Heterogeneous Cu/OMS-2 as an efficient catalyst for the synthesis of tetrasubstituted 1,4-enediones and 4H-pyrido[1,2-a]-pyrimidin-4-ones†
Jinqi Zhangac,
Xu Mengab,
Chaoying Yua,
Gexin Chena and
Peiqing Zhao*ab
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou 730000, China. E-mail: zhaopq@licp.cas.cn; Fax: +86 931 827700
bState Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou 730000, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 6th October 2015
Abstract
An efficient Cu/OMS-2-catalyzed oxidative heterogeneous protocol has been developed for the synthesis of tetrasubstituted unsymmetrical 1,4-enediones and 4H-pyrido[1,2-a]-pyrimidin-4-ones from 1,4-enediones and amines or 2-aminopyridines. The present catalytic system employs air as the terminal oxidant and tolerates a broad range of substrates using low loading copper (1.3 wt%). The Cu/OMS-2 was characterized by various methods, including XRD, XPS, BET as well as TEM/SEM, and the results of H2-TPR shows that the redox ability of the catalyst was improved by the combination of Cu and OMS-2. Moreover, the catalyst is recyclable.
Introduction
From economic and industrial points of views, heterogeneous catalysis is more attractive because of catalyst recyclability, handling and easy separation of catalyst from the products.1,2 In recent decades, manganese oxide octahedral molecular sieves (OMS-2) have gained attention mainly due to their unique nature in heterogeneous catalysis. OMS-2 is a microporous material with a composition of KMn8O16·nH2O. The structure of it contains 4.6 × 4.6 Å tunnels that arise from the 2 × 2 arrangement of octahedron and the average oxidation state of Mn is 3.8 with the presence of Mn4+, Mn3+, and Mn2+ ions in the framework.3 Consequently, the mixed valent, crystalline and semi-conductive properties make OMS-2 have a wide range of applications.4Our group has been committed to studying OMS-2-based heterogeneous catalysis and found that solid supported catalyst Cu/OMS-2 with the low-loading copper has the interaction of electron transfer between metal catalytic species and support.5 Based on the previous reports, it is believed that the catalytic ability of Cu/OMS-2 can be significantly improved by the electronic interaction, which might facilitate organic transformations and make them proceed under a low-energy pathway.6,7 Therefore, the development of efficient heterogeneous protocol for organic transformation using Cu/OMS-2 is appreciated.
In recent years, the 1,4-enedione skeleton has received great attention since it widely presents in bioactive natural products and pharmaceutical compounds (Fig. 1).8 More importantly, 1,4-enediones are significant synthetic drug precursors for further elaboration.9 Thus, numerous synthetic methods have been established to produce this unit.10 Nevertheless, to the best of our knowledge, only Wu's group reported a homogeneous method promoted by Cu(OAc)2 for the synthesis of tetrasubstituted unsymmetrical 1,4-enediones in 2013.11 Based on the above works and our previous studies,12 we herein describe a heterogeneous oxidative method for the synthesis of tetrasubstituted unsymmetrical 1,4-enediones using Cu/OMS-2 as catalyst under air.
Experimental section
Preparation of OMS-2 (ref. 4a)
5.89 g of KMnO4 in 100 mL of deionized water was added to a solution of 8.8 g of MnSO4·H2O in 30 mL of deionized water and 3 mL concentrated HNO3. The solution was refluxed at 100 °C for 24 hours, and the product was filtered, washed, and dried at 120 °C for 8 hours. Finally, the dry OMS-2 was calcined in a muffle furnace at 350 °C for 2 hours. Then, the black powder OMS-2 was obtained.Preparation of Cu/OMS-2 (ref. 5)
Support OMS-2 (2 g) was added to a 50 mL round-bottom flask. A solution of Cu(NO3)2·3H2O (0.15 g) in deionized water (10 mL) was added to OMS-2, and additional deionized water (10 mL) was added to wash down the sides of the flask. Then the flask was submerged into an ultrasound bath for 3 hours at room temperature and stirred for further 20 hours at room temperature. After that, the water was distilled under reduced pressure on a rotary evaporator at 80 °C for more than 2 hours. Finally, the black powder was dried into an oven at 110 °C for 4 hours followed by calcination at 350 °C for 2 hours. The Inductive Coupled Plasma Optical Emission Spectrum (ICP-OES) showed Cu content is 1.3 wt%.General procedure for Cu/OMS-2-catalyzed synthesis of tetrasubstituted 1,4-enediones 3
Cu/OMS-2 (30 mg, 2 mol%), 1,4-enediones 1 (0.3 mmol), N-substituted nucleophiles 2 (0.6 mmol), DMSO (1.8 mL) were added to a flask with a stirring bar. The flask was stirred at 80 °C for 12 hours under air. After cooling to room temperature, the mixture was diluted with ethyl acetate and filtered. The filtrate was removed under reduced pressure to get the crude product, which was further purified by silica gel chromatography (petroleum/ethyl acetate = 4/1 as eluent) to yield corresponding product. The identity and purity of the products was confirmed by 1H and 13C NMR spectroscopic analysis.General procedure for Cu/OMS-2-catalyzed synthesis of 4H-pyrido[1,2-a]-pyrimidin-4-ones 5
