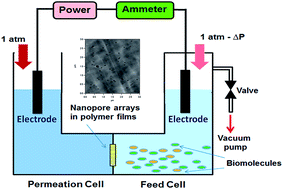
The separation and/or purification of biomolecules are of great importance in biotechnological analysis, characterization and related applications. In this work, a set of “U”-type devices containing a feed cell and a permeation cell connected by nanopore arrays was designed for controlled protein separation. Certain voltages (as a driving force) and pressure differences (ΔP, as resistance) were applied to make selected protein move from the feed cell into the permeation cell through nanopore arrays. The resultant driving forces applied to the proteins can be modulated by changing the parameters such as applied voltage, pH value and ΔP between two cells, thus, their translocation velocity can be controlled. By finding the P–V equilibrium points for bovine serum albumin (BSA) and bovine hemoglobin (Hb), the separation ratios (mass ratio) for BSA and Hb can be achieved as BSA : Hb = 12.5 and Hb : BSA = 14.3.

 Please wait while we load your content...
Please wait while we load your content...