Cu/OMS-2 (30 mg, 2 mol%), 1,4-enediones 1 (0.3 mmol), 2-aminopyridines 4 (0.6 mmol), DMSO (1.8 mL) were added to a flask with a stirring bar. The flask was stirred at 80 °C for 12 hours under air. After cooling to room temperature, the mixture was diluted with ethyl acetate and filtered. The filtrate was removed under reduced pressure to get the crude product, which was further purified by silica gel chromatography (petroleum/ethyl acetate = 2/1 as eluent) to yield corresponding product. The identity and purity of the products was confirmed by 1H and 13C NMR spectroscopic analysis.Catalyst durability
The Cu/OMS-2 catalyst was isolated by centrifugation after each run of reactions, then washed by ethanol and water. After drying at 110 °C for 5 hours, the catalyst was reused with fresh charge of solvent and reactant for subsequent reaction under the same conditions.Characterization
The specific surface areas (SBET) of the catalysts were measured from a multipoint Brunauer–Emmett–Teller (BET) analysis of the nitrogen adsorption isotherms at 77 K recorded on a Quantachrome Autosorn-1 apparatus. The morphologies of the samples were characterized by a TF20 transmission electron microscope (TEM) and SM-5600LV scanning electron microscope (SEM). The crystal phase and composition of catalysts were determined by power X-ray diffraction (XRD) using a X-Pert PRO X-ray diffractometer with Cu Kα radiation in the 2θ ranges of 10–90°. The surface properties of the catalyst were analyzed by a Thermo Scientific ESCALAB 250Xi X-ray photoelectron spectrometer (XPS). The reducibility of the catalysts was measured by the hydrogen temperature-programmed reduction (H2-TPR) technique. 50 mg of catalyst was placed in a quartz reactor that was connected to a TPR apparatus and the reactor was heated from r.t. to 550 °C with a heating rate of 10 °C min−1. The reducing atmosphere was the mixture of H2 and N2 with a total flow rate of 30 mL min−1 and the amount of H2 uptake during the reduction was measured by a thermal conductivity detector (TCD).Results and discussion
The catalyst was synthesized by wet impregnation method. The BET surface areas and porosities of Cu/OMS-2 were determined by N2 adsorption–desorption at 77 K and the results showed that the BET surface area is 127 m2 g−1, pore volume is 0.48 cm3 g−1 and pore size is 137.2 Å (Table S1, see ESI†).The morphology of Cu/OMS-2 was inspected by TEM and SEM (Fig. 2), the images show that the synthetic material is uniform nanorod with diameters of about 8 nm and lengths of 70–120 nm.13
Fig. 3 plots the XPD patterns of OMS-2 and Cu/OMS-2, the results show that no signals caused by copper oxide or copper metal (cluster) were observed and the XRD pattern of Cu/OMS-2 was identical to that of support OMS-2,14 which further illustrated copper oxide is highly dispersed on support OMS-2. Besides, the structure of reused catalyst was not altered (see below for the test of catalyst recyclability).
To explore the surface properties of the catalyst, Cu/OMS-2 was characterized by XPS (Fig. S2, see ESI†). The Mn 2p spectrum shows that Mn 2p1/2 peak and Mn 2p3/2 peak are centred at 654.0 eV and 642.4 eV respectively, indicating this sample contains both Mn3+ and Mn4+.15 The Cu 2p spectrum shows that the binding energies of Cu 2p1/2 and Cu 2p3/2 are about 954.0 eV and 934.3 eV, and the shake-up satellite peaks of Cu 2p1/2 and Cu 2p3/2 are at 962.1 eV and at 944.7 eV, respectively, which demonstrates that Cu2+ species are existed on the surface of the catalyst.15,19a
The redox ability of the catalyst was studied by H2-temperature programmed reduction (H2-TPR) and the profiles are presented in Fig. 4. For the support OMS-2, the overlapping peaks (marked as α, β) from 369 °C to 390 °C are ascribed to the reduction of MnO2 to MnO.16,18 Compared with OMS-2, the reduction peaks of Cu/OMS-2 shift to low temperature, suggesting that the existence of copper species improves the reducibility of manganese oxide by a hydrogen spillover effect.17,19a More importantly, the reduction peaks α′ and β′ of Cu/OMS-2 became obvious, which probably is caused by the enhanced mobility of oxygen.15,18 These results all confirm the interaction between the catalystic metal copper and the support OMS-2. Moreover, the extra peaks γ and δ appeared in the TPR profile of the Cu/OMS-2 are related to the reduction of CuO to Cu. The γ peak observed at 210 °C corresponds to the reduction of the finely dispersed CuO particles and the higher-temperature peak δ represents the reduction of the bulk CuO particles.19
Next, we employed 1,4-enedione 1a and pyrazole 2a as the model substrates to optimize the reaction conditions (Table 1). In the first attempt, the reaction was run using OMS-2 as the catalyst under air at room temperature, but no desired product was observed (Table 1, entry 1). To our delight, 58% yield of the target product was obtained when Cu/OMS-2 was used as the catalyst (Table 1, entry 2). Then, Cu(OH)x/OMS-2 was examined in the reaction and it did not better the reaction (Table 1, entry 3). Besides, various supports were tested, such as ATP (attapulgite), C (charcoal) and Al2O3, and it was found that OMS-2 is optimal for this reaction (Table 1, entries 4–6). Importantly, a very low yield of 3aa was observed when the reaction temperature was increased from r.t. to 120 °C with the Cu/Al2O3 as the catalyst. These observations perhaps indicate that Cu/Al2O3 is not able to lower the redox energy barriers while high temperature made the reaction work to some extent by overcoming the redox energy barriers (Table 1, entry 7). Next, we tried different solvents, which shows the reaction did not proceed in DMF (dimethyl formamide) and DCB (dichlorobenzene) (Table 1, entries 8 and 9). DMSO (dimethylsulfoxide) led to the best result and other solvents, like nitromethane, toluene, dichloromethane and THF (tetrahydrofuran), could give the desired product to some extent (Table 1, entries 10–13). Subsequently, the reaction temperature was optimized, and we found that 80 °C is the best choice (Table 1, entries 14–16). More experiments demonstrated that using O2 as the oxidant did not better the reaction obviously and only trace amount of 3aa was observed under N2 (Table 1, entries 17 and 18). Finally, the catalytic activity of physical mixture of bulk CuO and OMS-2 was much lower than this of Cu/OMS-2, which indicates highly dispersed Cu species played a crucial role in the reaction (Table 1, entry 19). Furthermore, without the external oxidant, equivalent unsupported CuO could also give moderate yield (Table 1, entry 20), while catalytic amount of CuO gave the poor yield of 3aa with oxygen as the external oxidant (Table 1, entry 21). These observations indicate that the reduced copper species cannot be reoxidized by O2 due to the high-energy barrier of direct reoxidation without the help of OMS-2.
| Entry | Cat. | Solvent | Temp. (°C) | Oxidant | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise specified, all reactions were carried out using 1a (0.3 mmol), 2a (0.6 mmol) and catalysts (30 mg) in 1.8 mL solvent under air for 12 h.b Isolated yields.c Cu content: 1.3 wt%.d CuO (2 mol%), OMS-2 (30 mg). | |||||
| 1 | OMS-2 | DMSO | r.t. | Air | 0 |
| 2c | Cu/OMS-2 | DMSO | r.t. | Air | 58 |
| 3 | Cu(OH)x/OMS-2 | DMSO | r.t. | Air | 0 |
| 4 | Cu/ATP | DMSO | r.t. | Air | 0 |
| 5 | Cu/C | DMSO | r.t. | Air | 0 |
| 6 | Cu/Al2O3 | DMSO | r.t. | Air | 0 |
| 7 | Cu/Al2O3 | DMSO | 120 | Air | 7 |
| 8c | Cu/OMS-2 | DMF | r.t. | Air | 0 |
| 9c | Cu/OMS-2 | DCB | r.t. | Air | 0 |
| 10c | Cu/OMS-2 | CH3NO2 | r.t. | Air | 36 |
| 11c | Cu/OMS-2 | Toluene | r.t. | Air | 43 |
| 12c | Cu/OMS-2 | CH2Cl2 | r.t. | Air | 34 |
| 13c | Cu/OMS-2 | THF | r.t. | Air | 27 |
| 14c | Cu/OMS-2 | DMSO | 40 | Air | 60 |
| 15c | Cu/OMS-2 | DMSO | 80 | Air | 72 |
| 16c | Cu/OMS-2 | DMSO | 100 | Air | 54 |
| 17c | Cu/OMS-2 | DMSO | 80 | O2 | 75 |
| 18c | Cu/OMS-2 | DMSO | 80 | N2 | <5 |
| 19d | CuO + OMS-2 | DMSO | 80 | Air | 15 |
| 20 | CuO (1 equiv.) | DMSO | 80 | N2 | 56 |
| 21 | CuO (0.1 equiv.) | DMSO | 80 | Air | 41 |
To examine whether the observed catalytic activity is generated from the solid catalyst Cu/OMS-2 or the leached copper species, the hot filtration experiment was carried out with 1,4-enedione 1g and pyrazole 2a as substrates (specific details see ESI†). The catalyst was removed after the reaction was run for 3 hours, then the reaction was kept on, but the yield did not increase. At the same time, the filtrate was analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES), and the result displayed that the concentration of copper species in the solution is 0.45 ppm. Hence, it is concluded that the copper species leached from the catalyst is catalytically inactive and the catalysis is truly heterogeneous. Additionally, the life span of the catalyst is another aspect of the heterogeneous catalysis. The reactions between 1,4-enedione 1g and pyrazole 2a was used as the model reactants to test the recyclability of Cu/OMS-2. The catalyst was recovered by filtration and centrifugation, and results showed that the catalyst can recycle at least 3 times with slightly decrease of catalytic activity (Fig. 5). Furthermore, the result of TEM indicated that the used Cu/OMS-2 was intact in morphology, implying the stability of Cu/OMS-2 (Fig. S1, see ESI†). From the results of the hot filtration experiment (specific details see ESI†), we can speculate that the loss of the catalytic activity most likely results from the leaching of copper from the support.
 | ||
| Fig. 5 The recyclability of Cu/OMS-2. Reaction conditions: 1g (0.3 mmol), 2a (0.6 mmol) and Cu/OMS-2 (30 mg, 2 mol%) in 1.8 mL DMSO under air for 12 h. | ||
With the optimized conditions in hand, we investigated the scope of this amination catalyzed by Cu/OMS-2 (Table 2). Generally, this catalytic system could tolerate a large range of substrates and lead to moderate to good yields of corresponding products. In terms of R1 substituents, good yields were gained with various substituted groups, such as –Me, –OMe and halogenated substituents (Table 2, entries 1–6). Delightedly, when R3 was alkyloxy groups (–OMe, –OEt), the reaction proceeded smoothly with good to excellent yields of tetrasubstituted unsymmetrical 1,4-enediones (Table 2, entries 7–19).
| Entry | 1 | R1 | R2 | R3 | 3 | Yieldb (%) |
|---|---|---|---|---|---|---|
a Unless otherwise specified, all reactions were carried out using 1 (0.3 mmol), 2a (0.6 mmol) and Cu/OMS-2 (30 mg, 2 mol%) in 1.8 mL solvent under air for 12 h.b Isolated yields.c A mixture of E/Z-isomers was obtained, E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio determined by 1H NMR. Z ratio determined by 1H NMR. |
||||||
| 1 | 1a | Ph | Ph | Ph | 3aa | 72 |
| 2 | 1b | 4-Me-Ph | Ph | Ph | 3ba | 57 |
| 3 | 1c | 4-OMe-Ph | Ph | Ph | 3ca | 68 |
| 4 | 1d | 4-Cl-Ph | Ph | Ph | 3da | 56 |
| 5 | 1e | 4-Br-Ph | Ph | Ph | 3ea | 59 |
| 6 | 1f | 4-F-Ph | Ph | Ph | 3fa | 75 |
| 7c | 1g | Ph | Ph | OEt | 3ga | 80 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 66 Z = 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34) 34) |
| 8c | 1h | 4-Me-Ph | Ph | OEt | 3ha | 78 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 77 Z = 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23) 23) |
| 9c | 1i | 4-OMe-Ph | Ph | OEt | 3ia | 87 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 67 Z = 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33) 33) |
| 10c | 1j | 4-NO2-Ph | Ph | OEt | 3ja | 67 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 87 Z = 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13) 13) |
| 11c | 1k | 4-CF3-Ph | Ph | OEt | 3ka | 88 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 90 Z = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
| 12c | 1l | 2-Naphthyl | Ph | OEt | 3la | 90 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 81 Z = 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) 19) |
| 13c | 1m | 4-Cl-Ph | Ph | OEt | 3ma | 84 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 88 Z = 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) 12) |
| 14c | 1n | 4-Br-Ph | Ph | OEt | 3na | 87 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 89 Z = 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11) 11) |
| 15c | 1o | 4-F-Ph | Ph | OEt | 3oa | 81 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 88 Z = 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) 12) |
| 16c | 1p | Ph | 4-OMe-Ph | OEt | 3pa | 78 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 63 Z = 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37) 37) |
| 17c | 1q | Ph | 4-F-Ph | OEt | 3qa | 86 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 86 Z = 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14) 14) |
| 18c | 1r | Ph | 4-OMe-Ph | OMe | 3ra | 91 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 74 Z = 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26) 26) |
| 19c | 1s | Ph | 4-Cl-Ph | OMe | 3sa | 83 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 92 Z = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
Next, the scope of N-substituted nucleophiles was tested under the standard conditions with 1,4-enedione 1g as the model substrate (Table 3). Anilines proceeded very well in this system and moderate to good yields of corresponding products were observed with electron-neutral (3gb), electron-donating (3gc, 3gd), electron-withdrawing (3ge, 3gf, 3gg) substituents. Besides, N-methylaniline (3gh) and 6-chloropyridin-2-amine (3gi) could also be used as suited substrates. To our delight, aliphatic N-nucleophiles were also tolerated in this reaction and gave moderate yields of desired products (3gj, 3gk) with excellent E/Z ratio.
| a Unless otherwise specified, all reactions were carried out using 1g (0.3 mmol), 2 (0.6 mmol) and Cu/OMS-2 (30 mg, 2 mol%) in 1.8 mL DMSO under air for 12 h, isolated yields. E/Z ratio determined by 1H NMR. |
|---|
 |
Surprisingly, a cyclized product 4H-pyrido[1,2-a]-pyrimidin-4-one was obtained in 91% yield when 1,4-enedione 1g reacted with 2-aminopyridine under our standard reaction conditions. It is believed after 2-aminopyridine and 1,4-enedione undergo the aza-Michael addition and aerobic dehydrogenation, the nitrogen in pyridine ring further undergoes intramolecular amidation under the present conditions to generate the heterocycles 5. It is an encouraging result because 4H-pyrido[1,2-a]-pyrimidin-4-ones are a class of nitrogen-containing heterocycles with pharmacological activities, and they extensively exist in natural products and pharmaceutical drugs.20 So far, a number of synthetic protocols for construction of this skeleton have been reported.21 However, as far as we know, this is the first example to synthesize 4H-pyrido[1,2-a]-pyrimidin-4-ones under the heterogeneous conditions. Therefore, the scope of cyclizations was examined with a series of different 1,4-enediones 1 and various substituted 2-aminopyridines 4 under the present reaction conditions (Table 4). Many 1,4-enediones with different substituted groups were employed as the substrates and good to excellent yields of desired products were obtained. We also examined the scope of 2-aminopyridines in this reaction. Specifically, 3-methylpyridin-2-amine afforded the corresponding product in good yield (5gb). However, 6-chloropyridin-2-amine provided the product 3gi via C–N bond-forming instead of cyclied product 5gc because of the steric hindrance.
| a Unless otherwise specified, all reactions were carried out using 1 (0.3 mmol), 4 (0.6 mmol) and Cu/OMS-2 (30 mg, 2 mol%) in 1.8 mL DMSO under air for 12 h, isolated yields. |
|---|
 |
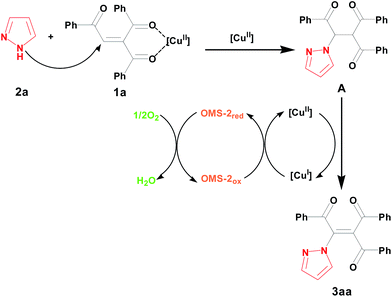
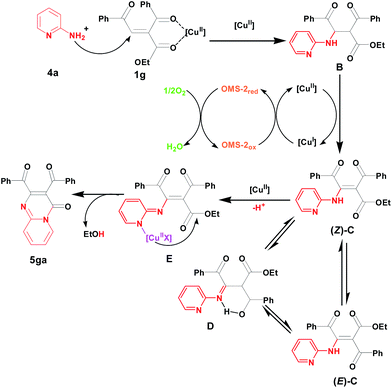
Based on the reported literatures and our results, we proposed a possible mechanism for these two kinds of reactions respectively. The first amination reaction consists of aza-Michael addition reaction between amine 2a and 1,4-enedione 1a with assistance of Cu and a following supported copper species catalyzed aerobic oxidative dehydrogenation of intermediate A under air (Fig. 6).14,22 For the cyclization, the first two steps are same as these of the amination. After that, the E-isomer amination intermediate (E)-C produces its Z-isomer amination intermediate (Z)-C through isomerization, finally the cyclized product 5ga is formed by supported copper species catalyzed intramolecular amidation (Fig. 7).21f In the two kinds of reaction mechanisms, the reduced copper species is reoxidized by OMS-2, and the reduced OMS-2 is reoxidized by O2 of air to finish the whole redox cycle.
Conclusions
In summary, we have developed an efficient heterogeneous strategy for the synthesis of tetrasubstituted 1,4-enediones and 4H-pyrido[1,2-a]-pyrimidin-4-ones catalyzed by Cu/OMS-2. The excellent catalytic ability of Cu/OMS-2 is attributed to OMS-2 which accelerates the reoxidation of reduced copper species through rapid electron transfer. This superiority might allow it to apply to other oxidative processes and reactions. Further applications of this strategy are underway in our laboratory.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (Grant No. 21403256, 21573261) and the Suzhou Industrial Technology and Innovation Project (Grant No. SYG201531).Notes and references
- (a) M. Pagliaro, V. Pandarus, R. Ciriminna, F. Béland and P. D. Carà, ChemCatChem, 2012, 4, 432 CrossRef CAS PubMed; (b) M. J. Climent, A. Corma and S. Iborra, RSC Adv., 2012, 2, 16 RSC; (c) R. A. Sheldon and H. V. Bekkum, Fine Chemicals through Heterogeneous Catalysis, Wiley-VCH, Weinheim, 2001 Search PubMed.
- For selected papers on heterogeneous catalysis, see: (a) X. Meng, X. Xu, T. Gao and B. Chen, Eur. J. Org. Chem., 2010, 5409 CrossRef CAS PubMed; (b) J. B. Bharate, S. K. Guru, S. K. Jain, S. Meena, P. P. Singh, S. Bhushan, B. Singh, S. B. Bharate and R. A. Vishwakarma, RSC Adv., 2013, 3, 20869 RSC; (c) F. Nemati, A. Elhampour, H. Farrokhi and M. B. Natanzi, Catal. Commun., 2015, 66, 15 CrossRef CAS PubMed; (d) B. Yang, Z. Mao, X. Zhu and Y. Wan, Catal. Commun., 2015, 60, 92 CrossRef CAS PubMed; (e) A. T. Nguyen, L. T. Pham, N. T. S. Phan and T. Truong, Catal. Sci. Technol., 2014, 4, 4281 RSC; (f) L. Zhang, P. Li, C. Liu, J. Yang, M. Wang and L. Wang, Catal. Sci. Technol., 2014, 4, 1979 RSC.
- J. R. Kona, C. K. Kingòndu, A. R. Howell and S. L. Suib, ChemCatChem, 2014, 6, 749 CrossRef CAS PubMed.
- (a) Y. C. Son, V. D. Makwana, A. R. Howell and S. L. Suib, Angew. Chem., 2001, 113, 4410 (Angew. Chem., Int. Ed., 2001, 40, 4280) CrossRef; (b) V. V. Krishnan and S. L. Suib, J. Catal., 1999, 184, 305 CrossRef CAS; (c) S. Sithambaram, Y. Ding, W. Li, X. Shen, F. Gaenzler and S. L. Suib, Green Chem., 2008, 10, 1029 RSC; (d) R. Kumar, S. Sithambaram and S. L. Suib, J. Catal., 2009, 262, 304 CrossRef CAS PubMed; (e) K. Yamaguchi, H. Kobayashi, T. Oishi and N. Mizuno, Angew. Chem., 2012, 124, 559 (Angew. Chem., Int. Ed., 2012, 51, 544) CrossRef PubMed; (f) K. Yamaguchi, Y. Wang and N. Mizuno, ChemCatChem, 2013, 5, 2835 CrossRef CAS PubMed; (g) K. Yamaguchi, H. Kobayashi, Y. Wang, T. Oishi, Y. Ogasawara and N. Mizuno, Catal. Sci. Technol., 2013, 3, 318 RSC; (h) Y. Wang, H. Kobayashi, K. Yamaguchi and N. Mizuno, Chem. Commun., 2012, 48, 2642 RSC; (i) X. Jin, K. Yamaguchi and N. Mizuno, RSC Adv., 2014, 4, 34712 RSC; (j) X. Jin, K. Yamaguchi and N. Mizuno, Angew. Chem., 2014, 126, 465 (Angew. Chem., Int. Ed., 2014, 53, 455) CrossRef PubMed; (k) K. Yamaguchi, Y. Wang, T. Oishi, Y. Kuroda and N. Mizuno, Angew. Chem., 2013, 125, 5737 (Angew. Chem., Int. Ed., 2013, 52, 5627) CrossRef PubMed; (l) X. Meng, J. Zhang, G. Chen, B. Chen and P. Zhao, Catal. Commun., 2015, 69, 239 CrossRef CAS PubMed.
- X. Meng, C. Yu, G. Chen and P. Zhao, Catal. Sci. Technol., 2015, 5, 372 CAS.
- T. Oishi, K. Yamaguchi and N. Mizuno, ACS Catal., 2011, 1, 1351 CrossRef CAS.
- (a) B. P. Babu, X. Meng and J. E. Bäckvall, Chem.–Eur. J., 2013, 19, 4140 CrossRef CAS PubMed; (b) Y. Endo and J. E. Bäckvall, Chem.–Eur. J., 2011, 17, 12596 CrossRef CAS PubMed; (c) B. P. Babu, Y. Endo and J. E. Bäckvall, Chem.–Eur. J., 2012, 18, 11524 CrossRef CAS PubMed; (d) M. Johansson, B. W. Purse, O. Terasaki and J. E. Bäckvall, Adv. Synth. Catal., 2008, 350, 1807 CrossRef CAS PubMed; (e) N. Gigant and J. E. Bäckvall, Org. Lett., 2014, 16, 1664 CrossRef CAS PubMed; (f) N. Gigant and J. E. Bäckvall, Chem.–Eur. J., 2013, 19, 10799 CrossRef CAS PubMed; (g) X. Meng, C. Li, B. Han, T. Wang and B. Chen, Tetrahedron, 2010, 66, 4029 CrossRef CAS PubMed; (h) J. Piera and J. E. Bäckvall, Angew. Chem., 2008, 120, 3558 (Angew. Chem., Int. Ed., 2008, 47, 3506) CrossRef PubMed.
- (a) X. F. Niu, X. Liu, L. Pan and L. Qi, Fitoterapia, 2011, 82, 960 CrossRef CAS PubMed; (b) A. Aiello, E. Fattorusso, S. Magno, M. Menna and M. Pansini, J. Nat. Prod., 1991, 54, 281 CrossRef CAS; (c) P. Cai, F. Kong, M. E. Ruppen, G. Glasier and G. T. Carter, J. Nat. Prod., 2005, 68, 1736 CrossRef CAS PubMed; (d) H. Abou-Gazar, E. Bedir, S. Takamatsu, D. Ferreira and I. A. Khan, Phytochemistry, 2004, 65, 2499 CrossRef CAS PubMed; (e) F. Lv, M. Xu, Z. Deng, N. J. de Voogd, R. W. M. van Soest, P. Proksch and W. Lin, J. Nat. Prod., 2008, 71, 1738 CrossRef CAS PubMed; (f) R. Ballini and P. Astolfi, Liebigs Ann., 1996, 11, 1879 CrossRef PubMed; (g) M. Fouad, R. A. Edrada, R. Ebel, V. Wray, W. E. G. Müller, W. H. Lin and P. Proksch, J. Nat. Prod., 2006, 69, 211 CrossRef CAS PubMed.
- (a) Y. Yang, M. Gao, C. Deng, D. X. Zhang, L. M. Wu, W. M. Shu and A. X. Wu, Tetrahedron, 2012, 68, 6257 CrossRef CAS PubMed; (b) Y. Yang, M. Gao, D. X. Zhang, L. M. Wu, W. M. Shu and A. X. Wu, Tetrahedron, 2012, 68, 7338 CrossRef CAS PubMed; (c) G. Yin, Z. Wang, A. Chen, M. Gao, A. Wu and Y. J. Pan, Org. Chem., 2008, 73, 3377 CrossRef CAS PubMed; (d) Y. Yang, M. Gao, L. M. Wu, C. Deng, D. X. Zhang, Y. Gao, Y. P. Zhu and A. X. Wu, Tetrahedron, 2011, 67, 5142 CrossRef CAS PubMed; (e) M. Gao, Y. Yang, Y. D. Wu, C. Deng, W. M. Shu, D. X. Zhang, L. P. Cao, N. F. She and A. X. Wu, Org. Lett., 2010, 12, 4026 CrossRef CAS PubMed; (f) W. M. Shu, Y. Yang, D. X. Zhang, L. M. Wu, Y. P. Zhu, G. D. Yin and A. X. Wu, Org. Lett., 2013, 15, 456 CrossRef CAS PubMed; (g) D. Zhang, Y. Yang, M. Gao, W. Shu, L. Wu, Y. Zhu and A. Wu, Tetrahedron, 2013, 69, 1849 CrossRef CAS PubMed; (h) L. Wu, Y. Yang, M. Gao, D. Zhang, W. Shu, Y. Zhu and A. Wu, Synlett, 2012, 23, 2137 CrossRef CAS; (i) Q. Gao, Y. Zhu, M. Lian, M. Liu, J. Yuan, G. Yin and A. Wu, J. Org. Chem., 2012, 77, 9865 CrossRef CAS PubMed.
- (a) M. K. Eberhardt, J. Org. Chem., 1993, 58, 497 CrossRef CAS; (b) J. Q. Yu and E. J. Corey, J. Am. Chem. Soc., 2003, 125, 3232 CrossRef CAS PubMed; (c) M. Gao, Y. Yang, Y. D. Wu, C. Deng, L. P. Cao, X. G. Meng and A. X. Wu, Org. Lett., 2010, 12, 1856 CrossRef CAS PubMed; (d) G. Yin, B. Zhou, X. Meng, A. Wu and Y. Pan, Org. Lett., 2006, 8, 2245 CrossRef CAS PubMed; (e) Z. Wang, G. Yin, A. Chen, S. Hu and A. Wu, Synth. Commun., 2007, 37, 4399 CrossRef CAS PubMed; (f) B. Crone and S. F. Kirsch, Chem. Commun., 2006, 764 RSC; (g) O. Prakash, A. Batra, V. Chaudhri and R. Prakash, Tetrahedron Lett., 2005, 46, 2877 CrossRef CAS PubMed; (h) A. M. Echavarren, M. Perez, A. M. Castano and J. M. Cuerva, J. Org. Chem., 1994, 59, 4179 CrossRef CAS; (i) S. K. Kang, Y. T. Lee and S. H. Lee, Tetrahedron Lett., 1999, 40, 3573 CrossRef CAS; (j) K. A. Runcie and R. J. K. Taylor, Chem. Commun., 2002, 974 RSC; (k) C. Deng, Y. Yang, M. Gao, Y. P. Zhu, A. X. Wu, J. R. Ma and G. D. Yin, Tetrahedron, 2012, 68, 3828 CrossRef CAS PubMed; (l) P. Wasnaire, T. de Merode and I. E. Markó, Chem. Commun., 2007, 4755 RSC; (m) A. Del Zotto, W. Baratta, G. Verardo and P. Rigo, Eur. J. Org. Chem., 2000, 2795 CrossRef CAS; (n) C. D. S. Lisboa, V. G. Santos, B. G. Vaz, N. C. de Lucas, M. N. Eberlin and S. J. Garden, J. Org. Chem., 2011, 76, 5264 CrossRef CAS PubMed.
- Y. Yang, F. Ni, W. M. Shu, S. B. Yu, M. Gao and A. X. Wu, J. Org. Chem., 2013, 78, 5418 CrossRef CAS PubMed.
- (a) X. Meng, Y. Wang, C. Yu and P. Zhao, RSC Adv., 2014, 4, 27301 RSC; (b) X. Meng, C. Yu and P. Zhao, RSC Adv., 2014, 4, 8612 RSC; (c) J. Liu, C. Yu, P. Zhao and G. Chen, Appl. Surf. Sci., 2012, 258, 9096 CrossRef CAS PubMed; (d) C. Yu, P. Zhao, G. Chen and B. Hu, Appl. Surf. Sci., 2011, 257, 7727 CrossRef CAS PubMed.
- S. Sithambaram, R. Kumar, Y. C. Son and S. L. Suib, J. Catal., 2008, 253, 269 CrossRef CAS PubMed.
- S. Sithambaram, L. Xu, C. H. Chen, Y. Ding, R. Kumar, C. Calvert and S. L. Suib, Catal. Today, 2009, 140, 162 CrossRef CAS PubMed.
- W. Y. Hernández, M. A. Centeno, S. Ivanova, P. Eloy, E. M. Gaigneaux and J. A. Odriozola, Appl. Catal., B, 2012, 123–124, 27 CrossRef PubMed.
- V. P. Santos, M. F. R. Pereira, J. J. M. Orfao and J. L. Figueiredo, Appl. Catal., B, 2010, 99, 353 CrossRef CAS PubMed.
- M. R. Morales, B. P. Barbero and L. E. Cadús, Fuel, 2008, 87, 1177 CrossRef CAS PubMed.
- Y. Yang, J. Huang, S. Zhang, S. Wang, S. Deng, B. Wang and G. Yu, Appl. Catal., B, 2014, 150–151, 167 CrossRef CAS PubMed.
- (a) X. S. Liu, Z. N. Jin, J. Q. Lu, X. X. Wang and M. F. Luo, Chem. Eng. J., 2010, 162, 151 CrossRef CAS PubMed; (b) M. F. Luo, Y. J. Zhong, X. X. Yuan and X. M. Zheng, Appl. Catal., A, 1997, 162, 121 CrossRef CAS; (c) M. F. Luo, Y. P. Song, J. Q. Lu, X. Y. Wang and Z. Y. Pu, J. Phys. Chem. C, 2007, 111, 12686 CrossRef CAS; (d) M. F. Luo, J. M. Ma, J. Q. Lu, Y. P. Song and Y. J. Wang, J. Catal., 2007, 246, 52 CrossRef CAS PubMed.
- (a) I. Hermecz and Z. Mészáros, Med. Res. Rev., 1988, 8, 203 CrossRef CAS PubMed; (b) I. Hermecz and L. Vasvari-Debreczy, Comprehensive Heterocyclic Chemistry III, ed. K. Jones Execut and A. R. Katritzky, 2007, pp. 77–217 Search PubMed; (c) C. Fenton and L. J. Scott, CNS Drugs, 2005, 19, 429 CrossRef CAS; (d) F. Awouters, J. Vermeire, F. Smeyers, P. Vermote, R. Van Beek and C. J. E. Niemegeers, Drug Dev. Res., 1986, 8, 95 CrossRef CAS PubMed; (e) L. E. J. Kennis, F. P. Bischoff, C. J. Mertens, C. J. Love, F. A. F. van den Keybus, S. Pieters, M. Braeken, A. A. H. P. Megens and J. E. Leysen, Bioorg. Med. Chem. Lett., 2000, 10, 71 CrossRef CAS; (f) R. L. Smith, R. J. Barrett and E. Sanders-Bush, J. Pharmacol. Exp. Ther., 1995, 275, 1050 CAS; (g) A. Pettersson, K. Gradin, T. Hedner and B. Persson, Naunyn-Schmiedeberg's Arch. Pharmacol., 1985, 329, 394 CrossRef CAS.
- (a) I. Hermecz, Adv. Heterocycl. Chem., 2003, 85, 173 CrossRef CAS; (b) J. Modranka and T. Janecki, Tetrahedron, 2011, 67, 9595 CrossRef CAS PubMed; (c) G. Bentabed-Ababsa, S. C. S. Ely, S. Hesse, E. Nassar, F. Chevallier, T. T. Nguyen, A. Derdour and F. Mongin, J. Org. Chem., 2010, 75, 839 CrossRef CAS PubMed; (d) P. Cěbasěk, D. Bevk, S. Pirc, B. Stanovnik and J. Svete, J. Comb. Chem., 2006, 8, 95 CrossRef PubMed; (e) A. Begouin, S. Hesse, M. J. R. P. Queiroz and G. Kirsch, Synthesis, 2006, 2794 CAS; (f) Y. Yang, W. M. Shu, S. B. Yu, F. Ni, M. Gao and A. X. Wu, Chem. Commun., 2013, 49, 1729 RSC.
- (a) O. A. Attanasi, G. Favi, P. Filippone, F. Mantellini, G. Moscatelli and F. R. Perrulli, Org. Lett., 2009, 12, 468 CrossRef PubMed; (b) L. W. Xu, J. W. Li, C. G. Xia, S. L. Zhou and X. X. Hu, Synlett, 2003, 2425 CAS; (c) D. Wang, Y. Shiraishi and T. Hirai, Chem. Commun., 2011, 47, 2673 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17351e |
| This journal is © The Royal Society of Chemistry 2015 |